Impact of Neem Oil on Malathion in the Fish Oreochromis mossambicus
DOI: http://dx.doi.org/10.12944/CWE.15.2.19
The far reaching dissemination and toxic nature of pesticides and their elements seriously affects the aquatic surroundings, and exerts negative consequences on the related organisms. The present study was carried out to investigate the effect of malathion and neem oil individually and also in combination on the fresh water fish, Oreochromis mossambicus to elucidate the change in the different target organs like liver and kidney with reference to biochemical and histopathological studies. Median lethal concentration (LC50) of neem oil and malathion was maintained for acute toxicity (96 hours) and chronic toxicity (21 days) studies. Further the fishes were segregated into 4 groups where in each group contained 6 fishes. Group I fishes were maintained in tap water, Group II in neem oil, Group III fishes were maintained in malathion , Group IV fishes were maintained in both neem oil and malathion. The LC50 dose of neem oil and malathion was found to be 0.9 ml/L and 3.52 mg/L respectively. The results of biochemical analysis revealed that total protein and lipid increased in combination of neem oil and malathion which was comparatively more than that of the neem oil and malathion maintained individually. The total free sugar showed a decrease in combination as well as individually in acute toxicity study .However there is an increase in total free sugar as observed in chronic toxicity study. Histopathological study in acute and chronic toxicity (i) in liver, fishes exposed to neem oil showed normal hepatocellular architecture while fishes exposed to malathion only and those of malathion combined with neem oil showed hepatocellular degeneration while the latter showed the signs of recovery (ii) in kidney the neem oil exposed fishes showed normal renal architecture , malathion exposed fishes showed epithelial cell degeneration and necrosis, while those exposed to neem oil and malathion combined showed mild degeneration. This study indicated the action of neem oil which has interacted with malathion and reveals a protective influence on harmful effects of the toxicant.
Copy the following to cite this article:
Jothigayathri D, Azeez A, Begum F. A, Ghazia C. M. L. Impact of Neem Oil on Malathion in the Fish Oreochromis mossambicus Curr World Environ 2020; 15(2). DOI:http://dx.doi.org/10.12944/CWE.15.2.19
Copy the following to cite this URL:
Jothigayathri D, Azeez A, Begum F. A, Ghazia C. M. L. Impact of Neem Oil on Malathion in the Fish Oreochromis mossambicus Curr World Environ 2020; 15(2). Available from: https://bit.ly/3iOIexw
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 22-03-2020 |
|---|---|
| Accepted: | 03-07-2020 |
| Reviewed by: | 
 Sunil Saini
Sunil Saini
|
| Second Review by: |

 Ashwani Khajuraho
Ashwani Khajuraho
|
| Final Approval by: | Dr. Umesh Chandra Kulshrestha |
Introduction
A greater part of the natural aquatic environment is faced with the risk of a shrinking genetic base and biodiversity due to the haphazard use of pesticides.1 Pesticides have been used for centuries in agriculture for increased food production by destroying undesirable bugs and to control disease vectors.2 Pesticides that reach the water frame can accumulate in fish and molluscs which might be dangerous to humans whilst ingested.3 Fishes are vulnerable to toxic substances and their bioaccumulation causes serious risk to life.4 The pesticides affect the aquatic environment by disrupting the aquatic food chain leading to the loss/ shift in enormous amount of natural invertebrate as well as vertebrate life forms in the water habitats.5, 6 The toxicity studies are extremely useful for vulnerable species of an ecosystem and can be used to track different forms of pollutants in assessing the indicator organisms. Toxicity tests are usually stated with respect to median lethal concentration LC50. Histopathological assessment has been increasingly recognized as an effective tool for evaluating the environmental effects.7, 8 An organophosphate pesticide leaves residues in soil and water after exposure for several days, posing a constant threat to non-target species, particularly fish.9 Malathion is a major source of environmental poisoning in the developing countries.10 Malathion (Diethyl 2-[(dimethoxyphosphorothioyl)sulfanyl]butanedioate) is an organophosphate insecticides widely used in agriculture and house to control variety of insects.11, 12 Neem extracts and pure compounds have been evaluated against more than 300 species of insect pests. Neem oil contains several triterpenoids like azadiractin, salanin, alkaloids, flavanoids, glycosides etc.13 Neem oil was found to be very effective as antirepellent and insecticide.14 It was selected as it is a biodegradable safe, efficient, versatile, ecofriendly, low cost and natural biopesticide. Oreochromis mossambicus a freshwater fish is tolerant to a broad range of environmental conditions. The purpose of this study was to assess whether neem oil can influence malathion and combat with the stress and toxic effects on the fish Oreochromis mossambicus and elucidate changes in the different target organs like liver and kidney with reference to histopathological and biochemical studies.
Materials and Methods
Oreochromis mossambicus were collected from the Hydrological Research Station, Chetpet, Chennai in a clean container of 10 litres capacity ensuring that they were not harmed either physically or physiologically during collection and transportation. The fishes were acclimatized to the laboratory condition for a week by keeping them in clean glass containers of 25 litres capacity containing dechlorinated and aerated tap water. Active fishes of almost same size 5-6 cm length were selected for the experiment , precautions were followed as per.15
In the present investigation, a biopesticide (Herb essential Pure Neem Oil) and an organophosphate pesticide (Malathion) were chosen. Malathion was obtained from International Institute of Biotechnology and Toxicology Research (IIBTR), Paddapai.
The bioassay procedure16, 17 followed in the present investigation to determine the LC50 for both malathion and neem oil are reported in literature.
Fishes weighing from 10-20g were used in the present study. The bioassay was carried out for malathion, neem oil and the combination of malathion and neem oil separately. After calculating the LC50 values, the fishes were segregated into 4 groups and each group contained 6 fishes. Group 1 fishes were maintained in tap water, Group II in neem oil, Group III fishes were maintained in malathion , Group IV fishes were maintained in both neem oil and malathion. The LC50 dose of neem oil was found to be 0.9 ml/L and that of malathion was found to be 3.52 mg/L.
The fishes were starved prior to the experiment for a period of 24 hours. After the said period of 96 hours (acute toxicity) and 21 days (chronic toxicity), the fishes both control and experimental were sacrificed. The tissues were excised immediately and used for the present study. The tissues selected for the study were the liver and kidney.
The biochemical constituents like total protein, total free sugar and lipids were analysed for median lethal toxicity in the tissues selected by using the following standard procedures. Total protein, total free sugar and lipids18, 19, 20 were estimated by the methods described in the literature.
For histopathological preparation the different organs of the fishes such as liver and kidney of both control and experimental groups were excised and immediately fixed in Bouin's fluid. The tissues were then processed as with Haematoxylin and Eosin stains.21 as described in the literature. Photo-micrographs were taken to substantiate observation and the results were discussed. Statistical analysis was carried out with two-way analysis of variances (ANOVA) followed by LSD tests using SPSS computer package.
Result
Results for biochemical analysis of protein, total free sugar and lipids from acute and chronic toxicity studies were obtained in all groups. The total protein content in the Group II fishes was observed to have a slight decrease while in the Group III fishes it’s significantly decreased. However an increase in Group IV was recorded which is comparatively more than Group III and this increase was found to be statistically significant. Acute and chronic toxicity study for total protein did not show a significant change (Table 1 and Fig 1). The total free sugar showed a decrease in all the groups subjected to acute toxicity study. However the chronic toxicity study showed an increase (Table 2 and Fig 2). The lipid content showed a slight decrease in Group III fishes more than Group II. However an increase in Group IV was recorded which is comparatively more than Group III and the increase was found to be statistically significant. Lipid content in all groups of acute toxicity was less when compared to chronic toxicity (Table 3 and Fig 3). The results were observed to be significant at p<0.0001.
Table 1: Protein content in kidney and liver of Oreochromis mossambicus of acute and chronic toxicity studies (mg/100mg of wet tissue)
|
Groups |
Acute Toxicity |
Chronic Toxicity |
||||||
|
Kidney |
Liver |
Kidney |
Liver |
|||||
|
Mean |
S.D |
Mean |
S.D |
Mean |
S.D |
Mean |
S.D |
|
|
I |
9.34 |
±0.09 |
10.58 |
±0.14 |
9.22 |
±0.07 |
10.31 |
±0.09 |
|
II |
7.25 |
±0.08 |
8.4 |
±0.17 |
7.19 |
±0.06 |
8.32 |
±0.08 |
|
III |
3.16 |
±0.05 |
4.23 |
±0.06 |
3.08 |
0.04 |
4.16 |
±0.04 |
|
IV |
5.2 |
±0.07 |
6.43 |
±0.11 |
5.11 |
±0.08 |
6.23 |
±0.07 |
 |
Figure 1: Protein content in kidney and liver of Oreochromis mossambicusof acute and chronic toxicity studies (mg/100mg of wet tissue)
|
Table 2: Total free sugars in kidney and liver of Oreocheomis mossambicus of acute and chronic toxicity studies (mg/100mg of wet tissue)
|
Groups |
Acute Toxicity |
|
Chronic Toxicity |
|||||
|
Kidney |
Liver |
Kidney |
Liver |
|||||
|
Mean |
S.D |
Mean |
S.D |
Mean |
S.D |
Mean |
S.D |
|
|
I |
11.14 |
±0.07 |
12.22 |
±0.07 |
10.29 |
±0.06 |
14.22 |
±0.07 |
|
II |
9.15 |
±0.10 |
10.24 |
±0.06 |
12.22 |
±0.08 |
16.38 |
±0.08 |
|
III |
4.39 |
±0.12 |
6.15 |
±0.09 |
6.24 |
±0.11 |
10.10 |
±0.03 |
|
IV |
6.28 |
±0.05 |
8.20 |
±0.02 |
8.22 |
±0.04 |
12.16 |
±0.16 |
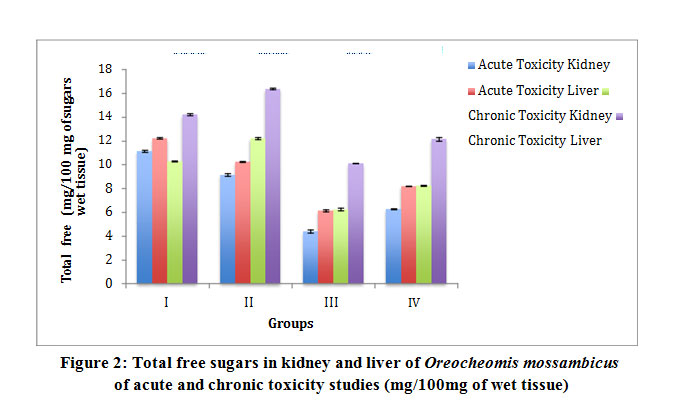 |
Figure 2: Total free sugars in kidney and liver of Oreocheomis mossambicus of acute and chronic toxicity studies (mg/100mg of wet tissue)
Click here to View Figure |
Table 3: Lipid content in kidney and liver of Oreochromis mossambicus of acute and chronic toxicity studies (mg/100mg of wet tissue)
|
Groups |
Acute Toxicity |
Chronic Toxicity |
||||||
|
Kidney |
Liver |
Kidney |
Liver |
|||||
|
Mean |
S.D |
Mean |
S.D |
Mean |
S.D |
Mean |
S.D |
|
|
I |
9.32 |
±0.10 |
10.24 |
±0.08 |
7.28 |
±0.05 |
8.37 |
±0.14 |
|
II |
7.33 |
±0.04 |
8.36 |
±0.10 |
5.26 |
±0.10 |
6.25 |
±0.11 |
|
III |
3.25 |
±0.06 |
4.31 |
±0.06 |
3.28 |
±0.04 |
3.21 |
±0.18 |
|
IV |
5.25 |
0.09 |
6.38 |
±0.12 |
4.26 |
±0.07 |
5.22 |
±0.04 |
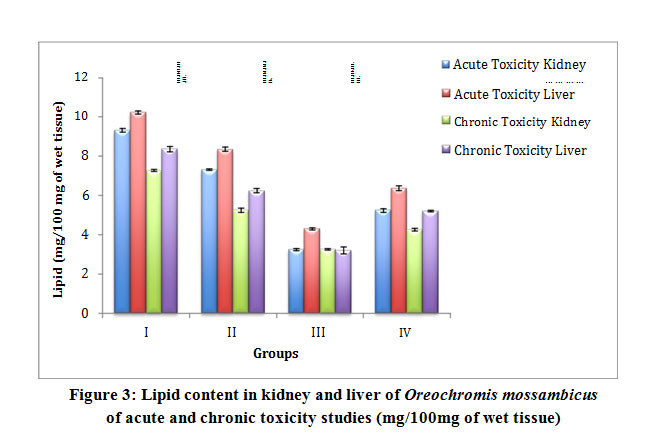 |
Figure 3: Lipid content in kidney and liver of Oreochromis mossambicus of acute and chronic toxicity studies (mg/100mg of wet tissue) |
Histopathology of liver for acute toxicity study of Group II fishes exposed to neem oil showed normal hepatocellular architecture and the Group III fishes exposed to malathion showed degeneration and coagulative necrosis of the hepatocytes. Group IV fishes showed the degeneration of hepatocytes while showing signs of recovery (Fig 4). In chronic toxicity, Group II fishes showed normal necrosis of the hepatic cells and the Group III fishes exposed to malathion showed mild degeneration and necrosis of the hepatocytes. Group IV fishes showed slight degeneration and multifocal fatty changes in the hepatocytes (Fig 5).
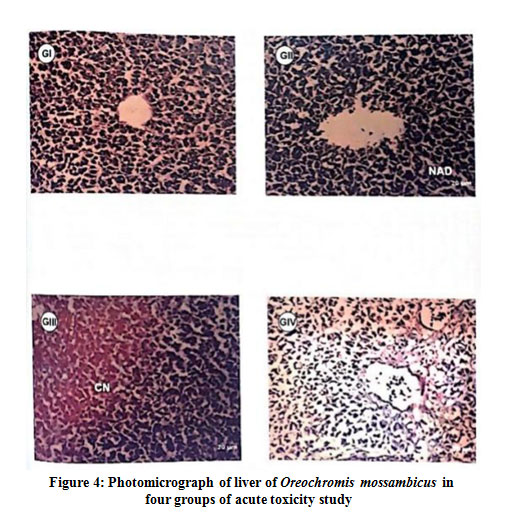 |
Figure 4: Photomicrograph of liver of Oreochromis mossambicus in four groups of acute toxicity study |
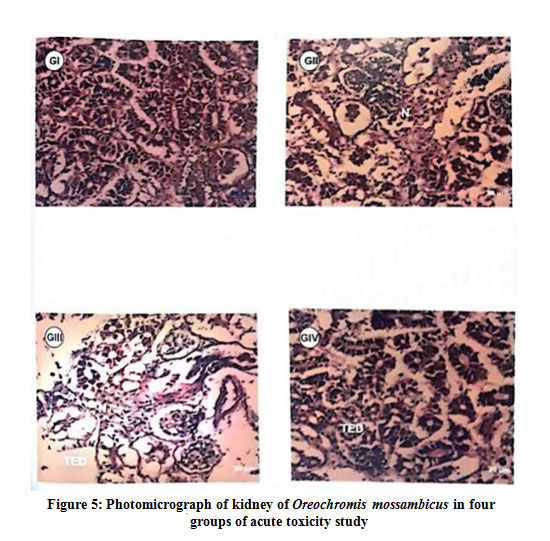 |
Figure 5: Photomicrograph of kidney of Oreochromis mossambicus in four groups of acute toxicity study |
In the histopathology studies of kidney in the acute toxicity study, Group II fishes showed necrosis and Group III fishes showed tubular epithelial cell degeneration and the necrosis and the Group IV fishes showed tubular epithelial cell degeneration (Fig 6). In chronic toxicity study, Group II fishes showed normal renal cellular architecture and the Group III fishes showed tubular epithelial cell degeneration and necrosis and Group IV fishes showed mild tubular epithelial cell degeneration and necrosis (Fig 7).
 |
Figure 6: Photomicrograph of liver of Oreochromis mossambicus in four groups of chronic toxicity study |
 |
Figure 7: Photomicrograph of kidney of Oreochromis mossambicus in four groups of chronic toxicity study |
Discussion
The present investigation shed light on the biochemical and histopathological changes in kidney and liver of Oreochromis mossambicus through acute and chronic toxicity studies of an organophosphate insecticide, malathion and neem oil. The Food and Drug Administration (FDA) and the EPA allow for the existence of a limit of around 8 ppm of malathion as a residue in specific food products. Studies in herbaceous plant Centella asiatica reported its highest residual level of malathion at 19.78μg / kg (0.198 ppm).22 Biochemical changes caused by toxicant stress may lead to the disturbance in the metabolism in animals and man. Reduced protein levels can be due to stress induced immobilization of these compounds in order to fulfill an energy-enhancing function to cope with toxicant-exposed environmental conditions.23 The decrease in protein level in liver of Group III and Group IV fishes of both acute and chronic toxicity may be due to necrotic action caused by the pesticides as evident by the histomorphological damage in liver cells and the study is in agreement with the work of24 in the cybermethrin treated rats. But the decrease in Group III and Group IV is not much when compared to Group I indicating. The recovery in the structural and the biochemical changes in the tissues of liver and kidney may be due to preventive nature of neem which has interacted with malathion in combination and thereby reducing the detoxifying effect of malathion. The decline in protein content was more in prevalent in the liver, since liver is a major metabolic centre and has a greater variation in protein content than the kidney. Reduction of carbohydrates indicates the possibility of active glycogenolysis in the glycolytic pathway to provide surplus energy in stress conditions.25 The decrease in the total free sugar in liver and kidney in Group III may be due to greater mobilization of glycogen from liver for metabolic purpose under pesticidal stress. The decrease in total free sugar in Group IV was not much as in Group III since resynthesis of RNA has taken place which showed an increase when compared to Group III indicating to a certain extent the protective influence of neem oil which may have detoxified the effect of malathion. Liver is the most active and effective tissue capable of detoxifying pesticides of chemical followed by kidney. 26 The total free sugar were found to be increased in the chronic toxicity study of Group II, Group III and Group IV since hyperglycemia an index of stress response occurs in fish challenges with stressors.27,28 The lipid serves as protective reservoir helping to prevent toxicant from reaching the target organs in larger fraction. 29 It may be noted from the result that there is an increase in the lipid content in Group II and again a decrease in Group III and further an increase in Group IV fishes. Decrease in lipid content may be attributed either due to the oxidation or hydrolysis of lipids, The present study is in agreement with the studies of30 where in the neem extract acts as a protective agent against the chemicals .A gradual recovery was observed in biochemical parameters in liver and kidney in Group IV but still complete recovery was not observed which may be due to short time duration. From the result of histopathological study in acute and chronic toxicity study the neem oil has a capacity to detoxify the organophosphate compound malathion by reducing its toxic nature or inhibitory effect and their by interacting in the combination of neem oil contains constituents which can degrade the malathion and proves the ameliorative effect of neem oil.
Conclusion
In future, instead of a single or dual chemical for synergetic study, combination of a chemical or pesticide along with a herbal compound may be encouraged to encounter the toxic effects on the non-target species, so that the pesticides acts on the target organisms and the herbal compound and further it may take a longer period for an effective decline of toxicity caused by pesticide.
Acknowledgement
We thank the Justice Basheer Ahmed Sayeed College for Women for the technical and logistic support provided to carry out the research work.
Funding Source
The authors received no financial support for the research work and authorship of the article.
Conflict of Interest
The authors do not have any conflict of interest.
References
- Rahman M.Z, Hossain Z, Mollah M.F.A and Ahmed G.U. Effect of Diazunum 60EC on Anabas testudineus, Channa punctatus and Bardodes goniontus “Naga”, The ICLARM Quarterly. 2002; 25:8-12.
- Prakasam A, Sethupathy S, Lalitha S. Plasma and RBCs antioxidant status in occupational male pesticide sprayers. Clin. Chim. Acta.2001; 310:107-112.
- Hayes W.J. Pesticide study in men. Williams & Williams, Baltimore and London. 1982. 301
- Jaroli D.P and Sharma B.L. Effect of Organophosphate insecticide on the organic constituents in the liver of Channa punctatus. Asian. J. Exp. Sci. 2005;19(1):121-129.
- Ventura B.C, Angelis D.F, Marin-Molares M.A. Mutagenic and genotoxic effects of atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pesticide Biochem. Physiol., 2008; 90:42-51.
- Vargas R, Ponce-Canchihuaman J. Emerging various environmental threats to brain and overview of surveillance system with zebrafish model. Toxicol. Rep., 2017; 4:467-473.
- Chourpagar R. Atul and Kulkarni G.K. Effect of mercuric chloride on the gill structure of a freshwater female crab, Barytelphusa cunicularis (Westwood). Journal of Global Biosciences., 2014; Vol.3(2):423-427.
- Paruruckumani P.S, Maharajan A, Ganapiriya V,Narayanaswamy Y amd Raja Jeyasekar R. Surface ultrastructural changes in the gill and liver tissue of Asian sea bass Lates calcarifes (Bloch) exposed to copper. Biol. Trace. Elem. Res.2015. 168:50000-5007.
- Magare S.R, Patil H.T. Effects of pesticides on oxygen consumption, red blood count and metabolites of fish, Punitus. Environ. Ecol. 2000;18:891-894.
- WHO, World Health Organisation. Lindane in drinking water . Background document for preparation of WHO guidelines for drinking water quality, Geneva, World Health Organisation (WHO/SDE/WSH/03.04.102). 2003. 3-5.
- Al-Akel AS, Alkahem-Al-Balawi HF, Al-Misned F, Mahboob S, Ahmad Z, Suliman EM. Effects of dietary copper exposure on accumulation, growth and haematological parameters in Cyprinus carpio. Toxicol.Environ.Chem. 2010, 92:1865-1878
- Alkahem Al-Balawi HF, Ahmad Z, Al-Akel AS, Al-Misned F, Suliman EM, Al-Ghanim KA. Toxicity bioassay of lead acetate and effects of sub-lethal exposure on growth, haematological parameters and reproduction in Clarias gariepinus. Afr. J. Biotechnonol. 2011; 10:11039-11047.
- Sindhu D.S. Neem in agroforestry: As a source of plant derived chemicals for pest management. Ind. Forestry. 1995. 12(11):1012-1021.
- Ali S.I, Singh O.P and Misra U.S. Effectiveness of plant oils against pulse beetle, Callasobruchus chinensis Linn. Ind. J. Entomol. 1983; 45:6-9
- Behringer M.P.R. Techniques and materials in biology. McGraw Hill Publ. 1972; 120-122.
- Weil C.S. Table for convenient calculation of median effective dose (LC50 or ED50) and instructions in their use. Biometrics. 1952. 8:249-263
- Gandhi M, ramesh Lal A, Sankaranarayanan C. Banerjee and Sharma P.L. Acute toxicity study of the oil from Azadirachta indica seed (neem oil). J. Ethanopharm. 1988; 23:39-51.
- Lowry O.H, Rosebrough N.J, Farr A.L and Randall R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951; 265-275.
- Roe J.H. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955; 212:335-343.
- Folch J, Lees M and Sloane Stanely G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957. 266(1):497-509.
- Pearse A.G.E Histochemistry: Theoretical and applied, Vol. 1 and 2, 4th ed; Churchill Livingstone, Edinburgh. 1980.
- Latifah A.M, David Musa R, Latiff P.A. Gas chromatography mono spectrometry study of Malathion residues in Centella asiatica. Iran. J. Environ. Health. Sci. Eng. 2011; 8(1):57-64.
- Jenkins F, Smith J. Effect of sublethal concentration of endosulfan on haematological and serum biochemical parameters in the carp, Cyprinus carpio. Bull. Environ. Contam. Toxicol. 2003; 70:993-947.
- Shakoori A.R, Ali S.S and Abaleem M.A. Effect of six months feeding of cypermethrin on the liver of albino rats. Ind. J. Pharm. 1988. 25(2):24-28.
- Reddy M.M, Kumar V.A, Reddy P.l.S and Reddy S.N.L. Phenol induced metabolic alterations in the brainand muscle of freshwater fish, Channa punctatus during sublethal toxicosis. J.Ecotox.Environ.Monit. 1993; 3(1):7-11.
- Bordeur J and Dubois K.P. Studies on factors influencing the acute toxicity of malathion and malaoxon in rats. Can. J. Physiol and pharm. 1967; 45(4) 621-623.
- Barton B.A. Stress in finfish: Past, present and future –A historical perspective. Fish stress and health in aquaculture. Cambridge University press, New York. 1997; 34.
- Iwama G.K, Afonso L.O.B and Vijayan M.M. Stress in fishes The physiology of fishes. CRC press, Boca Raton. 2006; 319-343.
- Geyer H.j, Scheunert I, Broggemann R, Matthies M, Chiston E, Steingerg and Garrison W. The revelance of aquatic organisms’ lipid content to the toxicity of lindane to different fish species. Ecotoxicol. Environ. Saf. 1994; 28:53-70.
- Bhanwra S, Singh J and Khosla P. Effect of Azadirachta indica (neem) leaf aqueous extract on paracetamol induced liver damage in rats. Ind.J.Physiol. Pharmacol. 2000. 44(1): 64-68.






