Preparation and Utilization of the Precursor Activated Carbon from Carbon Enriched Fuel Oil Fly Ash: Part II Removal and Speciation of Chromium(III and VI) from Wastewater
1
Environmental Sciences Department,
Faculty of Meteorology Environment and Arid Land Agriculture,
Jeddah,
Saudi Arabia
2
Chemistry Department,
Faculty of Science King Abdulaziz University,
Jeddah,
Saudi Arabia
DOI: http://dx.doi.org/10.12944/CWE.12.3.08
Activated carbon (AC) prepared from residual fly (FA) was activated by combined CO2-steam gasification and physically modified with procaine hydrochloride (PQ+Cl-). Modified AC was used as low cost and effective solid phase extractor (SPE) for removal, total determination and chemical speciation of chromium(III&VI) in wastewater. Pseudo-second order rate equation is proposed for assigning the kinetics of chromium(VI) sorption in aqueous HCl (1.0 mol L-1) by PQ+Cl-- modified AC . The data were correlated with surface structure of the SPE. A dual separation mechanism of chromium(VI) as a binary complex ion associate [PQ+.CrO3Cl-] in/on the modified AC via "weak base anion exchanger" and an added component of “surface adsorption” is proposed. At 2 mL min-1 flow rate Sorption of spiked chromium(VI) at known concentrations (0.5-20 mg mL-1) from water onto modified AC packed columns at 2 mL min-1 was achieved. The sorbed chromium(VI) species were successfully recovered (106.0±2- 110 ±3%, n=3) from AC packed column with NaOH (10 mL, 1.0 mol L-1). Chromium(III) after oxidation to chromium(VI) was also enriched and eluted from the AC packed column and analyzed. Modified PQ+Cl- AC packed column was also used for total determination and speciation of chromium(III & VI) species at trace levels in environmental water samples. Good apparent recovery percentage (94.6±6.3-102.0± 8.5 %) of chromium species was achieved confirming the analytical utility of the modified AC-packed column for removal, total determination and speciation of chromium(III & VI) species in environmental water samples.
Copy the following to cite this article:
Abu-Rizaiza A, Kadi M. W, El-Shahawi M. S. Preparation and Utilization of the Precursor Activated Carbon from Carbon Enriched Fuel Oil Fly Ash: Part II Removal and Speciation of Chromium(III and VI) from Wastewater. Curr World Environ 2017;12(3). DOI:http://dx.doi.org/10.12944/CWE.12.3.08
Copy the following to cite this URL:
Abu-Rizaiza A, Kadi M. W, El-Shahawi M. S. Preparation and Utilization of the Precursor Activated Carbon from Carbon Enriched Fuel Oil Fly Ash: Part II Removal and Speciation of Chromium(III and VI) from Wastewater. Curr World Environ 2017;12(3). Available from: http://www.cwejournal.org?p=1040/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-09-27 |
|---|---|
| Accepted: | 2017-10-16 |
Introduction
Heavy metal ions at trace levels in water represents a major of great concern for environmental engineers and chemists.1-3 Wastewater (Industrial and domestic) affects the health of people and causes severe damage to the environment if not managed properly.4,5 Chromium(VI) is highly toxic and it has great mutagenic and genotoxic effects on biological systems whereas chromium(III) is essential trace nutrient.6,7 Biological reduction of Cr6+species to intermediates e.g. Cr5+ and Cr4+ play a crucial role in its toxicity.8 In drinking water, the permissible concentration level of chromium(VI) reported by WHO set at 0.05 µg L-1 9 whereas USA-Environmental Protection Agency (EPA) guide recommended a level of 100.0 µg/L.10 Allowable limit of discharge of Cr3+ in surface- and seawater varies from 0.5 to 2.0 mg/L, respectively.10 Manufacturing industries of aluminum, dye, paint, ink and tannery etc are the main sources of Cr6+.11 Removal and chemical speciation of Cr3+ and Cr6+ species from wastewater by low cost adsorbent before disposal are of great interest in water due to the bio dependence of Cr(III) oxidation in plants, soil sand sediments.11-17
The potential risk of FA generated in the local desalination power stations is considered as a solid waste that creates pollution problem in its landfill and surroundings areas.18 Coal, oil fuel and heavy fuel oil are the most common fuel used in thermal power stations in most countries.19,20 Utilization of various fossil fuels e.g. burning coal or heavy fuel oil in power generation plants and seawater desalination units are the major source of bottom ash and fly ash.21 Fly ashes seemed to too high, their chemical analysis indicated a high level of toxic metals; hence their utilization for the production of the precursor AC as a low cost solid phase extractor (SPE) for removal of heavy metal ions, dyes, phenols, chrysoidine Y, polyaromatic hydrocarbons in wastewater and capture of atmospheric gaseous species e.g. CO2 has been implemented and /or proposed recently in the literature.21-37 Large amount of FA is directly discharged as landfills and ash ponds, whereas a percentage (20 %) of FA is used in concrete production, road basement material, waste stabilization/solidification, amendments of soft soil and in geo polymers.37,38
In drinking water, the trace level of chromium is not compatible with the limit of detection of numerious spectrochemical methods. Thus, enrichment steps of chromium(VI) are of great importance to enhance the detection of the reported methods.39-45 Thus, numerous SPE including AC have been published for separation and subsequent speciation of low levels of chromium species.37-59
Various AC exhibit capacity performance towards chromium(VI) and other inorganic and organic analyte, because of their excellent textural properties (pore pore sizes and structures, large surface areas). Agricultural by-products and FA materials are the major sources for commercial AC.20-27 Based on the significant growth in Saudi Chemical industries23,24, this reports: i) Characterization of AC as an efficient SPE; ii) The sorption profile and kinetics of the uptake of chromium(VI) from aqueous HCl media onto PQ+.Cl- modified AC; and finally iii) Determination and speciation of chromium ions (III & VI) by modified AC packed column.
Materials and Methods
Sampling
FA samples (C1-C6) were randomly collected from three locations around Rabigh (one of the smallest towns in Saudi Arabia and lies at latitude 23o N and longitudes 400 30’E along the Red Sea coast in the west central part of the Arabian Shield) water desalination power station. Residual FA samples of heavy fuel oil (Vacuum gas oil, Bunker “C”) generated from the power station were dried in air for 21 days. FA samples were mixed with HCl (37%v/v) solution at FA waste/HCl weight ratio of 1: 20 (m/v) in a polytetrafluoroethylene beaker for 16-17 h at 25 oC. The solution mixtures were filtered and washed several times with water.The samples were dried at 105 oC for one day and finally stored in a desiccators.
Reagents and Materials
Glassware’s and polyethylene (LDPE) bottles were washed with con HNO3, and rinsed with doubly deionized water before use. A.R. grade chemicals were used as received. polyethylene (LDPE) bottles Standard solutions (1000.0 µg mL-1) of Cr (VI) and Cr(III) were prepared from BDH (Poole, England) Na2CrO4 and Cr(NO3)3 in de-ionized water, respectively. Diluted solutions of chromium(0.05- 20 μgmL-1) were prepared from the stock by dilution and was stored in LDPE bottles. BDH H2O2 (30% v/v) was used for oxidizing Cr(Ш) to chromium(VI) in aqueous alkaline solution.46,47 At 25 oC, the solutions were stored in amber glass bottles. The reagent [2-(diethylamino)ethyl 4 aminobenzoate] hydrochloride (1.0 %w/v) commercially named as procaine hydrochloride (PQ+.Cl-) and HCl were prepared individually in water (100 mL).
Apparatus
N2 adsorption measurements were performed at P/Po range 0.005 to 0.99 on a NOVA 2200e instrument (Quntachrome) at liquid nitrogen temperature of the FA and AC samples. A GSL-1800S60, MTI Inc., USA, Richmond, California tube furnace connected to an electric heating boiler was used for the heat treatment of the samples. Nitrogen gas (AHG, 99.99%) was used as an adsorbate. The equipment was connected to a rotary van vacuum pump (Pfeiffer vacuum) to obtain exhaust pressure of 250-1500 mbar and a final pressure ≤ 6.0×10-3 mbar [59]. A Perkin Elmer inductively coupled plasma – optical emission (ICP- OES), Optima 4100 DC (Shelton, CT, USA) and UV-visible (190-1100 nm) spectrometers were operated at the optimized parameters. ICP-OES is optimized daily and operated as recommended for analysis of Cr. The pH measurements were recorded on a Thermo Fisher Scientific Orion model 720 pH Meter (Milford, MA, USA). Milli-Q Waters Plus system (Milford, MA, USA) and digital micropipettes (Volac) were used for preparation of the solutions in deionized water. A mechanical shaker (Chicago, CH, USA) with 10 – 250 rpm shaking rate and glass columns (5.0 cm × 0.5 cm i.d) were used.
Preparation of AC
Dried FA samples (»3-4 g) were dipped into H2O2 (100 mL, 30%v/v) for 24 h to oxidize the volatile organic impurities , filtered and washed with water. At 100 °C, the samples were heated for 60 min to remove the moisture content, respectively. Heating of The samples were then heated in a tube furnace connected to an electric heating boiler for passing combined CO2 (grade 2.5-B) -steam at 4 psi at absolute pressure inside the tube below 0.12 MPa (Mega Pascal). The furnace was maintained at a heating rate of 5 oC min-1 and 6 psi. Inside the tube, the absolute pressure was always kept below 0.12 MPa (Mega Pascal). Samples were heated between 850-950 oC at 15 oC/min heating rate for 3 h at 120 mLmin-1 flow rate to a final carbonization process and activation at 950 oC. AC samples were left to cool down, washed with hot distilled water (until the pH is neutral) and dried in the oven at 105 oC for 24 h . The calculated weight loss of FA samples during activation was in the range 16.9-18% m/m.
Preparation of PQ+Cl- modified AC
Initially, PQ+ .Cl- reagent was dissolved in 100 mL of deionized in water (1.0% w/v). This solution was shaken with the AC (» 5.0 g) with efficient stirring in a jar test for 30 min as reported.59 The synthesized PQ+.Cl- treated AC was separated out from the liquid phase after centrifugation, washed at least three times with acetone and water and finally dried for 4 h under N2 gas.
Recommended procedures
Batch Experiments
In dry LDPE bottle, aqueous solution (100 mL) containing known concentration (20.0µg mL-1) of chromium(VI) at various pH were shaken with accurate weights (0.1 ± 0.001 g) of modified AC at room temperature for 1 h. After shaking, chromium(VI) remained in the aqueous solutions was analyzed spectrophotometrically.57 At trace level of chromium(VI) lower than the limit of detection (LOD) of DPC method,57 ICP-OES was used. At equilibrium, Sorbed chromium (VI), at equilibrium, qe onto modified AC was determined and the distribution ratio (D) and the extraction percentage (% E) of chromium (VI) uptake were determined.46,47 Similarly, the influence of mineral acids, HCl acidity, shaking time, media polarity and surfactant on chromium(VI) sorption was also studied following the same procedures. Triplicate measurements at 25±0.1ºC were performed and the results of % E and D of chromium(VI) sorption were calculated as means ± standard deviation (n=3).
Separation of chromium(VІ) by modified AC packed column
Various concentrations (0.5-20 μg mL-1) of chromium(VI) in aqueous HCl (100 mL, 1.0 mol/L) were percolated through the adsorbent (0.5± 0.001 g) packed column at 2.0 mL min-1 flow rate versus blank. AC packed columns of the blank and sample were washed with HCl solution (100 mL). Chromium(VI) in the effluent solution was then determined spectrophotometry [57] or by ICP-OES via standard curve of Cr. The analyte sorbed analyte on the AC packed column was then stripped with NaOH (10 mL, 1.0 mol/L) and analyzed versus reagent blank.
Separation of chromium(III)) by modified AC packed column
In aqueous HCl (1.0 mol/L) solution), various known concentrations (0.5-20 μg mL-1) of chromium(III) were transferred to conical flask (50.0 mL). The aliquots were, reacted with H2O2 (2 mL, 10% w/v) in alcoholic NaOH (1.0 mol/L), boiled for 20 min, cooled and the solution mixtures were adjusted to pH ~zero by HCl (1.0 -2.0 mol L-1). The solutions were percolated through the modified AC packed column and analyzed for chromium(VI).57 Sorbed analyte were stripped from the column with NaOH (1.0 1.0 mol/L) and quantified spectrophotometry.57
Total determination and speciation of chromium(ІІІ & VI) species
Various solutions (0.5L ) of chromium (III) & (VI) mixtures at a total trace levels ≤ 0.1 µg mL-1 were passed through the modified AC packed column at 2 mL min-1 flow rate. Chromium(VI) species in the effluents were analyzed as mentioned for chromium(VI) [57]. Another aliquot samples (0.5L) were oxidized to chromium(VI), percolated through the modified AC packed column and analyzed as mentioned above for chromium(III). The absorbance (A1) or the ICP-OES signal (I1) of the first aliquot is a measure of chromium(VI) ions whereas the absorbance (A2) or the signal intensity (I2) of the second aliquot is equivalent for the sum of total chromium(III & VI) species in the mixture. Hence, the absorbance (A2-A1) or ICP-OES signal (I2-I1) intensities is equivalent to of chromium(III) in the aliquots
Analytical Application
In LDPE bottles, samples of industrial wastewater (1.0 L) were filtered through a 0.45 μm membrane filter (Milex, Millipore Corporation). Samples were immediately spiked with various amounts (0.05-0.5 μg) of chromium(III & VI), treated with HNO3-H2O2 (100 mL, 1:1 v/v) and boiled for 10 min. The aliquots were centrifuged for 5 min and percolated through adsorbent packed column and analyzed as described for chromium(III). Sorbed Cr species were then stripped with NaOH and analyzed by ICP-OES.
Precision and accuracy
The precision of the extraction and recovery in the overall procedures, was tested by subjecting three sub samples to the sequential procedure. The limit of detection (LOD) (µg/g of dry ash) was defined as LOD = 3Sy/x/b where Sy/x = standard deviation of y- residual and b is the slope of the standard plot. Precision is low as the analyte concentration approaches the LOD and it improves for higher concentrations.
Results and Discussion
FA represents a major issue in the coming decades since electricity generation would remain predominantly heavy fuel and coal-based burning. Thus, FA solid waste is current increasing/year and in 2016, it is expected to increase to 300-400 MT/year. Utilization of power generation FA wastes represents one of the most common problems of thermal power plants for heavy oil and solid fuel burning. Thus, great efforts performed for better utilization and application of FA generated from heavy fuel oil FA for the production of AC adsorbent for use as a SPE for removal and/or minimization of chromium (VI) from wastewaters.
Characterization of FA and AC
Chemical analysis of FA samples revealed considerable amount of V, Fe and Ni. On the other hand, Na, S, K, Si, Al in addition to other trace elements are the next abundant elements . The average content of V, Fe and Ni content lies in the range 3625.1 ±21.8; 4057.0±32.7 and 751.2 ±13.7 µg/ g, respectively. The content of Fe and V is comparable with the data reported except for Ni.42 Digestion of FA containing Ni requires enough time resulting in an incomplete digestion of FA and it is also too tedious. Moreover, Ni able to form organo Ni compounds with the oxygenated and organocarbon species forming stable complex species of Ni in the FA samples. C, H, N, total CHN contents and weight loss of FA samples were in the range 87.93± 5.3; 0.15± 0.03; 1.51± 0.27, 89.51± 2.14% and 91.45%, respectively. These values are in agreement with FA of other heavy fuel oil.24,59
The major particle sizes of FA were in the range 1.8-16.4 µm with 50 frequencies in comparable with FA generated from heavy fuel power plants.58,59 XRD patterns (Fig. 1) of FA at room temperature revealed similar broad peaks which are highly disordered and amorphous.58,59 Anorthite (CaAl2Si2O8) was the only aluminosilicate found with a FOM close to 20 whereas calcium containing minerals e.g. CaSO4, KCaCl3 were noticed [58]. On the other hand, the composition of FA was porous spheroid of unburned carbon with diameters within a range from 10.0 to 120 mm larger than that of black carbon and contains pores few mm in sizes.
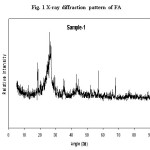 |
Figure 1: X-ray diffraction pattern of FA sample. |
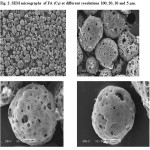
SEM images (Fig. 2) of FA (sample C3) showed many of iron spheres most likely composed of Fe2O3 mixed with amorphous alumino-silicate. Calcium associated with S and/ or P in distinct particles was also noticed not with amorphous alumino-silicate. Small pores may have formed during the combustion process of heavy oil FA. The morphology of FA particles is mainly spherical, porous spheroid of unburned carbon.These particles are porous, hollow and granular in size. The burn of FA at 850 and 950 0C lies in the range 16.15-25.39 and 16.67-64.6%, respectively.
|
|
|
Major particle size distributions (PSD) of AC were in the range 1.8-16. µm with frequencies 90, respectively. The surface area and pore volume of AC were 775.9 m2/g and 0.138 cc/g, respectively were reported earlier [59]. The calculated pore volume (0.138cc/g) of the AC represents the most successful approach for activation of FA at 950 0C for 2 h. The pore volume decreased or did not develop quite well at temperature of 850 0C for different time intervals. The data suggest further modification of AC by ion pairing reagent like procaine hydrochloride (PQ+.Cl-) for use as a SPE for removal of chromium(VI) from water. SEM images (Fig. 3) of AC before and after modification with PQ+.Cl give evidence to the more porous nature of the template carbons. In the AC and modified AC little porosity is also seen with their respective fraction of mesoporisity.
|
|
|
Sorption characteristics of chromium(VI) onto modified AC
Chromium (VI) species in HCl (1.mol L-1) present as halochromate (CrO3Cl-) and it can be extracted into SPE by complex ion associate formation as reported earlier [46, 47].Thus, preliminary batch experiments on chromium(VI) uptake from aqueous HCl media by PQ+.Cl- modified AC were critically performed. Considerable adsorption of chromium(VI) by the PQ+.Cl- modified AC adsorbent was achieved. Sorbed chromium(VI) was found to depend on extraction media pH. Thus, the use of PQ+.Cl- modified AC for the extraction of chromium(VI) at different pH was critically studied out.
The adsorption of chromium(VI) at two different concentrations (5, 7.5 µg/mL) from aqueous solution (100mL, 10mg mL-1) at different pH by PQ+.Cl- modified AC was examined over a wide range of pH after 1 h shaking period. Maximum % E and the D of chromium(VI) from the aqueous solution was achieved at pH ~ zero and lowered at higher pH of the solution pH. Thus, the influence of mineral acid e.g. HCl, HNO3 and H2SO4 at 1.0 mol/L concentration on chromium(VI) uptake was studied. Of which, only HCl showed satisfactory extraction of chromium(VI) onto AC adsorbent. The formation of chlorochromate [CrClO3]- anion which is able to form complex ion associate on/in PQ+.Cl- modified AC may account for the observed trend.
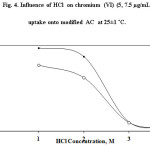
HCl concentration and the formation of [CrClO3]- anion play a crucial role on chromium(VI) uptake. Thus, the effect of various concentrations (1-5 mol L-1) of HCl on chromium(VI) sorption by the used adsorbent was studied (Fig. 4). On raising HCl concentration up to 2.0molL-1 , chromium (VI) retention increased and remained constant up to 3.0 mol L-1. Hydrolysis, instability or the incomplete formation of the produced [PQ+.CrClO3]-AC ion associate on/in the adsorbent may account for the observed decrease in chromium(VI) extraction. Thus, in the next experiments, the concentration of HCl was adopted at 2 molL-1as a suitable extraction medium.
|
|
|
Based on the achieved results at pH and HC and the data reported [46], a "weak base ion exchange" of the ion associate [CrO3Cl-. PQ+] is proposed. For analyte uptake by the modified AC. Based on the results of pH, mineral acids, HCl and the surface characteristics of the AC a dual – mode of chromium(VI separation as [PQ+.CrO3Cl-] ion associate via a "weak base anion exchanger" and an added component for "surface adsorption" is most likely proposed mechanism.
The most common specie at HCl ≤ 3.0 mol L-1 is hydrogen chromate oxoanion (HCrO4−) [46, 47] which in HCl media converted to chlorochromate anions [CrO3Cl-] as follows:
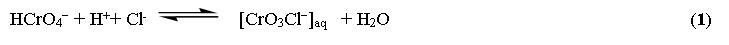
The bulky anion [CrO3Cl]− is retained in/on the modified AC as follows:
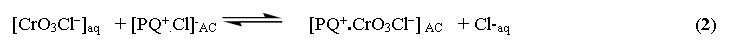
The solvent polarity on chromium (IV) extraction by the modified AC was also studied at various proportions of ethanol (1-10 %v/v). Chromium(VI) uptake increased linearly on adding ethanol up to 5% (v/v) and remained constant. The change in media polarity makes the binding sites of the AC sorbent around chromium (VI) more hydrophilic and diminishing the need of water for solvating molecules. Thus, the analyte uptake decreased in/on the used adsorbent.47
The effect of Triton-X 100, tetraethyl ammonium chloride or sodium dodecyl sulphate (SDS) on chromium (VI) adsorption was tested. In the presence of tetraethylammonium chloride up to 0.1% (w/v), chromium(VI) sorption increased and leveled off at higher concentrations of surfactant. The change in the viscosity of the environment of the around the complex anion associate [CrO3Cl]- is most likely account for the observed trend.46,47 The solution viscosity enhances aggregation of the associate thus, reducing the diffusion rate of chromium(VI).
Kinetics of chromium(VI) adsorption by modified AC
Chromium (VI) adsorption from aqueous HCl media (≤3.0 Mol L-1) onto the modified AC was found fast and reached equilibrium within short time (~20 min). Thus, Weber-Morris equation61 is proposed:
Qt =Rd (t)1/2 (3)
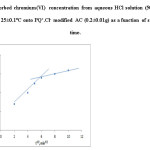
the amount of chromium(VI) adsorbed on the modified AC was plotted versus square root of time (min), where Rd = the rate constant of intraparticle transport in mg g-1 min-1/2. The diffusion rate was rapid in the initial stage and slightly decreased on passage of time .The plot of qt versus time was linear (Fig. 5, R2= 0.986) in the initial stage for chromium (VI) retention onto adsorbent up to 10 ± 1 min and deviate on increasing time of shaking. The diffusion rate was high and decreased on time passage indicating that the rate of adsorption is film diffusion at the early stage of extraction [49]. The values of Rd determined from the two slopes of the plot were found 2.796 ± 1.01 and 0.793 ± 0.02 with r2= 0.989 and 0.992 , respectively. The existence of different pore size is most likely account for the change in the slope [60, 61].
|
|
|
Chromium(VI) sorption was further subjected to the linear form of Lagergren model for pseudo – first order is expressed as follows [60]:

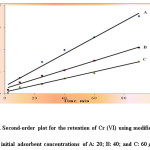
where, qe is the amount of Cr (VI) sorbed at equilibrium per unit mass of sorbent (μ moles g-1); KLager = first order overall rate constant for the sorption process per min and t is the time in min.The values of KLager and qe computed from the slopes and intercepts of the linear plots of log (qe − qt) versus t at various analyte concentrations (20, 40, and 60 µg mL−1) were in the range 0.028 - 0.030 min-1 and 9.03 - 23.3 mg g-1, respectively with correlation coefficients (R2=0.91-0.94) (Table 1). Because of the large difference between the calculated and experimental values of qe , this model is not often used for the estimation of qe [60]. Thus, the Cr (VI) adsorption onto the modified AC at 25 ± 1 0C was subjected to the second order kinetic model [61, 62]] :

|
|
|
Table 1: Calculated kinetic parameters for pseudo first-order and second-order kinetic models for the adsorption of Cr (VI) onto PQ+.Cl- modified as an adsorbent
|
S. No |
Cr (mgL-1) |
qe (mg g−1) experimental |
First-order kinetic model |
second-order kinetic model |
||||
|
K1(min-1) |
qe |
R2 |
K2×103 |
qe |
R2 |
|||
|
1 |
20 |
13.92 |
0.028 |
9.03 |
0.91 |
7.66 |
13.7 |
0.98 |
|
2 |
40 |
32 |
0.028 |
17.9 |
0.96 |
1.88 |
33 |
0.98 |
|
3 |
60 |
20.1 |
0.030 |
23.3 |
0.94 |
0.88 |
24 |
0.99 |
Separation of Chromium(VI) by PQ+.Cl--AC packed columns
The sorption profile and the kinetics of chromium(VI) by the proposed modified AC suggested the use of the modified AC adsorbent in packed column for chromatographic separation of chromium(VI). Known concentrations (0.5-20 μg mL-1, 0.1 L) of chromium(VI) were passed through modified AC packed column at 2 mL/min flow rate at optimized conditions. ICP-OES analysis of Cr in the effluent revealed complete sorption of chromium(VI). Sorbed chromium(VI) species were finally quantitatively stripped (106.0±2- 110 ±3%) with NaOH (Table 2).
The proposed modified AC packed column was also used for recovery of chromium(III) after its oxidation to chromium(VI) [46]. Known concentration (0.5-20 μg mL-1) of chromium(III) after oxidation were passed through the modified AC packed column. The retained chromium(VI) species were stripped from the AC packed column and an acceptable apparent recovery percentage (90.0 ±6-96.0±0.6) of chromium(III) was achieved (Table 2). Incomplete oxidation of chromium(III) may account for the decrease in the recovery percentage. Total determination of chromium(III & VI) ions in their mixtures were also performed and analyzed following the optimized conditions for chromium(III) ions. The signal intensity represents a measure of total chromium(III & VI) species in the mixture and a recovery percentage in the range 92 (±3.9)-104.3 (±4.4) % was achieved.
Table 2: Analytical results for chromium(III & VI) in deionized water sample
|
Added, µg mL-1 |
Found, µgmL-1 |
Recovery*, % |
|||
|
Cr(III) |
Cr(VI) |
Cr(III) |
Cr(VI) |
Cr(III) |
Cr(VI) |
|
0.5 |
0.5 |
0.45 ±0.03 |
0.53±0.01 |
90.0 ±6 |
106.0±2 |
|
10 |
10 |
9.6 ±0.09 |
10.7±0.07 |
96±0.9 |
107±0.7 |
|
20 |
20 |
19.2 ±0.12 |
21.6 ±0.5 |
96.0±0.6 |
110 ±3 |
* Average of three measurements ±standard deviation (n=3).
Analytical Applications
The analytical utility of the proposed AC packed column was tested by the recovery study of a total concentration of chromium(III) and/or chromium(VI) species ≤ 50µg/ L (0.5L) spiked to tap and wastewater. Various concentrations of chromium(III) and/or (VI) were spiked into tap- and wastewater samples and analyzed following the optimized procedures for chromium(III). Complete adsorption of chromium was achieved as indicated from ICP-OES analysis of chromium in the effluent. Sorbed chromium species were recovered with NaOH (10 ml, 1 mol/L) and analyzed by ICP-OES. The results are demonstrated in Table 3. Apparent recovery percentage (94.6±6.3-102.0± 8.5 %) of chromium confirming the analytical utility of the proposed AC packed column for complete removal and/or determination of chromium species in environmental water samples.
Table 3: Determination of total chromium(VI) in distilled and tap water by the developed PQ+.Cl- treated AC *
|
Sample |
Cr(VI), µg L-1 |
||
|
Added |
Found |
Recovery, % |
|
|
Wastewater |
100 |
102.6± 8.50 |
102.0± 8.5 |
|
Tap water |
500 |
480.4±2.9 |
96.0 ± 0.58 |
|
Tap water |
100 |
94.6±6.3 |
94.6±6.3 |
Conclusion
The manuscript provides a snapshot of the field of FA utilization. Modified AC has a great potential as an effective SPE for removal, enrichment and/or total determination in environmental water samples. Utilization of FA in analytical chemistry for speciation of chromium(III & VI) species and other heavy metal ions represents challenges. The proposed PQ+Cl-. AC column offers LOD lower than maximum permissible levl (MAL) of chromium(VI) in water set by WHO and favorably compared with LOD of many spectrochemical and chromatographic techniques. AC adsorbent is worthwhile alternative SPE to the commercial active carbon. The used AC packed column could also be extended for trace enrichment of Cr and other toxic metals in water samples by on-line collection from large sample volumes of water. AC packed column could be reused for 3-4 times without decrease in its performance.
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) – King Abdulaziz City for Science and Technology –the Kingdom of Saudi Arabia- award number (8-ENV124-3). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support. One of the authors (M.S. El-Shahawi) would like to thank the Department of Chemistry, KAU for the facilities provided. Special thanks to the students and technicians whom assisted the authors to perform the experimental work.
References
- Mohanty, M. Jha, B.C. Meikap, M.N. Biswas “Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride”. Chem. Eng. Sci., 60 (2005) 3049 - 3059.
- Ali, H. Y. Aboul-Enein, Instrumental methods in metal ions speciation: Chromatography, Capillary Electrophoresis and Electrochemistry,. Taylor & Francis Ltd., New York, USA (2006), ISBN: 0-8493-3736-4. 4.
- I. Ali, H. Y. Aboul-Enein, V. K. Gupta, Nanochromatography and Nanocapillary Electrophoresis: Pharmaceutical and Environmental Analyses, Wiley & Sons, Hoboken, USA (2009), ISBN: 978-0-470-17851-5.
- R.K. Dwari, M.N. Biswas, B.C. Meikap “Performance characteristics for particles of Sand-FCC and Fly-Ash in a novel hydrocyclone”. Chem. Eng. Sci., 59 (2004) 671–684.
- J. Threeprom, S. Purachaka, L. Potipan “Simultaneous determination of Cr(III)-EDTA and Cr(VI) by ion interaction chromatography using a C18 column”. J. Chromatogr. A, 1073 (2005) 291-295.
- Y. Yang, J. He, Z. Huang, N. Zhongb, Z. Zhua, R. Jiang, J. You, X. Lu, Y. Zhu, S. He “Analysis of hexavalent chromium in Colla corii asini with on-line sample pretreatment valve-switching ion chromatography” J. Chromatogr. A, 1305 (2013) 171- 175.
- L. Elci, U. Divrikli, A. Akdogan, A. Hol, A. Cetin, M. Soylak, Selective extraction of chromium(VI) using a leaching procedure with sodium carbonate from some plant leaves, soil and sediment samples, J.Hazard.Mater.173 (2010) 778-782.
- I. Ali, V K Gupta, Advances in Water Treatment by Adsorption Technology, Nature Protocol, 1 (2006) 2661-2667.
- I. Ali, New Generation Adsorbents for Water Treatment, Chem. Rev., 112 (10) (2012) 5073–5091.
- I. Ali, The Quest for Active Carbon Adsorbent Substitutes: Inexpensive Adsorbents for Toxic Metal Ions Removal from Wastewater, Sep. Purf. Rev., 39 (2010) 95-171.
- I. Ali, M. Asim, T.A. Khan, Low cost adsorbents for removal of organic pollutants from wastewater, J. Environ. Manag., 113 (2012) 170-183.
- I. Ali, Water Treatment by Adsorption Columns: Evaluation at Ground Level, Sep. Purf. Rev., 43 (2014) 175-205 .
- X. Han, X.-T. Zhoh, S.-W. Xu, Y. Li, Y.-F. Wang, Y. Liu” Removal of Cr(VI) and phenol coupled with the reduction of sulfate by sulfate-reducing bacteria sludge:, Int. J. Environ. Sci. Technol 14 (2017) 2173-2180.
- P. Miretzky, A. F. Cirelli, Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: a review, J. Hazard. Mater. 180 (2010) 1-19.
- S. Sadeghi, A. Z.Moghaddam, Task-specific ionic liquid based in situ dispersive liquid-liquid microextraction for the sequential extraction and determination of chromium species: optimization by experimental design, RSC Advances. 5 (2015) 60621- 60629.
- D. Mohan, K.P. Singh, V.K. Singh Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth, J. Hazard. Mater.B135 (2006) 280-295.
- N.F. Fahim, B.N. Barsoum, A.E. Eid, M.S. Khalil Removal of chromium(III) from tannery wastewater using activated carbon from sugar industrial waste J. Hazard. Mater. B136 (2006) 303-309.
- C.M. Alonso-Hernández, J. Bernal-Castillo, Y. Bolanos-Alvarez, M. Gómez-Batista, M. Diaz-Asencio, Heavy metal content of bottom ashes from a fuel oil power plant and oil refinery in Cuba, Fuel 90 (2011) 2820–2823.
- M. Pires, X. Querol, Characterization of Candiota (South Brazil) coal and combustion by-product, Intern. J. Coal Geology 60 (2004) 57-72.
- Y. S. Al-Degs, A. Ghrir, H. Khoury, G. M.Walker,M. Sunjuk, M. A. Al-Ghouti, Characterization and utilization of fly ash of heavy fuel oil generated in power stations, Fuel Processing Technol., 123 (2014) 41- 46.
- H. Tao, T. Lei, G. Shi, Xia-Nan Sun, Xue-Yan Wei, Li-Juan Zhang, Wei-Min Wub, Removal of heavy metals from fly ash leachate using combined bio electrochemical systems and electrolysis, J. Hazard. Mater., 264 (2014) 1- 7.
- M. Izquierdo, X. Querol, Leaching behavior of elements from coal combustion fly ash: An overview, International Journal of Coal Geology 94 (2012) 54- 66.
- Y. A. Alhamed, S.U. Rather, A.H. El-Shazly, S.F. Zaman, M.A. Daous, A.A. Al-Zahrani, Preparation of activated carbon from fly ash and its application for CO2 capture. Korean J. Chem. Eng. 32 (4) (2015) 723-730.
- S. Salehin, A. S. Aburizaiza, M.A. Barakat, Activated carbon from residual oil fly ash for heavy metals removal from aqueous solution, Desalination and Water Treatment 57 (1) (2016) 278-287.
- D. Gómez, M. Dos Santos, F. Fujiwara, G. Polla, J. Marrero, L. Dawidowski, P. Smichowski, Fractionation of metals and metalloids by chemical bonding from particles accumulated by electrostatic precipitation in an Argentine thermal power plant, Microchem. J., 85 (2007) 276–284.
- V.K. Gupta, A. Mittal, A. Malviya, J. Mittal “Adsorption of carmoisine A from wastewater using waste materials-Bottom ash and deoiled soya, J. Colloid and Interface Sci 335 (2009) 24-33.
- M. Visa, A. Duta, TiO2/fly ash novel substrate for simultaneous removal of heavy metals and surfactants, Chem. Eng J 223 (2013) 860-868.
- M. Visa, A-M. Chelaru “Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment’ Applied Surface Sci 303 (2014) 14-22.
- A. Mittal, D. Kaur, A. Malviya, J. Mittal, V.K. Gupta “Adsorption studies on the removal of coloring agent phenol red from wastewater using wastewater as adsorbents” J. Colloid. Interface Sci 337 (2009) 345-354.
- A. Mittal, J. Mittal, A. Malviya, V.K. Gupta “Removal and recovery of chrysoidine Y from aqueous solution by waste materials” J. Collod. Interface Sci., 344 (2010) 497-507.
- T. A. Khan, S. Sharma, I. Ali, Adsorption of Rhodamine B dye from aqueous solution onto acid activated mango (Magnifera indica) leaf powder: Equilibrium, kinetic and thermodynamic studies J. Toxicol. Environ. Health Sci.,3(10) (2011) 286-297.
- I. Ali, Z.A. Al -Othman, A. Alwarthan, Uptake of propranolol on ionic liquid iron nanocomposite adsorbent: Kinetic, thermodynamics and mechanism of adsorption, J. Mol. Liq, 236 (2017) 205-203.
- I. Ali, Z.A. Al-Othman, A. Alwarthan, Molecular uptake of congo red dye from water on iron composite nano particle, J. Mol. Liq., 224 (2016) 171-176.
- I. Ali, Z.A. Al-Othman, A. Alwarthan, Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water, J. Mol. Liq., 221 (2016) 1168-1174.
- I. Ali, Z.A. Al-Othman, A. Alwarthan, Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water, J. Mol. Liq., 219 (2016) 858-864.
- I. Ali, Z.A. Al-Othman, O.M. Alharbi, Uptake of pantoprazole drug residue from water using novel synthesized composite iron nano adsorbent, J. Mol. Liq., 218 (2016) 465-472.
- J. Caoa, X. D. L. Li, Y. Donga, S. Hampshire, Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity, J. Eur. Ceramic Society 34 (13) (2014) 3181–3194.
- D. Mohan, K.P. Singh, V.K. Singh, Removal of hexavalent chromiumfrom aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth, Ind. Eng.Chem. Res. (ACS) 44 (2005) 1027–1042.
- S. Rengaraj, C.K. Joo, Y. Kim, J. Yi, Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins:1200H, 1500H and IRN97H, J. Hazard. Mater. 102 (2003) 257–275.
- H. Shaalan, M. Sorour, S. Tewfik, Simulation and optimization of a membrane system for chromium recovery from tanning wastes, Desalination 14 (2001) 315 - 324.
- C.A. Kozlowski, W. Walkowiak, Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes, Water Res. 36 (2002) 4870 - 4876.
- J.J. Testa, M.A. Grela, M.I. Litter, Heterogeneous photocatalytic reduction of chromium (III) over TiO2 particles in the presence of oxalate: involvement of Cr(VI) species, Environ. Sci. Technol. 38 (2004)1589 -1594.
- V.K. Gupta, A.K. Shrivastava, N. Jain, Biosorption of chromium (VI) from aqueous solutions by green algae spirogyra species, Water Res. 35 (2001) 4079 - 4085.
- R. Arvindhan, B. Madhan, J.R. Rao, B.U. Nair, T. Ramasami, Bioaccumulation of chromium from tannery wastewater: an approach for chrome recovery and reuse, Environ. Sci. Technol. 38 (2004) 300 - 306.
- M.H. Liu, H. Zhang, X.S. Zhang, Y. Deng, W.G. Lu, H.Y. Zhan, Removal and recovery of chromium (III) from aqueous solutions by spheroidal cellulose adsorbent, Water Environ. Res. 73 (2001) 322 - 328.
- M. S. El-Shahawi, † A. S. Bashammakh, M. Abdelmageed,“ Chemical Speciation of Chromium(III) and (VI) using Phosphonium Cation Impregnated Polyurethane Foams Prior to Their Spectrometric Determination” , Analytical Sci. (Japan), 27 (7) (2011) 757 -763.
- M.S.El-Shahawi, S.S.M. Hassan, A.M.Othman, M.A.El-Sonbati "Retention Profile and Subsequent Chemical Speciation of Chromium (III) and (VI)) in Industrial Wastewater Samples Employing some Onium Cations Loaded Polyurethane Foams" Microchem. J., 89 (2008) 13 -319.
- Z. Reddad, C. Gerenete, Y. Andres, P. LeCloeiric, Mechanism of Cr(III) and Cr(VI) removal from aqueous solutions by sugar beet pulp, Environ. Technol. 24 (2003) 257 - 264.
- S. Deng, R. Bai, Removal of trivalent and hexavalent chromium with aminated polyacrylo nitrile fibers: performance and mechanism, Water Res. 38 (2004) 2424 - 2432.
- J. Rivera-Utrilla, I.B. Toledo, M.A. Ferro-Garcia, C. Moreno-Satilla, Biosorption of Pb(II), Cd(II) and Cr(VI) on activated carbon from aqueous solutions, Carbon 41 (2003) 323 -330.
- V.K. Gupta, I. Ali, Removal of lead and chromium from wastewater using bagasse fly ash a sugar industry waste, J. Colloid Interface Sci. 271 (2004) 321 - 328.
- R. Leyva-Ramos, L. Fuentes-Rubio, R.M. Guerrero-Coronado, J.Mendoza-Barron, Adsorption of Cr(III) from aqueous solutions onto activated carbon, J. Chem. Technol. Biotechnol. 62 (1995) 64 -67.
- R. Leyva-Ramos, J. Martinez, R. Guerrero-Coronado, Adsorption of Cr(IV) from aqueous solutions onto activated carbon, Water Sci. Technol.30 (1994) 19 - 197.
- S. S. Baral, S. N. Das, P. Rath, G. Roy Chaudhury, Y. V. Swamy, Removal of Cr (VI) from aqueous solution using waste weed, Salvinia cucullata, Chem. Ecology, 23(2007) 105-117.
- S. S. Baral, Namrata Das, G. Roy. Chaudhury, S. N. Das A preliminary study on the adsorptive removal of Cr(V) using seaweed, Hydrilla verticillata, J. Hazard. Mater. ,171 (2009) 358 -369.
- S. S. Baral, S. N. Das, P. Rath, G. Roy Chaudhury, Y. V. Swamy, Biosorption of Cr (VI) using Thermally Activated Weed Salvinia Cucullata, Chemical Eng. J., 139 (2008) 245-255.
- Z. Marczenko, M. Balcerzak “Separation, Preconcentration and Spectrophotometry in Inorganic Analysis” Elsevier Amsterdam- Lausanne-New York- Oxford-Shannon-Tokyo, 1st edn.2000.
- Y. Liu, L. Zheng, X. Li, S. Xie. SEM/EDS and XRD Characterization of raw and washed MSWI fly ash sintered at different temperatures, J. Hazard. Mater., 162 (2009) 161-173.
- A.Abu-Rizaiza, M.W. Kadi, M.S. El-Shahawi, Activated carbon from fly ash of heavy fuel oil: Characterization and its utilization for removal and determination of chlorophenonls in water, Bioscience Biotechnol. Res. Asia 14 (3) (2017) in press.
- Y.S. Ho, Citation review of Lagergren kinetic rate equation on adsorption reactions, Akadémiai Kiadó (Budapest) 59 (1) (2004) 171-177.
- Y.S. Ho, G. Mckay , A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents, Process Safety and Environmental Protection, 76B (1998) 332-340.
- Y.S. Ho, G. Mckay, G. , The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat, Can. J. Chem. Eng., 76 (1998) 822-827.