Synthesis and Characterization of a Chemically-Activated Novel Mesoporous Silica for Cobalt Decontamination from Polluted Water
Corresponding author Email: s.matavos@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.14.2.12
Mesoporous silica was synthesized by a chemical process and its efficiency was investigated for removal of cobalt (Co2+) ions from contaminated water in a laboratory scale. The characteristics of synthesized mesoporous were analyzed by SEM/TGA. Optimal conditions were determined for important parameters such as solution pH, the absorbent dose, the initial Co2+ concentration, and contact time by a single-variable method through the batch experiments. The SEM results confirmed the synthesized silica had high porosity with a honeycomb-like structure. The results showed that with an increasing adsorbent dose and contact time to the optimum, the efficiency of Co2+ adsorption increased. However, with increasing concentration of Co2+, the removal efficiency decreased. At optimal contact time (8 h), 85 % of Co2+ was removed. The maximum adsorption efficiency at pH =7, initial Co2+ concentration of 5 ppm, and at the adsorbent dose 0.3 g/50 ml, was 89%. The study of adsorption isotherm and kinetic models showed that the adsorption process followed the Freundlich isotherm (R2 = 0.9359) and the second-order kinetic model (R2=0.999). Therefore, the synthesized mesoporous silica presented a chemical adsorption mechanism for Co2+ removal from aqueous media and can be utilized in wastewater treatment containing divalent heavy metals such as Co2+.
Copy the following to cite this article:
Matavos-Aramyan S, Soukhakian S. Synthesis and Characterization of a Chemically-Activated Novel Mesoporous Silica for Cobalt Decontamination from Polluted Water. Curr World Environ 2019; 14(2). DOI:http://dx.doi.org/10.12944/CWE.14.2.12
Copy the following to cite this URL:
Matavos-Aramyan S, Soukhakian S. Synthesis and Characterization of a Chemically-Activated Novel Mesoporous Silica for Cobalt Decontamination from Polluted Water. Curr World Environ 2019; 14(2). Available from: https://bit.ly/2kj2T3n
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 18-06-2019 |
|---|---|
| Accepted: | 27-08-2019 |
| Reviewed by: | 
 Dr. Asabuwa fahanwi
Dr. Asabuwa fahanwi
|
| Second Review by: |

 Dr. Masafumi Tateda
Dr. Masafumi Tateda
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Heavy metals, due to their inherent toxicity and the ability to accumulate in living tissues, are often hazardous to organisms and environment.1 They can cause many problems for the health of humans and animals as well as the environment.2,3 Cobalt (Co2+) ion is an essential element for metabolic activities in low concentrations and may also be effective in regulating pro-oxidants in the blood4-6; however, in high concentrations, it causes acute effects of lung toxicity and asthma, inflammation of the lungs, and chest tightness.7,8 The International Agency for Research on Cancer (IARC), has identified cobalt as a potential carcinogen, and its maximum level in drinking water is 40 μg/L based on the recommendation of EPA.9
Industrial wastewater is the main source of water pollution to heavy metals, and as a result, removal of these pollutants before discharge into the environment is of utmost importance in view of public health and pollution control.10,11 Recently, various methods such as chemical treatments, ion exchange, membrane separation, evaporation, electrochemical regeneration, coagulation and flocculation, flotation, biological purification have been used for the removal of heavy metals from the contaminated solutions.12-16 Although there are several methods for removing metals from aqueous media, most of the aforementioned processes have noticeable disadvantages, such as the need for high energy, resulting in costly processes, low efficiency, production of high amounts of sludge, sludge disposal problems containing large amounts of heavy metal, the need for specific and costly chemicals.17,18 Among these methods, adsorption technology has attracted a lot of attention19-21 because it is a simple, low-cost, and effective method for the removal of heavy metal ions in low and medium concentrations. Surface adsorption on the porous adsorbents is an environmentally-compatible technology that is considered for the removal of organic and inorganic pollutants, such as heavy metal ions from contaminated water and wastewater.22,23 Among the porous adsorbents, the efficiency of nanoporous or mesoporous in the field of adsorption is very effective, and in some cases unique. These nanoparticles have cavities in the size of 2 to 50 nm and are commonly known as mesoporous compounds.24,25 The uniform nanometric pores of mesoporous make it possible to remove the different type of pollutants based on the difference in dimensions.26
One of the important burdens of chemical engineers is the synthesis and design of a suitable adsorbent, which, in addition to being capable of adsorption contaminants, has a high adsorption capacity and also to be sufficiently stable in the acid and alkaline medium.27,28 In the present study, mesoporous silica was synthesized by the chemical method, used to remove Co2+ ions from contaminated aqueous solutions in the batch adsorption process and obtain the optimum effective parameters on the adsorption process. By utilization of silica instead of typical carbon-based structure in this adsorbent, it is believed the adsorption capacity be much higher than typical carbon-based adsorbent, and due to the novel chemical activation exploited to modify the utilized silica, it is also believed that this adsorbent is highly stable in the acid and alkaline medium in comparison to other typical adsorbents.
Materials and Methods
Chemicals and Instruments
Pure ethanol, polyethylene glycol (PEG), tetraethyl orthosilicate (TEOS), sodium hydroxide, hydrogen chloride, and cobalt nitrate hexahydrate (Co (NO3)2.6H2O) 99.9% was parched from Merck Co, Germany. The solution of NaOH and HCl 0.1 or 1 N were used for pH adjustment. The amounts of solutions pH were measured by pH meter (Hanna instrument). The residual Co2+ ions in the solution was measured after adsorption by atomic absorption spectrophotometer (Spectra 20 AA Varian). The shaker incubator (Innova 40) was used for mixing and proper contact of Co2+ ions with adsorbent in all adsorption experiments. The separation of nano adsorbents from the suspension was also used with a centrifuge (Hettich EA20) after the adsorption process. The mesoporous characteristic was studied by SEM images (Siemens, Germany).
Synthesis of mesoporous silica
To synthesis mesoporous-silica (MCM-48), firstly, 3.8 ml of polyethylene glycol (P 123) was dissolved in 40 ml mixture of distilled water and ethanol (1:1), and then 34.3 ml of hydrogen chloride was added to the mixture in a 2 h period time. The mixture was shaken under 30°C at 220 rpm by a shaker-incubator. Then, 7 ml of TEOS (tetraethyl orthosilicate) was added to the solution with the molar ratio of mixture calculated to be TEOS: 0.0349, HCl: 0.08, and H2O: 5.8. The mixture was again heated for one hour in a shaker-incubator under the 30°C at 220 rpm. Finally, the achieved green sediment (MCM-48) was separated from the solution by a 0.45 µ filter paper and dried at 70°C for 2 h after washing with deionized water.
Adsorption Experiments
To determine the adsorption efficiency of the synthesized mesoporous silica in removal of Co2+ ions from contaminated solutions, the effective parameters such as pH in different values (3, 7, 9), adsorbent concentration (0.1, 0.3, 0.5, g in 50 mL), various initial concentrations of Co2+ ions (5, 10, 20, 30 and 50 mg/L), and contact time (0.5, 1, 5, 8, 10, and 12 h) were examined in single-variable DoE method through the batch experiments. For adsorption experiments, a stack solution 1g /L Co2+ ions were prepared by adding 0.259 g of cobalt nitrate to 100 ml of distilled water. The required initial concentrations for working solutions were prepared from the stock solution in 50 ml samples, and then the determined amount of adsorbent was added to each container. The effect of contact time on the removal efficiency of Co2+ ions by synthesized mesoporous silica was investigated in a concentration of 5 mg/L of Co2+ ions at 0.5, 1, 5, 8, 10, and 12 h. At this step, 0.1 g of the adsorbent was added in 50 ml, and solution pH was adjusted to 3. To determine the effect of pH on the adsorption process, pH was investigated at three levels of 3, 7, and 9 in the initial Co2+ ions concentration of 5 mg/L and the optimum contact time. For each test, 0.1 g of adsorbent was added to the 50 ml solution with the adjusted above pH values by 0.1 N HCl or NaOH. The effect of initial Co2+ ions concentration was investigated on the removal efficiency at pH 7, adsorbent weight of 0.1 g in 50 ml solutions with concentration of 5, 10, 20, 30 and 50 mg/L of Co2+ ions. Also, to investigate the effect of adsorbent weight on Co2+ ions removal efficiency, 0.1, 0.3 and 0.5 g were used in contaminant solution with a concentration of 5 mg/L Co2+ ions. In all experiments, for measuring the concentration of residual Co2+ ions in solutions, the adsorbent particles were separated from the suspension after the termination of adsorption process. Then, the atomic absorption spectrometer was calibrated with standard solutions of Co2+ ions at a wavelength of 240 nm, and the residual Co2+ ions were measured in the filtrate samples under the same atomic absorption conditions, eventually.
The adsorption isotherm was obtained at 8 h, adsorbent weight of 0.3 g, a temperature of 25°C, various Co2+ ions concentrations of 5, 10, 30 and 50 mg/L and other optimal conditions. To determine the kinetics of Co2+ ions adsorption on mesoporous silica, experiments were conducted in the contact times (0.5, 2, 3, 5, 8, 16 h) at other optimum condition. All experiments were carried out at a constant laboratory temperature (25 ± 2°C), mixing speed of 150 rpm and a constant volume of 50 ml.
Adsorption isotherm equations
The study of isotherms can describe the behavior of adsorbent with adsorbate. In fact, the isotherm equation provides a correlation between the concentration of Co2+ ions in the solution and the amount of Co2+ adsorbed on the solid phase surface, in which both phases are in equilibrium.29 In the present study, the equilibrium data for adsorption of Co2+ ions by mesoporous silica were tested for the Langmuir, Freundlich, and Temkin isotherm models. The linear equation of the Langmuir isotherm model for single-layer adsorption is shown in Eq.123:

where qe is the amount of Co2+ adsorbed on the unit of adsorbent (mg/g), Ce is the equilibrium concentration of Co2+ ions in solution (mg /L), KL the constant that refers to the bonding energy of sorption in l/mg and qm is the maximum adsorption capacity (mg/g). The Langmuir equilibrium constants are obtained by plotting Ce/qe against Ce based on the empirical data.
The Freundlich equation is completely empirical and is based on adsorption on the heterogeneous surface in which the equation is as Eq. 228:

where KF and n are Freundlich adsorption constants are related to the adsorption capacity and intensity, respectively. The Freundlich equilibrium constants are obtained by plotting log qe against log Ce based on the empirical data diagram.
The Temkin isotherm model is as Eq. 330:
qe= BT Ln KT + BT Ln Ce … (3)
where the BT and KT are Temkin constants in the linear isotherm model. The Temkin equilibrium constants are obtained by plotting qe against LnCe based on the empirical data.
Adsorption kinetic equations
By considering the chemical kinetics, the progress of a reaction will be more accurate over the time. In other words, the speed of Co2+ ions adsorption process on the mesoporous silica and the effect of contact time on the removal efficiency are well studied in the study of adsorption kinetics.29 In order to investigate the adsorption kinetics, the experimental data were fitted to the pseudo-first order (Eq. 4) and pseudo-second order (Eq. 5) equations.24
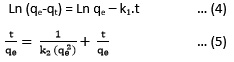
Where qe and qt are the amounts of Co2+ ions adsorbed at the equilibrium and t time (mg/g), respectively. The k1 (min-1) and k2 (g./mg.min) are the pseudo-first and pseudo- second order rate constants, respectively.
Results and Discussion
Characteristics of synthesized mesoporous silica
The structure and size of the mesoporous silica particles were investigated using SEM images which are shown in Fig. 1. As can be seen from the image, the synthesized mesoporous silica has a honeycomb-like structure and porous structure. Hence it results in a higher surface area for adsorption of metal ions from aqueous environments29 and was suitable to adsorb divalent heavy metals from aqueous solutions.
 |
Figure 1: SEM image of synthesized mesoporous silica MCM-48 Click here to view Figure |
Also, the TGA test results have shown a highly stable thermal structure of MCM-48 compared to other typical absorbents. As can be seen in Table 1, the synthesized absorbent of this study shows a tremendous value of higher thermal stability of up to 57% compared to second most thermal-stable absorbent. This data also suggest the role of TEOS utilization in the activation step which has resulted in a highly thermal-stable yet mesopore absorbent.
Table 1: Comparison of TGA test results of MCM-48 and other typical absorbents
|
Sample |
Ref. |
Initial degradation temperature (°C) and weight loss (%) |
Final degradation temperature (°C) and weight loss (%) |
Final residue (%) |
||
|
(°C) |
(%) |
(°C) |
(%) |
|||
|
Mangosteen shell |
[36] |
413 |
36 |
587 |
21 |
28 |
|
Modified silica gel |
[38] |
388 |
29 |
495 |
17 |
22 |
|
Montmorillonite |
[40] |
293 |
33 |
440 |
18 |
25 |
|
Modified kaolinite |
[42] |
255 |
27 |
420 |
16 |
21 |
|
Expanded perlite |
[44] |
228 |
21 |
360 |
12 |
16 |
|
MCM-48 |
This Study |
520 |
17 |
800 |
10 |
12 |
The effect of contact time
The effect of contact time on Co2+ ions removal efficiency is shown in Fig. 2. As can be seen, with increment in time, the removal rate increases and 85% of the Co2+ ions were removed at 8 h contact time. As a result, the contact time of 8 h was chosen as the optimal time. Increasing the removal efficiency of Co2+ ions by increasing the contact time can be related to increasing opportunities for adsorption and, in fact, increasing the collision and contact between the pollutant and the adsorbent in the adsorption medium.23 Furthermore, the surface area of the mesoporous silica is larger than 30 m2/g. So, the fast sorption at initial times is related to ion exchange onto the mesopores surface of the synthesized mesoporous silica. Diffusion flow of metal ion through solution balk to surfaces provides the significant rate of this stage. These results are consistent with the result of Kosa et al.,31 and Saif et al.,32 study.
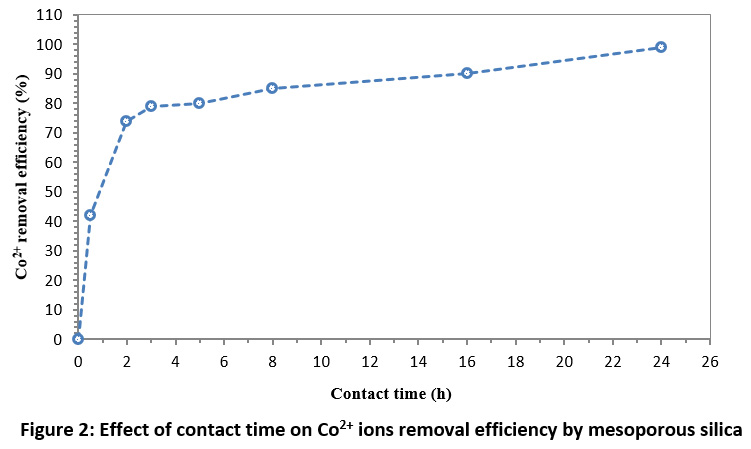 |
Figure 2: Effect of contact time on Co2+ ions removal efficiency by mesoporous silica Click here to view Figure |
The effect of initial pH solution
The pH factor is one of the most important factors in the adsorption process since it causes the change in the ionic state of the compounds, ionization, and also charge of the adsorbent surface, which can affect the reaction between the adsorbent with the adsorbat.23 Fig. 3 shows the effect of pH on the removal efficiency of Co2+ ions by mesoporous silica. The results pointed out that at pH 7, Co2+ ions removal efficiency was 70% that was higher than other pHs. With increasing pH from 3 to 7 (acidic pH), the removal efficiency increases and then the removal efficiency decreases in alkaline medium which is in accordance with findings of others that used the modified alumina nano-particles of diatomite nitrogen-phenylhydrazine for removal of heavy metals.33 A reason e for the low efficiency of acidic pH is related to producing a positive charge on the adsorbent at the acidic pH, which creates an electrostatic repulsion between the adsorbent and Co2+ ions cations, and also the amount of hydrogen ions increases in the solution and it instead of the Co2+ ions on the adsorbent surface as a result, and so the removal efficiency decreases in acidic solution. As shown in Fig. 3, the adsorption efficiency decreases in higher pH ranges due to the formation of cobalt hydroxide.34,35
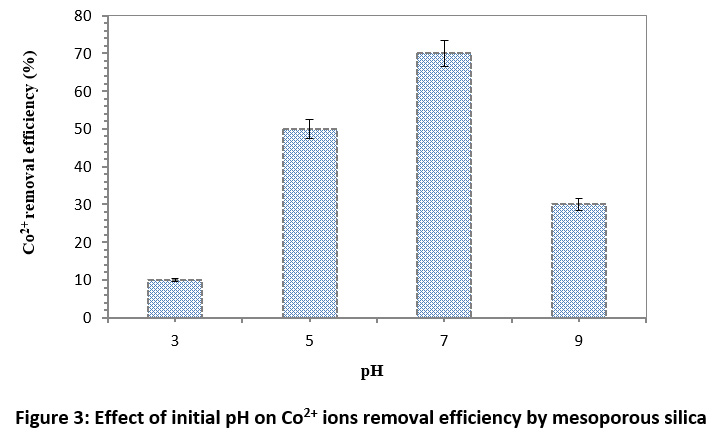 |
Figure 3: Effect of initial pH on Co2+ ions removal efficiency by mesoporous silica Click here to view Figure |
The effect of the initial Co2+ concentration
The effect of initial Co2+ ions concentration on the removal efficiency of the synthesized mesoporous silica is shown in Fig. 4. As can be seen, with the increase in Co2+ ions concentration from 5 to 30 mg/l, the removal efficiency decreased from 88% to 40%. This is consistent with the results of the study of Vojoudi et al., on the use of mesoporous silica in the removal of heavy metals from aqueous solution23 and study of Anbia et al., on the removal of heavy metals by mesoporous silica MCM-48.24 The decreasing in removal efficiency can be due to repulsion between the Co2+ ions present in the solution and on the adsorbent surface at high concentration. In fact, at low concentrations, a single layer of metal ions forms on the surface of the adsorbent, but at high concentrations, multiple layers are formed on the surface to the point it limits the number of adsorbent sites, and therefore the adsorption efficiency decreases.36
 |
Figure 4: Effect of initial Co2+concentration on Co2+ removal efficiency by mesoporous silica Click here to view Figure |
Effect of adsorbent dose
Fig. 5 shows the effect of adsorbent dose on Co2+ ions removal efficiency. The results showed that by increasing the amount of adsorbent from 0.1 to 0.3 g in 50 ml, the removal efficiency increased from 41 to 75%. From this amount forth, it was observed a little increase, but removal efficiency had not changed significantly. The increasing adsorbent weight causes the number of active sites in adsorption process, or increasing the ratio of number of active sites to the adsorbent. As a result, it causes an increase in the contact surface between the adsorbent and the pollutant, then the removal efficiency of Co2+ ions increases. However, due to the accumulation of adsorbent in a batch process, the active sites of the adsorbent decrease with increasing mass of adsorbent; therefore, there was no linear relationship between adsorbent weight and removal efficiency. A study on the modified mesoporous silica (SBA-15) to remove mercury ion from the aqueous solution showed that an optimum adsorbent dose was 15 mg and then with increasing the adsorbent dose to 45 mg, the removal efficiency was constant.37 The other study which used nano-sized silica-amine for metal ions removal, showed that with an increase in adsorbent dose, removal efficiency increases up to the optimum amount38 and these result was consistent with this study.
 |
Figure 5: Effect of adsorbent dose on Co2+ removal efficiency by mesoporous silica Click here to view Figure |
Adsorption Isotherm Model
Langmuir, Freundlich and Temkin isotherm models are shown in Fig 6. The conformity of the Co2+ ions adsorption by mesoporous silica with isotherm models was determined by the determination coefficient. Based on Fig 6, the determination coefficient for Freundlich, Langmuir, and Temkin were 0.9359, 0.8704 and 0.8251, respectively.
Table 2 shows the calculated isotherm parameters of three models for the Co2+ adsorption. According to Table 2, there was the highest correlation between the Freundlich isotherm model and experimental data for Co2+ ions adsorption. Thus, this isotherm was selected as the best model for describing the adsorbent properties of mesoporous silica in the adsorption of Co2+ ions from aqueous solutions.
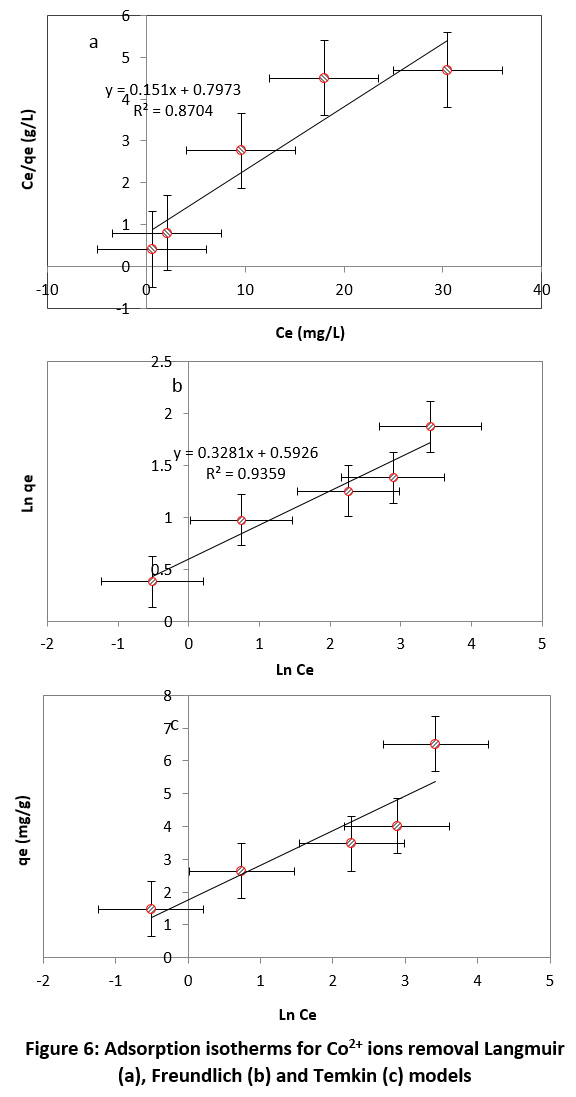 |
Figure 6: Adsorption isotherms for Co2+ ions removal Langmuir (a), Freundlich (b) and Temkin (c) models Click here to view Figure |
Table 2: Adsorption parameters of the Langmuir, Freundlich and Temkin isotherm models for the adsorption of Co2+ on mesoporous silica
|
Isotherms |
Langmuir |
Freundlich |
Temkin |
||||||
|
Parameter Value |
qm(mg/g) |
KL(L/mg) |
R2 |
KF(L/mg) |
n |
R2 |
KT(L/mg) |
BT(L/mg) |
R2 |
|
6.62 |
0.19 |
0.870 |
1.8 |
3 |
0.935 |
6.3 |
2326 |
0.825 |
|
Also, experimental data were followed for the Langmuir isotherm that assumed the adsorption is done in a single layer. The necessary and feasible properties of the Langmuir isotherm can be expressed in terms of the dimensionless constant called the separation factor that is calculated by Eq. 6.29

where RL is isolation factor, C0 is the initial concentration of Co2+ ions, KL is Langmuir constant. If RL >1, adsorption is unfavorable. If RL = 1 adsorption is linear and if RL <1 is adsorption irreversible and if 0< RL< 1, adsorption is favorable.29 In the present study, RL for optimal concentration obtained 0.19 and, since it is between 0 and 1, it indicates that the adsorption process is favorable. Also, the Freundlich isotherm adsorption constant n is related to the adsorption intensity. If 1/n is lower than 1, then the Freundlich isotherm is favorable.39 In the present study, 1/n was 0.33 that indicated the adsorption process of Co2+ onto mesoporous silica has been desirably performed.
Table 3: Comparison of maximum adsorption capacity of different adsorbents for Co2+ ions
|
Adsorbent |
qmax (mg/g) |
References |
|
Modified SBA-15 mesoporous silica |
5.8 |
[36] |
|
Mangosteen shell |
0.34 |
[37] |
|
Magnetic multi walled carbon nanotube/ iron oxide composites |
10.61 |
[38] |
|
Modified silica gel |
8.43 |
[39] |
|
Barley straw ash |
4.15 |
[40] |
|
Montmorillonite |
6.92 |
[41] |
|
Modified montmorillonite |
22.3 |
[42] |
|
Modified kaolinite |
9 |
[43] |
|
Poly[N-(4-[4-(aminophenyl)methylphenylmethacrylamide])] |
7.19 |
[44] |
|
Expanded perlite |
1.05 |
[45] |
|
Coir Pith |
12.82 |
[46] |
|
Natural bentonite |
9.911 |
[47] |
|
Natural Jordanian sorbent |
4.5 |
[48] |
|
Mesoporous silica |
6.62 |
Present study |
Maximum adsorption capacities (qmax) of different adsorbents for Co2+ adsorption reported in literature are presented in Table 3. Comparing the qmax of other adsorbents with mesoporous silica used in this study shows that qmax of mesoporous silica is relatively suitable to adsorb Co2+ ions. But the use of different methods for modification of adsorbents such as functionalization and nanoparticle impregnating on the adsorbent surface can also be useful for improving and increasing the maximum adsorption capacity of adsorbent.40-42
Adsorption Kinetic Model
One of the most important factors for the design of the adsorption system is the prediction of adsorption rate that is controlled by the system kinetic.43
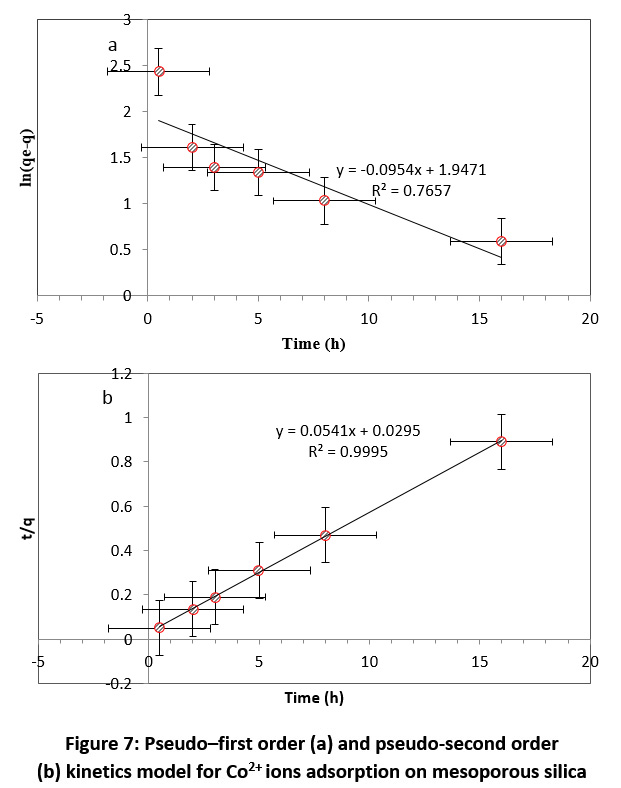 |
Figure 7: Pseudo–first order (a) and pseudo-second order (b) kinetics model for Co2+ ions adsorption on mesoporous silica Click here to view Figure |
The adsorption kinetics was examined based on pseudo–first order (Fig. 7a) and pseudo–second order (Fig. 7b). As can be seen from Fig. 7, the determination coefficients (R2) for both pseudo–first and second order models were obtained 0.8898 and 0.9826, respectively. Based on the results (Table 4), the highest accordance was observed by the pseudo–second order model and this model was selected as the best model for describing the kinetic behavior of mesoporous silica in adsorption of Co2+ ions from aqueous media. The pseudo–second order model implies that two factors or a second–order of a factor effect on the adsorption process rate. Then, it seems that the Co2+ ions and functional groups on the surface had the important role in the kinetic rate of adsorption.
Table 4: Pseudo-first and Pseudo-second order parameters for the Co (II) adsorption on the mesoporous silica
|
Kinetic |
Pseudo-first order |
Pseudo-second order |
||||
|
parameter |
K1 (min-1) |
qe (mg/g) |
R2 |
K2 (g/mg.min) |
qe (mg/g) |
R2 |
|
value |
0.0954 |
0.66 |
0.765 |
0.1 |
18.94 |
0.999 |
Adsorption Free Energy Change
The equilibrium constants were used to calculate the Gibbs free energy change (ð›¥G°) of adsorption. It is calculated from the Eq. 7.
∆G°=-RTLnK … (7)
Where R represent the universal gas constant (8.314 J/mol.°K) and T is the absolute temperature (°K). The constant K is related to KC (Thermodynamic constant), KF (Freundlich constant), KL (Langmuir constant) and KT (Temkin constant).44 The calculated values for ð›¥G° are given in Table 5. The values of free energy change are negative which conferment the feasibility and the spontaneous nature of adsorption process of Co2+ ions on the synthesized mesoporous.
Table 5: The calculated ð›¥G° of adsorption Co (II) adsorption on the mesoporous silica
|
Equilibrium constant |
Values |
ð›¥G° (kJ/m) |
|
KC |
0.61 |
- 1.510 |
|
KF |
1.8 |
- 1.476 |
|
KL |
0.19 |
- 4.127 |
|
KT |
6.3 |
- 1.160 |
Adsorption Mechanism
The adsorption mechanisms are shown in the Fig. 8. According to the first reaction, the protonated surface of mesoporous is not favorable for adsorption of Co2+ ions. The dominance of Co2+ ions adsorption in the second reaction is probably caused by exchangeable cations to the layers of the mesoporous silica and the subsequent occupation of exchange sites on the surface by other ions.45 During the third reaction of the fast metal sorption, ionic interactions with functional groups on the mesoporous silica inside ion exchange are believed to take place. Ionic attraction deceleration in the close mesopores of mesoporous silica is connected by more intensive sorption in comparison with exchange in the surface mesopores. Fast and slight adsorption of Co2+ ions in the third stage confirms that the Co2+ ions adsorption occurs mainly in the functional groups on the surface. Conformations and proportions of the kinetic model are same at different initial metal concentrations. It is indicated that the increasing of the Co2+ ions concentrations in solutions leads to their large intensity, and hence to more intensive attraction between ions and the active sites on the surface.46,47
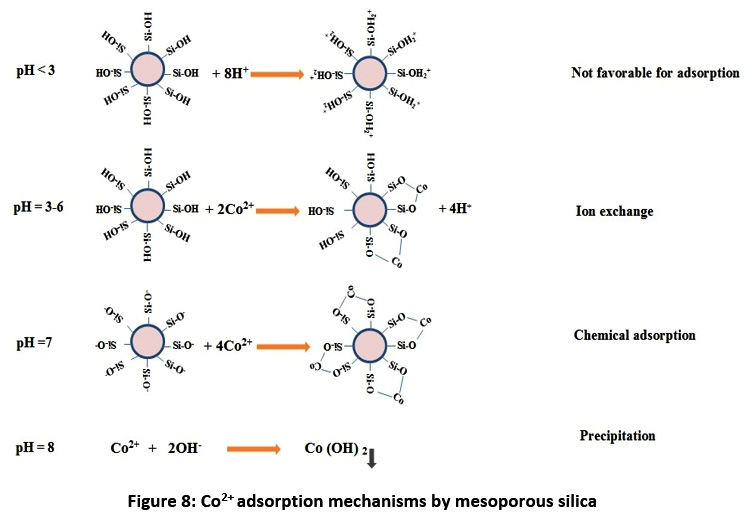 |
Figure 8: Co2+ adsorption mechanisms by mesoporous silica Click here to view Figure |
Also, according to the Fig. 8 and the effect of the solution pH on the adsorption of Co2+ ions by mesoporous silica (Fig. 3), the adsorption of metal ions depends on the solution pH, which influences electrostatic binding of ions to corresponding OH– groups decreasing the solution pH values; as the protonated hydroxyl groups to varied degrees were increased, the numbers of binding sites available for Co2+ ions were then decreased. Consequently the extent of Co2+ ions removal was low in the high concentrations of protons. With adjusting pH to 7, OH– groups were de-protonated and forming negatively charged sites. At pH values higher than 8.0, Co2+ ions precipitated out because of the high concentrations of OH– ions in the aqueous solution.48-50 The adsorption capacity of mesoporous silica for Co2+ adsorption first increased with the increase of the solution pH from pH 3 to 7. But the adsorption capacity of mesoporous decreased afterwards when the pH value reached 7.0.
Conclusions
In the present study, mesoporous silica was successfully synthesized by a single chemical method as a low cost adsorbent for Co2+ ions adsorption from aqueous solutions. Experiments showed that the change in the pH from 3 to 7 (acidic pH), the removal efficiency of adsorbent for Co2+ ions increases, and decreases in the alkaline condition (pH > 7). By increasing the amount of adsorbent and the contact time to optimal levels, the removal efficiency of Co2+ ions increased. Also, with an increase in the initial Co2+ ions concentration from 5 to 30 mg/L, the percent of removal efficiency decreased. The isotherms study showed that the Co2+ ions adsorption follows the Freundlich isotherm and the adsorption kinetics data was better described by a pseudo–second order model. Considering that heavy metals are usually soluble in acidic pH ranges and the wastewaters have a heavy metal problem, mesoporous silica can be a good adsorbent for the removal of two valent heavy metals such as Co2+ ions.
Acknowledgements
The authors would like to express their sincere gratitude to the Financing Department of Raazi Environmental Protection Foundation, Iran Office, for their financial assistant provided to conduct this research.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Schweitzer L, Noblet J (2018) Chapter 3.6 - Water Contamination and Pollution. In: Török B, Dransfield T (eds) Green Chemistry. Elsevier, pp 261-290. doi:https://doi.org/10.1016/B978-0-12-809270-5.00011-X
CrossRef - Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L (2017) Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 387:43-56. doi:https://doi.org/10.1016/j.tox.2017.05.015
CrossRef - Fernández-Rodríguez J, García A, Coz A, Labidi J (2015) Spent sulphite liquor fractionation into lignosulphonates and fermentable sugars by ultrafiltration. Separation and Purification Technology 152:172-179. doi:https://doi.org/10.1016/j.seppur.2015.08.017
CrossRef - Awual MR, Yaita T, Okamoto Y (2014) A novel ligand based dual conjugate adsorbent for cobalt(II) and copper(II) ions capturing from water. Sensors and Actuators B: Chemical 203:71-80. doi:https://doi.org/10.1016/j.snb.2014.06.088
CrossRef - Chen H, Li J, Shao D, Ren X, Wang X (2012) Poly(acrylic acid) grafted multiwall carbon nanotubes by plasma techniques for Co(II) removal from aqueous solution. Chemical Engineering Journal 210:475-481. doi:https://doi.org/10.1016/j.cej.2012.08.082
CrossRef - Li X, Zeng C, Jiang J, Ai L (2016) Magnetic cobalt nanoparticles embedded in hierarchically porous nitrogen-doped carbon frameworks for highly efficient and well-recyclable catalysis. Journal of Materials Chemistry A 4 (19):7476-7482. doi:10.1039/C6TA01054G
CrossRef - Abbas M, Kaddour S, Trari M (2014) Kinetic and equilibrium studies of cobalt adsorption on apricot stone activated carbon. Journal of Industrial and Engineering Chemistry 20 (3):745-751. doi:https://doi.org/10.1016/j.jiec.2013.06.030
CrossRef - Shahat A, Awual MR, Naushad M (2015) Functional ligand anchored nanomaterial based facial adsorbent for cobalt(II) detection and removal from water samples. Chemical Engineering Journal 271:155-163. doi:https://doi.org/10.1016/j.cej.2015.02.097
CrossRef - Clancy JL, Bukhari Z, McCuin RM, Matheson Z, Fricker CR (1999) USEPA method 1622. Journal - American Water Works Association 91 (9):60-68. doi:10.1002/j.1551-8833.1999.tb08697.x
CrossRef - Moussavi M, Matavos-Aramyan S (2016) Chelate-modified fenton treatment of sulfidic spent caustic. Korean J Chem Eng 33 (8):2384-2391. doi:10.1007/s11814-016-0080-z
CrossRef - Matavos-Aramyan S (2017) Advances in Fenton and Fenton Based Oxidation Processes for Industrial Effluent Contaminants Control-A Review. International Journal of Environmental Sciences & Natural Resources 2 (4). doi:10.19080/ijesnr.2017.02.555594
CrossRef - Minamisawa M, Minamisawa H, Yoshida S, Takai N (2004) Adsorption Behavior of Heavy Metals on Biomaterials. Journal of Agricultural and Food Chemistry 52 (18):5606-5611. doi:10.1021/jf0496402
CrossRef - Behl M, Stout MD, Herbert RA, Dill JA, Baker GL, Hayden BK, Roycroft JH, Bucher JR, Hooth MJ (2015) Comparative toxicity and carcinogenicity of soluble and insoluble cobalt compounds. Toxicology 333:195-205. doi:https://doi.org/10.1016/j.tox.2015.04.008
CrossRef - Matavos-Aramyan S, Neysari S, Jazebizadeh MH (2017) Fabrication, Application and Mathematical Modeling of a new Diamine/Trimesoyl Chloride Reverse Osmosis Composite Membrane for Copper Sulfate Desalination from Wastewaters. Silicon 9 (6):829-840. doi:10.1007/s12633-017-9582-5
CrossRef - Tofighy MA, Mohammadi T (2011) Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. Journal of hazardous materials 185 (1):140-147. doi:https://doi.org/10.1016/j.jhazmat.2010.09.008
CrossRef - Yousefi A, Matavos-Aramyan S (2018) Mix Design Optimization of Silica Fume-Based Pervious Concrete for Removal of Heavy Metals from Wastewaters. Silicon 10 (4):1737-1744. doi:10.1007/s12633-017-9663-5
CrossRef - Matavos-Aramyan S, Moussavi M, Matavos-Aramyan H, Roozkhosh S (2017) Cryptosporidium-contaminated water disinfection by a novel Fenton process. Free Radical Biology and Medicine 106:158-167.
doi:http://dx.doi.org/10.1016/j.freeradbiomed.2017.02.030
CrossRef - Lameiras S, Quintelas C, Tavares T (2008) Biosorption of Cr (VI) using a bacterial biofilm supported on granular activated carbon and on zeolite. Bioresource technology 99 (4):801-806. doi:https://doi.org/10.1016/j.biortech.2007.01.040
CrossRef - Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, Gupta VK (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicology and environmental safety 148:702-712. doi:https://doi.org/10.1016/j.ecoenv.2017.11.034
CrossRef - Qu R, Zhang Y, Qu W, Sun C, Chen J, Ping Y, Chen H, Niu Y (2013) Mercury adsorption by sulfur- and amidoxime-containing bifunctional silica gel based hybrid materials. Chemical Engineering Journal 219:51-61. doi:https://doi.org/10.1016/j.cej.2012.12.070
CrossRef - Chen M, Ding S, Gao S, Xu S, Yang C, Wu Y, Gong M, Wang D, Wang Y (2019) Long-term effects of sediment dredging on controlling cobalt, zinc, and nickel contamination determined by chemical fractionation and passive sampling. Chemosphere 220:476-485. doi:https://doi.org/10.1016/j.chemosphere.2018.12.138
CrossRef - Chen Y-H, Li F-A (2010) Kinetic study on removal of copper(II) using goethite and hematite nano-photocatalysts. Journal of colloid and interface science 347 (2):277-281. doi:https://doi.org/10.1016/j.jcis.2010.03.050
CrossRef - Vojoudi H, Badiei A, Bahar S, Mohammadi Ziarani G, Faridbod F, Ganjali MR (2017) A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. Journal of Magnetism and Magnetic Materials 441:193-203. doi:https://doi.org/10.1016/j.jmmm.2017.05.065
CrossRef - Anbia M, Kargosha K, Khoshbooei S (2015) Heavy metal ions removal from aqueous media by modified magnetic mesoporous silica MCM-48. Chemical Engineering Research and Design 93:779-788. doi:https://doi.org/10.1016/j.cherd.2014.07.018
CrossRef - Li W, He R, Tan L, Xu S, Kang C, Wei C, Tang Y (2016) One-step synthesis of periodic ion imprinted mesoporous silica particles for highly specific removal of Cd2+ from mine wastewater. Journal of Sol-Gel Science and Technology 78 (3):632-640. doi:10.1007/s10971-016-3987-2
CrossRef - Anbia M, Mohammadi N (2009) A nanoporous adsorbent for removal of furfural from aqueous solutions. Desalination 249 (1):150-153. doi:https://doi.org/10.1016/j.desal.2008.06.027
CrossRef - Malik DS, Jain CK, Yadav AK (2017) Removal of heavy metals from emerging cellulosic low-cost adsorbents: a review. Applied Water Science 7 (5):2113-2136. doi:10.1007/s13201-016-0401-8
CrossRef - Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. Journal of Molecular Liquids 230:496-504. doi:https://doi.org/10.1016/j.molliq.2017.01.064
CrossRef - Wang X, Pei Y, Lu M, Lu X, Du X (2015) Highly efficient adsorption of heavy metals from wastewaters by graphene oxide-ordered mesoporous silica materials. Journal of Materials Science 50 (5):2113-2121. doi:10.1007/s10853-014-8773-3
CrossRef - MomÄilović MZ, RanÄ‘elović MS, Zarubica AR, Onjia AE, Kokunešoski M, Matović BZ (2013) SBA-15 templated mesoporous carbons for 2,4-dichlorophenoxyacetic acid removal. Chemical Engineering Journal 220:276-283. doi:https://doi.org/10.1016/j.cej.2012.12.024
CrossRef - Kosa SA, Al-Zhrani G, Abdel Salam M (2012) Removal of heavy metals from aqueous solutions by multi-walled carbon nanotubes modified with 8-hydroxyquinoline. Chemical Engineering Journal 181-182:159-168. doi:https://doi.org/10.1016/j.cej.2011.11.044
CrossRef - Saif MMS, Kumar NS, Prasad MNV (2012) Binding of cadmium to Strychnos potatorum seed proteins in aqueous solution: Adsorption kinetics and relevance to water purification. Colloids and Surfaces B: Biointerfaces 94:73-79. doi:https://doi.org/10.1016/j.colsurfb.2012.01.039
CrossRef - Afkhami A, Saber-Tehrani M, Bagheri H (2010) Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2,4-dinitrophenylhydrazine. Journal of hazardous materials 181 (1):836-844. doi:https://doi.org/10.1016/j.jhazmat.2010.05.089
CrossRef - Hakami O, Zhang Y, Banks CJ (2012) Thiol-functionalised mesoporous silica-coated magnetite nanoparticles for high efficiency removal and recovery of Hg from water. Water research 46 (12):3913-3922. doi:https://doi.org/10.1016/j.watres.2012.04.032
CrossRef - Li G, Zhao Z, Liu J, Jiang G (2011) Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica. Journal of hazardous materials 192 (1):277-283. doi:https://doi.org/10.1016/j.jhazmat.2011.05.015
CrossRef - Kanthimathi G, Kotteeswaran P, Thillai Arasu P, Govindaraj P, Kottaisamy M (2012) A Comparative Study of the Adsorption Efficiency of the Newly Synthetic Nano Iron Oxide and Commercial Activated Charcoal Towards the Removal of the Nickel(II) Ions. E-Journal of Chemistry 9 (4). doi:10.1155/2012/567428
CrossRef - Esmaeili Bidhendi M, Nabi Bidhendi GR, Mehrdadi N, Rashedi H (2014) Modified Mesoporous Silica (SBA–15) with Trithiane as a new effective adsorbent for mercury ions removal from aqueous environment. Journal of Environmental Health Science and Engineering 12 (1):100. doi:10.1186/2052-336X-12-100
CrossRef - Heidari A, Younesi H, Mehraban Z (2009) Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chemical Engineering Journal 153 (1):70-79. doi:https://doi.org/10.1016/j.cej.2009.06.016
CrossRef - Dehghani MH, Ghadermazi M, Bhatnagar A, Sadighara P, Jahed-Khaniki G, Heibati B, McKay G (2016) Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan. Journal of Environmental Chemical Engineering 4 (3):2647-2655. doi:https://doi.org/10.1016/j.jece.2016.05.011
CrossRef - Chang Y-C, Chang S-W, Chen D-H (2006) Magnetic chitosan nanoparticles: Studies on chitosan binding and adsorption of Co(II) ions. Reactive and Functional Polymers 66 (3):335-341. doi:https://doi.org/10.1016/j.reactfunctpolym.2005.08.006
CrossRef - Repo E, Warchol JK, Kurniawan TA, Sillanpää MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chemical Engineering Journal 161 (1):73-82. doi:https://doi.org/10.1016/j.cej.2010.04.030
CrossRef - Bhattacharyya KG, Gupta SS (2008) Adsorption of Fe(III), Co(II) and Ni(II) on ZrO–kaolinite and ZrO–montmorillonite surfaces in aqueous medium. Colloids and Surfaces A: Physicochemical and Engineering Aspects 317 (1):71-79. doi:https://doi.org/10.1016/j.colsurfa.2007.09.037
CrossRef - Labib SA, Yousif AM, Ibrahim IA, Atia AA (2018) Adsorption of rhodium by modified mesoporous cellulose/silica sorbents: equilibrium, kinetic, and thermodynamic studies. Journal of Porous Materials 25 (2):383-396. doi:10.1007/s10934-017-0449-3
CrossRef - Bhuyan MS, Bakar MA, Akhtar A, Hossain MB, Ali MM, Islam MS (2017) Heavy metal contamination in surface water and sediment of the Meghna River, Bangladesh. Environmental Nanotechnology, Monitoring & Management 8:273-279. doi:https://doi.org/10.1016/j.enmm.2017.10.003
CrossRef - Pan X, Wang J, Zhang D (2009) Sorption of cobalt to bone char: Kinetics, competitive sorption and mechanism. Desalination 249 (2):609-614. doi:https://doi.org/10.1016/j.desal.2009.01.027
CrossRef - Bui TX, Choi H (2009) Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. Journal of hazardous materials 168 (2):602-608. doi:https://doi.org/10.1016/j.jhazmat.2009.02.072
CrossRef - Cashin VB, Eldridge DS, Yu A, Zhao D (2018) Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: a review. Environmental Science: Water Research & Technology 4 (2):110-128. doi:10.1039/C7EW00322F
CrossRef - Guo W, Chen R, Liu Y, Meng M, Meng X, Hu Z, Song Z (2013) Preparation of ion-imprinted mesoporous silica SBA-15 functionalized with triglycine for selective adsorption of Co(II). Colloids and Surfaces A: Physicochemical and Engineering Aspects 436:693-703. doi:https://doi.org/10.1016/j.colsurfa.2013.08.011
CrossRef - Ngwabebhoh FA, Erdem A, Yildiz U (2016) Synergistic removal of Cu(II) and nitrazine yellow dye using an eco-friendly chitosan-montmorillonite hydrogel: Optimization by response surface methodology. Journal of Applied Polymer Science 133 (29). doi:10.1002/app.43664
CrossRef - Erdem A, Ngwabebhoh FA, Yildiz U (2017) Novel macroporous cryogels with enhanced adsorption capability for the removal of Cu(II) ions from aqueous phase: Modelling, kinetics and recovery studies. Journal of Environmental Chemical Engineering 5 (1):1269-1280. doi:10.1016/j.jece.2017.02.011
CrossRef






