Response Surface Optimization of Fixed Bed Adsorption of Cr+6 Onto Low-cost Adsorbent
Corresponding author Email: Padmajamegham@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.15.3.28
Copy the following to cite this article:
Padmaja M, Bhavani R. Response Surface Optimization of Fixed Bed Adsorption of Cr+6 Onto Low-cost Adsorbent. Curr World Environ 2020;15(3). DOI:http://dx.doi.org/10.12944/CWE.15.3.28
Copy the following to cite this URL:
Padmaja M, Bhavani R. Response Surface Optimization of Fixed Bed Adsorption of Cr+6 Onto Low-cost Adsorbent. Curr World Environ 2020;15(3). Available from: https://bit.ly/32WTNfs
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 26-03-2020 |
|---|---|
| Accepted: | 29-10-2020 |
| Reviewed by: | 
 Rajaa G
Rajaa G
|
| Second Review by: |

 Dr. Anoop Krishnan
Dr. Anoop Krishnan
|
| Final Approval by: | Dr. Gopal K |
Introduction
Tannery effluents are rampant as the most noteworthy pollutants among every industrial waste generating vast amounts of Chromium. For example, in India alone, around 2000–3000 tons of Cr+6 escapes into the water environment every year from tanneries. The permissible Chromium suggested is 2 mg/l, whereas it is extending in the range of 2000 and 5000 mg/l in the wastewaters [1].
The elevated concentrations of Chromium may cause many toxic, mutagenic and carcinogenic health effects. Out of the three oxidation states of Chromium, the Cr+3 is the most stable. The Cr+3 is essential in trace amounts required by humans, though unstable in a water environment because of its lower solubility in water. The Cr+6 concentration ranges in 0.5 to 270 mg/L in industrial effluents, which is highly toxic to many living beings, including plants and animals. [2]. The discharges of Cr+6 have tolerance limits of 0.1 mg/L (into inland surface waters), 0.05 mg/L (into potable water) [3,4].
Numerous Chromium removal technologies are employed which include reduction [5], chemical precipitation, electrochemical precipitation, ion exchange, adsorption, solvent extraction, and bio-sorption[6], membrane separation [7], reverse osmosis and Nanofiltration [8], emulsion per traction technology [9]. Out of various demerits of these methods, the most important being uneconomical. Adsorption technology is considered because of its cost-effectiveness and regenerative nature. Adsorption can remove variable pollutants such as heavy metals, dyes, phenols etc.[10, 11].
Response surface method was used by many researchers in the optimization of various process parameters in the adsorption process. Several studies are detrimental towards the use of many low-cost adsorbents including agricultural wastes, fruit wastes, microbes, industrial wastes, sludge and so on [12-20].
In this paper, the efficiency of Shrimp shells powder in the elimination of Chromium the from aqueous media was studied. The removal efficiency of SSW powder is tested for variable particle sizes, bed depths and a comparative study has also been done [20].To optimize and reduce the experimental complexity, time, the response surface methodology was used for process optimization.
Materials and Methods
Preparation of Stock Solution
A known quantity of 0.2828 gms of Potassium Dichromate (K2Cr2O7) was dissolved in 100ml of de-ionized water to prepare the standard solutions of Cr(VI) of 1000 ppm concentration. Subsequent dilutions were made from the standard solutions for performing the experiments; the initial (50 mg/L) and final concentrations were obtained from UV-Vis spectrophotometer having wavelength range between 520-550 nm [21].
The pH of the samples was regulated with acid (HCl) and base (NaCl). The oxidation of Cr+3 to Cr+6 is required to be done before the analysis of each sample to reduce Cr+6 to Cr+3.
Preparation of Shrimp Shell Powder
The Shrimp shell waste was obtained from a seafood processing unit; after collection, they were washed, dried and roughly ground to powder. Afterwards, the powder was soaked in HCl overnight, then treated with a base (NaCl) to remove any traces of acid and then rinsed with de-ionized water thoroughly to attain neutral pH. Now washed material was sun-dried and then in an oven at 103±2â—¦ C. The so dried powder was named as Shrimp shell waste (SSW) powder was further crushed, and after sieving, the obtained particle sizes were 150 microns, 300 microns and 600 microns. These three powders having variable sizes were stored in air-tight bags for experimental use.
Continuous Fixed bed Column Studies
A column with 2.5 cm internal diameter and having bed height 50 cm was set-up in a laboratory for conducting continuous fixed-bed column studies. A known quantity of freshly ground SSW powder was filled in the column bed for each set of experiments with varying bed depths and adsorbent sizes, respectively. The metal solution of known initial concentration(Ci ) selected based on industrial range [22] was continuously pumped using a peristaltic pump with a flow rate of (V
) selected based on industrial range [22] was continuously pumped using a peristaltic pump with a flow rate of (V = 5 mL /min, for all theexperimental sets. The metal adsorption capacity can be estimated by the equation given below:
= 5 mL /min, for all theexperimental sets. The metal adsorption capacity can be estimated by the equation given below:

Where, Ce and M being theequilibrium concentration of the Cr+6ion and weight of the adsorbent used in the column.
Central composite design (CCD)
The extensively employed model for fitting is CCD in Response Surface Methodology. In this method, only a minimum number of experiments are required for experimental modelling. Usually, the Central Composite Design (CCD) involves a 2nfactorial and nc centre runs (six replicates). The response surface models using ANOVA (Analysis of Variance) have arrived from the respective experimental responses and thecorresponding optimized factors. Also, the statistical parameters were estimated using response surface methods. Essentially, theprocess optimization comprises of three chief steps, to carry out the design of experiments (DOE), using a mathematical model for coefficient calculation and response prediction and to examine the appropriateness of the model.
Results and Discussion
Influence of Size on Adsorption Capacity
Replicates of column experiments with the three selected adsorbent sizes 150, 300 and 600 microns were conducted with an initial Cr+6 concentration of 50 ppm. A total of nine experimental sets were constructed in the study with varying factors, as discussed earlier. Table.1 Shows the concentration of Cr+6with time from 0.5 to 8.0 hrs in column adsorption. It was noticed that the metal ion removal increased till a specific time increment and later on, it gradually decreased as it reached saturation of the adsorbent material. A fresh adsorbent was used for each experimental set-up, and the adsorption capacity was observed for the factors mentioned above with three levels. The mean of the replicates calculated for all the three adsorbent sizes and the values are given in Table.1. The column breakthrough analysis gives an idea on the saturation time of an adsorbent and the extent of adsorption, which can therefore be compared with other adsorbents for their suitability analysis. The breakthrough curves for the selected adsorbent size were plotted for 20 cm,40 cm and 60 cm bed depths as shown in Fig.1 a), b) & c). It is evident that the smallest sized adsorbent, i.e. 150 µm reached the breakthrough (equilibrium) concentration first than others. The highest performance (removal) was 2.23 ppm, 5.13 ppm and 7.45 ppm shown in the order 150 µm > 300 µm > 600 µm size, respectively.The above particulars illustrate, smaller the adsorbent size, greater the adsorption capacity and though the surface area of the smaller particles is less but more number of such particles occupy the column might be the reason.
Table 1: Adsorbate Concentration w.r.t Bed Depth atvarioustime intervals
|
Time (Hrs) |
Cr+6Conc. (mg/L) = 50 mg/L |
||||||||
|
BD=20 cm |
BD= 40 cm |
BD= 60 cm |
|||||||
|
A |
B |
C |
A |
B |
C |
A |
B |
C |
|
|
0.5 |
16.42 |
18.53 |
22.98 |
14.77 |
27.34 |
30.99 |
20.98 |
23.75 |
34.32 |
|
1 |
9.67 |
8.43 |
17.65 |
8.02 |
14.78 |
18.43 |
14.23 |
17 |
21.76 |
|
1.5 |
7.49 |
6.45 |
13.09 |
5.87 |
10.54 |
14.19 |
12.05 |
14.82 |
17.52 |
|
2 |
6.24 |
5.87 |
8.54 |
4.59 |
6.54 |
9.76 |
10.8 |
15.88 |
13.09 |
|
2.5 |
5.56 |
7.9 |
7.65 |
3.91 |
5.32 |
8.97 |
8.12 |
12.65 |
12.3 |
|
3 |
4.65 |
16.96 |
7.34 |
3.33 |
7.54 |
13.87 |
3.87 |
8.55 |
7.45 |
|
3.5 |
3.98 |
26.09 |
10.67 |
2.99 |
12.76 |
22.76 |
2.23 |
5.13 |
10.43 |
|
4 |
3.56 |
34.45 |
16.86 |
18 |
20.66 |
27.45 |
9.65 |
14.78 |
16.75 |
|
4.5 |
5.56 |
42.68 |
22.87 |
24.01 |
27.44 |
34.87 |
13.87 |
21.66 |
22.9 |
|
5 |
12.76 |
43.21 |
29.33 |
30.47 |
32.89 |
38.76 |
18.55 |
27.31 |
28.09 |
|
5.5 |
19.64 |
44.9 |
35.89 |
37.03 |
38.54 |
41.66 |
27.43 |
32.65 |
32.67 |
|
6 |
28.64 |
45.96 |
39.65 |
40.79 |
42.3 |
43.78 |
34.87 |
39.76 |
38.15 |
|
6.5 |
37.96 |
44.67 |
43.02 |
44.16 |
43.99 |
44.74 |
39.77 |
40.32 |
40.43 |
|
7 |
45.89 |
43.86 |
42.66 |
43.8 |
44.67 |
45.43 |
41.22 |
41.17 |
41.21 |
|
7.5 |
47.65 |
43.33 |
41.76 |
42.9 |
45.31 |
46.55 |
43.86 |
42.33 |
42.77 |
|
8 |
47.08 |
43.05 |
41.34 |
42.48 |
46.76 |
47.44 |
44.12 |
43.44 |
43.9 |
Where, A-150 µm, B-300 µm, C-600 µm, BD-Bed Depth
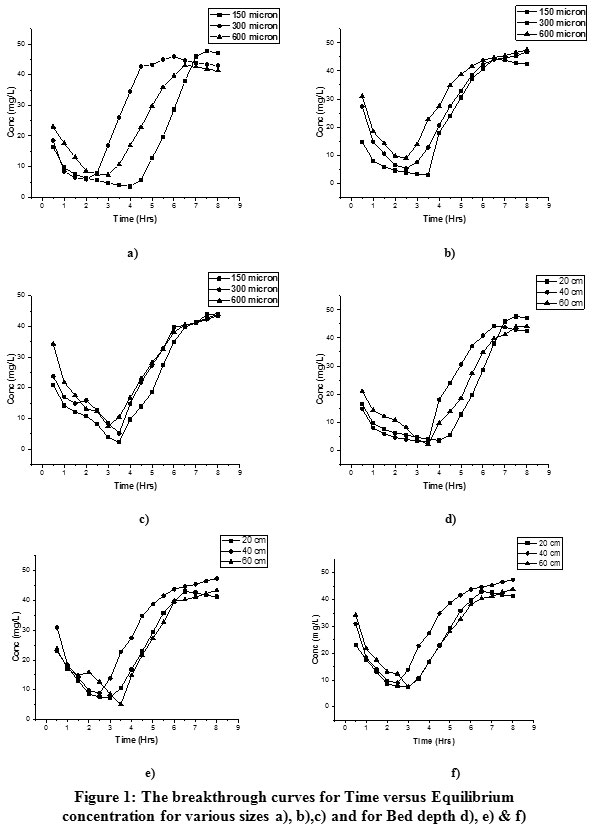 |
Figure 1: The breakthrough curves for Time versus Equilibrium concentration for various sizes a), b),c) and for Bed depth d), e) & f) Click here to view figure |
Influence of Bed Depth on Adsorption Capacity
Table.1 shows the influence of bed depth onthe outlet Chromium concentrationin the adsorption. The maximum adsorption was found to be at 60 cm. The maximum adsorption capacity was 231.65 ppm, at 150 µm, respectively.This adsorption indicates that as the depth of the adsorption bed increases the removal also increases. The reason behind this might be because of an extra dose of adsorbent in the glass column. More the depth of the adsorbent, the metal water passes through the additional layers of adsorbent and increases the travel time along with adsorption. The breakthrough curves can be seen in Fig.1 d), e) & f) show that in all the three cases, the adsorption capacity increased with bed depth.
Optimization using Response surface
CCD in Response surface method full factorial is used to optimize the experiments, and Table.2 shows the details of runs conducted in the test. The maximum adsorption capacity was obtained in the 3rd run at 231.65 mg/g for adsorbent size 150 µm and a bed depth of 60 cm. It is evident from the 3D response surface plot in Fig. 2 that the optimum parameters for effective adsorption are 150 µm and 60 cm and the design values are almost on par with the experimental values. The P-value and F-value in ANOVA are also shown in Table.3 where P<0.0.5specify model is significant. The fit statistics from Table.4 shows that R2=0.96, which is a good fit for the selected model.
Table 2: CCD for Cr+6 for factors size and bed depth
|
Factor 1 |
Factor 2 |
Response 1 |
|||
|
Std |
Group |
Run |
a: Size |
B: Bed Depth |
Adsorption Capacity |
|
µm |
cm |
mg/g |
|||
|
9 |
1 |
1 |
150 |
40 |
126.45 |
|
8 |
1 |
2 |
150 |
40 |
125.34 |
|
2 |
2 |
3 |
150 |
60 |
231.65 |
|
1 |
2 |
4 |
150 |
20 |
105.43 |
|
19 |
3 |
5 |
375 |
40 |
87.44 |
|
18 |
3 |
6 |
375 |
40 |
85.44 |
|
17 |
3 |
7 |
375 |
40 |
86.31 |
|
13 |
4 |
8 |
375 |
60 |
98.45 |
|
12 |
4 |
9 |
375 |
20 |
67.44 |
|
16 |
5 |
10 |
375 |
40 |
75.76 |
|
14 |
5 |
11 |
375 |
40 |
77.32 |
|
15 |
5 |
12 |
375 |
40 |
76.45 |
|
7 |
6 |
13 |
375 |
40 |
75.34 |
|
5 |
6 |
14 |
375 |
40 |
77.32 |
|
6 |
6 |
15 |
375 |
40 |
75.34 |
|
10 |
7 |
16 |
600 |
40 |
56.45 |
|
11 |
7 |
17 |
600 |
40 |
57.34 |
|
4 |
8 |
18 |
600 |
60 |
65.34 |
|
3 |
8 |
19 |
600 |
20 |
51.22 |
Table 3: Response in CCD design values for Cr+6 adsorption
|
Source |
Term df |
Error df |
F-value |
p-value |
|
|
Whole-plot |
3 |
3.99 |
19.69 |
0.0075 |
significant |
|
a-Size |
1 |
4.07 |
50.68 |
0.0019 |
|
|
a² |
1 |
3.94 |
4.02 |
0.1165 |
|
|
B² |
1 |
4.02 |
2.16 |
0.2157 |
|
|
Subplot |
2 |
8.79 |
69.02 |
< 0.0001 |
significant |
|
B-Bed Depth |
1 |
8.79 |
84.07 |
< 0.0001 |
|
|
aB |
1 |
8.79 |
53.97 |
< 0.0001 |
Table 4: Fit Statistics for Cr+6 adsorption
|
Std. Dev. |
13.70 |
R² |
0.9671 |
|
Mean |
89.57 |
Adjusted R² |
0.9461 |
|
C.V. % |
15.29 |
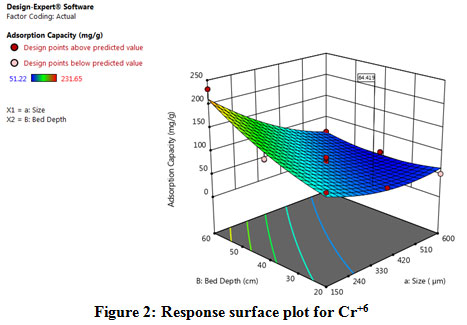 |
Figure 2: Response surface plot for Cr+6 Click here to view figure |
Conclusion
Chromium ion was eliminated from aqueous media fixed in a continuous column using various particle sizes of SSW powder. The chosen adsorbent can be considered as a reusablewaste material for industrial effluent treatment in terms of economic sense. Theadsorption capacity is less for low concentrations of Cr+6, however, it increased with time for the lower initial concentration. Fresh SSW powder with 150 micron size used in column adsorption for 8 hours can bring down Chromium concentration from 50 ppm to 3 ppm. The adsorption capacity of Cr+6 using SSW adsorbent were as follows: 150 microns> 300 microns> 600 microns. The response surface optimization model was successful in the study using SSW as adsorbent material. ANOVA in the response surface modelling results indicated a good fit for the adsorption.The adsorbent regeneration is irrelevant in the case of low-cost adsorbents as vast amounts of sea waste is being generated and dumped daily. TheSSWas an adsorbent addresses a two-fold advantage in terms of reuse of waste as well as the waste disposal aspect mainly in developing countries.
References
- Nazir, Ruqia, et al. "Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam Kohat." Journal of pharmaceutical sciences and research 7.3 (2015): 89.
- Bharagava, Ram Naresh, and Sandhya Mishra. "Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries." Ecotoxicology and Environmental Safety 147 (2018): 102-109.
CrossRef - Hossain, Md Anwar, et al. "Quality and metallic pollution level in surface waters of an urban industrialized city: a case study of Chittagong city, Bangladesh." Journal of Industrial Safety Engineering 4.2 (2017): 9-18.
CrossRef - Igwe, P. U., et al. "A review of environmental effects of surface water pollution." International Journal of Advanced Engineering Research and Science 4.12 (2017): 237340.
CrossRef - Zhao, Zengying, et al. "Progress on the photocatalytic reduction removal of chromium contamination." The Chemical Record 19.5 (2019): 873-882.
CrossRef - Peng, Hao, and Jing Guo. "Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review." Environmental Chemistry Letters (2020): 1-14.
CrossRef - Mohammed, Kemal, and Omprakash Sahu. "Recovery of chromium from tannery industry waste water by membrane separation technology: Health and engineering aspects." Scientific African 4 (2019): e00096.
CrossRef - Mnif, Amine, et al. "Hexavalent chromium removal from model water and car shock absorber factory effluent by nanofiltration and reverse osmosis membrane." International Journal of Analytical Chemistry 2017 (2017).
CrossRef - Ferreira, Larysse Caixeta, et al. "Mn (II) removal from water using emulsion liquid membrane composed of chelating agents and biosurfactant produced in loco." Journal of Water Process Engineering 29 (2019): 100792.
CrossRef - Khalifa, E. Ben, et al. "Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent." Chemometrics and Intelligent Laboratory Systems 189 (2019): 18-26.
CrossRef - Afroze, Sharmeen, and Tushar Kanti Sen. "A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents." Water, Air, & Soil Pollution 229.7 (2018): 225.
CrossRef - Khalifa, E. Ben, et al. "Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent." Chemometrics and Intelligent Laboratory Systems 189 (2019): 18-26.
CrossRef - Bhatti, Ijaz Ahmad, et al. "Chromium adsorption using waste tire and conditions optimization by response surface methodology." Journal of environmental chemical engineering 5.3 (2017): 2740-2751.
CrossRef - Kumar, Dhananjay, Lalit K. Pandey, and J. P. Gaur. "Metal sorption by algal biomass: from batch to continuous system." Algal research 18 (2016): 95-109.
CrossRef - Van Thuan, Tran, et al. "Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel." Surfaces and Interfaces 6 (2017): 209-217.
CrossRef - Rout, Prangya Ranjan, Rajesh Roshan Dash, and Puspendu Bhunia. "Nutrient removal from binary aqueous phase by dolochar: highlighting optimization, single and binary adsorption isotherms and nutrient release." Process Safety and Environmental Protection 100 (2016): 91-107.
CrossRef - Åžahan, Tekin. "Application of RSM for Pb (II) and Cu (II) adsorption by bentonite enriched with SH groups and a binary system study." Journal of Water Process Engineering 31 (2019): 100867.
CrossRef - Mahmoodi, Niyaz Mohammad, Mohsen Taghizadeh, and Ali Taghizadeh. "Activated carbon/metal-organic framework composite as a bio-based novel green adsorbent: Preparation and mathematical pollutant removal modeling." Journal of Molecular Liquids 277 (2019): 310-322.
CrossRef - Magoling, Bryan John Abel, and Angelica Angeles Macalalad. "Optimization and response surface modelling of activated carbon production from Mahogany fruit husk for removal of chromium (VI) from aqueous solution." BioResources 12.2 (2017): 3001-3016.
CrossRef - Khan, Saif Ullah, et al. "Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation." Process Safety and Environmental Protection 122 (2019): 118-130.
CrossRef - Zhao, Nan, et al. "Adsorption and coadsorption mechanisms of Cr (VI) and organic contaminants on H3PO4 treated biochar." Chemosphere 186 (2017): 422-429.
CrossRef - Pantazopoulou, E., and A. Zouboulis. "Chemical toxicity and ecotoxicity evaluation of tannery sludge stabilized with ladle furnace slag." Journal of environmental management 216 (2018): 257-262.
CrossRef






