Comparison of the Quality of Chitin and Chitosan From Shrimp, Crab and Squilla Waste
DOI: http://dx.doi.org/10.12944/CWE.12.3.18
Chitin and Chitosan obtained from the crustaceans are having more biological value such as physiological compatibility, non-toxicity, bio digestibility, adsorption and chelating capacity. These biological values of chitosan depend on the quality parameters which are directly related to the source of the raw material. In this study, three commercially available crustacean shell waste such as shrimp, crab and squilla were used for the extraction of chitin and chitosan. The chemical treatment of demineralization, deproteinization and deacetylation were used for the production of chitosan. The viscosity quality parameter of the shrimp chitosan (5300cPs) was better than the crab and squilla chitosan. It is due the high solubility (97.65%) of the shrimp chitosan in 1% acetic acid. The degree of deacetylation of the shrimp chitosan (81.24%) directly relates the solubility of the chitosan. The chitosan with these quality parameters considered to be the excellent biological value. The yield of shrimp chitosan (15.4%) was also more when compare to crab and squilla chitosan. These result showed that utilisation of shrimp shell waste for the production of chitin and chitosan will give more economical and biological value along with reduction of environmental pollution.
Copy the following to cite this article:
Parthiban F, Balasundari S, Gopalakannan A., Rathnakumar K, Felix S. Comparison of the Quality of Chitin and Chitosan From Shrimp, Crab and Squilla Waste. Curr World Environ 2017;12(3). DOI:http://dx.doi.org/10.12944/CWE.12.3.18
Copy the following to cite this URL:
Parthiban F, Balasundari S, Gopalakannan A., Rathnakumar K, Felix S. Comparison of the Quality of Chitin and Chitosan From Shrimp, Crab and Squilla Waste. Curr World Environ 2017;12(3). Available from: http://www.cwejournal.org/?p=18529
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2016-12-09 |
|---|---|
| Accepted: | 2017-11-16 |
Introduction
World population growth continuously overtakes the rate of fish production. The FAO Code of Conduct for Responsible Fisheries was concerned about the clear signs of over-exploitation of fish stocks and to recommend new approaches to fisheries management which included conservation, environmental, social, and economic considerations.14 The quantity of processing waste generated around 20-80% depends on the species, processing stage, and the technology used.3 The Indian seafood export was Rs. 9.5lakhs ton for the year 2015-16 dominated by crustaceans and molluscs.19 The industries reject approximately up to 75 % of total weight of raw material, these can create serious pollution and disposal problems.9 The crustacean shell wastes obtained from seafood industries have only a low economic value and they are used as either animal feed or organic manure [26]. Squilla is a another underutilized trash fish contributes to an estimated quantity of 1.8% of the total marine landings of India and converting into fishmeal, poultry feed and manure.27 The shellfish waste contains 8- 10% chitin, 30-65% protein 10-20 % and calcium on a dry weight basis.23 Chitin considered as the second most abundant biopolymer after cellulose found in the nature.22 Chitosan has attracted consideration due to the unique cationic nature, which is obtained after the process called N- deacetylation of chitin,28 Chitin and chitosan are commercial attracted due to their physiological compatibility, non-toxicity, bio digestibility, and adsorption and chelating capacity. These characteristics of chitosan have many biotechnological industrial applications such as clinical, cosmetics, food, pharmaceutical, agriculture, aquaculture and environmental engineering,24 The type and quality of chitin and chitosan varies with different source, though different concentration of wide range of acid and alkai are used to extract chitin and chitosan from shell waste.13 Nguyen Van Toan28 reported that the chitin and chitosan produced with 3% HCl for demineralisation, 4% NaOH for deprotenisation and 50% NaOH for deacetylation was resulted in good quality chitin and chitosan. By adopting Nguyen Van Toan28 chemical method, this study was designed to compare the quality of chitin and chitosan obtained from shrimp, crab and squilla using this processing method.
Materials and Methods
Materials
Fresh local Indian white shrimp (Fenneropenaeus indicus), Mud crab (Scylla serrata) and Squilla (Squilloides leptosquill) shells were collected from Pulicat lake, Tamil Nadu, India. The proximate composition of the fresh shells was estimated on wet weight basis. The shell wastes were sun dried after cleaning. The dried shells were packed in sealed polythene bags and kept at ambient temperature (28±2°C) to carry out the experiments.
Composition analysis
The proximate composition of crustacean shell waste was estimated by standard protocols as follows; moisture, protein, ash5 and lipid.7 There were three replicates sampling procedure adapted for the analyses various parameters. The percentage of moisture content was estimated by dehydration of sample (approx. 5g) in an oven at 105°C for 24 hours. For the estimation of crude protein, around 1g of shell waste sample was used to estimate the nitrogen content in semi-automatic Kjeldahl apparatus. The percentage of crude protein calculated from the total nitrogen by the multiplication factor of 6.25. Ash content was estimated using muffle furnace by burning the shell sample at 550°C for 6 hours. The crude lipid content was estimated using Soxhlet apparatus by extracting with the solvent acetone from the known quantity of dried shell waste. Analyses were made in three replicates.
Preparation of chitin and chitosan
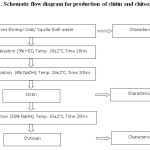
The preparation of chitin was followed by 2 (two) treatment steps namely demineralization, deproteinization and the production of chitosan by the additional treatment step called deacetylation. The schematic diagram for the above processing steps are explained below and shown in Fig.1.
Demineralization
Demineralization of shell wastes were treated with 3% HCl with a solvent to solid ratio 5:1 (v/w) at room temperature (28±2°C) for 16 hrs.28 The residual HCl was removed by repeated washing by portable water to reach the neutral pH.
Deproteinization
After demineralization, 4% NaOH with a solvent to solid ratio 5:1 (v/w) for 20 hours at ambient temperature (28±2°C) carried out for the deproteinization of shells.28 The residual NaOH was removed by repeated washing by portable water to reach the neutral pH.
The filtered chitin was dehydrated and made into powder to enable deacetylation process.
Deacetylation
Removal of acetyl groups from chitin obtained from the shell wastes were treated with 50% NaOH with a solvent to solid ratio 10:1 (v/w) for 20 hours at 65°C temperature.28 The residual NaOH was removed by repeated washing by portable water to reach the neutral pH. The filtered chitosan was dehydrated at hot air oven for 4 hours at 65±50° C to enable the characterization.
Determination of degree of deacetylation
The acid-base titration method was carried out to estimate the degree of deacetylation (DD).13 Around 0.1g of chitosan was dissolved in 30 ml of 0.1 M HCl solution with 5–6 drops of methyl orange indicator at room temperature. The red coloured reaction mixture was titrated with standardised 0.1mol NaOH solution until it turned to orange colour. The formula used for calculating DD% as follows
DD % = {(C1V1-C2V2) x 0.016} /{ M x 0.0994}
Where, C1 = (mol/l) concentration of standard HCl acid solution, C2 = (mol/l) concentration of standard NaOH solution , V1= (ml) volume of the standard HCl solution, V2= (ml) volume of standard NaOH solution, and M= (g) weight of chitosan. 0.016= gram equivalent weight of amino (NH2) group in 1 ml of standard 1 mol/l HCl acid solution and 0.0994 = the proportion of amino (NH2) group by weight in chitosan. The Degree of acetylation was calculated by subtracting the value of degree of deacetylation from 100%
Solubility
Unlike chitosan, chitin is insoluble in organic solvents but chitosan is soluble in acidic condition below pH 6.0. organic acids such as acetic, formic and lactic acids can solubilise the chitosan. But, 1 % acetic acid solution at pH 4.0 is most commonly used as a reference solution to solubilise chitosan. The higher concentration of acetic acid solutions at very high temperature leads to the depolymerization of chitosan. Usage of inorganic acid for dissolving chitosan is very limited.6
Viscosity
Viscosity of chitosan was estimated with a viscometer model ML-98965-40, Cole - Parmer, Vernon Hills, IL 60061, US. The 1% chitosan solution was prepared using 1% acetic acid on a dry basis was used to measure the viscosity. The viscometer fixed with No. 5 spindle and operated at 50 rpm at 25° C on 1% solutions. Analyses were made in three replicates. The values were reported in centipoises (cPs) units.
Results
The table 1shows the proximate composition value of moisture, ash, crude protein and crude fat content of various shell waste used in this experiments. The yield of the shell waste explains the quantity of shell waste generated from the crustacean during seafood processing. The shell waste obtained from crab shows the maximum yield of 55.58±0.18 %.
Table 1: Analysis of shell wastes
|
Shell waste |
Yield (%) |
Parameter (%) |
|||
|
Moisture |
Ash |
Protein |
Fat |
||
|
Shrimp |
45.14±0.15 |
21.51±0.13 |
20.45±0.01 |
32.13±0.08 |
9.13 |
|
Crab |
55.58±0.18 |
20.57±0.08 |
25.43±0.03 |
30.41±0.05 |
10.14 |
|
Squilla |
52.26±0.09 |
22.59±0.19 |
22.56±0.02 |
33.18±0.10 |
9.82 |
The table 2 explains the composition of chitin obtained from shrimp, crab and squilla shell waste. The result on chitin yield shows that shrimp waste can produce more yield of 17.36±0.07 % when compare to crab and squilla. The crude fat content of chitin was not detectable level for all shell waste.
Table: 2 Analysis of chitin
|
Chitin |
Yield (%) |
Parameter (%) |
|||
|
Moisture |
Ash |
Protein |
Fat |
||
|
Shrimp |
17.36±0.07 |
8.31±0.11 |
0.48±0.02 |
0.80 ±0.15 |
ND |
|
Crab |
14.52±0.12 |
9.32±0.13 |
0.96 ±0.01 |
0.82±0.12 |
ND |
|
Squilla |
13.45±0.21 |
9.10±0.16 |
0.76±0.03 |
0.87±0.09 |
ND |
ND: Not detectable
The composition and quality parameters of the chitosan were showed in table 3. The solubility of chitosan obtained from shrimp is very high (97.02±0.24 %) which is the best value among the waste involved in the experiment. The solubility of the chitosan influenced by the degree of deacetylation (71.58 ±0.09%), which exhibit the better viscosity 5300±32 cps value.
Table: 3 Analysis of chitosan
|
Chitosan |
Yield (%) |
Parameter (%) |
Viscosity(cps) |
|||||
|
Moisture |
Ash |
Fat |
solubility |
DA |
DD |
|||
|
Shrimp |
15.40 ±0.16 |
7.56 ±0.07 |
0.36 ±0.01 |
ND |
97.02 ±0.24 |
28.52 ±0.07 |
71.58 ±0.09 |
5300 ±32 |
|
Crab |
13.25 ±0.09 |
7.62 ±0.14 |
0.76 ±0.02 |
ND |
85.25 ±0.31 |
36.54 ±0.09 |
63.53 ±0.10 |
465 ±15 |
|
Squilla |
12.56 ±0.14 |
7.67 ±0.12 |
0.58 ±0.1 |
ND |
89.65 ±0.25 |
34.57 ±0.11 |
65.54 ±0.06 |
2600 ±34 |
Note: DA - Degree of acetylation, DD- Degree of deacetylation
ND: Not detectable
Discussion
Moisture content
The composition of shell waste, chitin and chitosan obtained from three different sources is given in in Table 1, 2 & 3. Hossain and Iqbal15 quantified the moisture of shrimp shell waste as 69.3%. But this study showed that the moisture content of shrimp, crab and squilla were, 21.51%, 20.57% and 22.59% respectively. The variation in moisture content may be due to the season, climate, weather and raw material condition,16 The lower result of moisture content of the raw material was mainly due to the collection procedure. Here the raw materials collected were used after straining the liquid. The moisture content of chitin obtained from shrimp, crab and squilla were 8.3, 9.32 and to 9.10% respectively. These results had shown lower than the moisture content chitin obtained by Abdulkarim et al. in mussel shell (12.90%),1 the reason may be due to the drying conditions of chitin, where the experimental chitin was dried in the hot air oven. Hygroscopic nature of chitosan leads to absorb moisture content from atmosphere when it exposed to outside. According to Sukumaran et. al.,27 commercial chitosan products should contain less than 10% moisture. The result of final moisture content of shrimp, crab and squilla chitosan were within the limit viz. 7.56%, 7.62 and 7.67%. These results of the study are evident to prove the moisture content of the chitosan is within the standard.
Protein and lipid content
No and Lee20 reported that crude protein content of shell fish waste is (7.06% to 7.97%) on dry matter. It was found that the crude proteins of the selected shell waste were ranged from 30 to 33 % on wet weight basis. Deproteinisation could not remove 100% protein from the shell waste. The residues of amino group may express as protein.25 Protein is bound by covalent bonds forming stable complex with chitin and chitosan. Even after deprotenisation, the chitin had trace amount (0.8%) of protein. It may be due to the amino (-NH2) group of the chitosan.20 The crude lipid of the shell wastes was around 10% that describes the relation with crude protein content of the shells. Crude lipid was found neither in chitin nor in chitosan. These results were comparable with 9% crude lipid in shrimp shell waste by Trung and Phuong.29 Brine and Austin10 also stated about the lower solubility in relation with incomplete removal of protein. Marwa et. al.17 found the maximum level of deproteinization was around 85% and 91% for P. segnis and P. kerathurus shells, respectively.
Ash content
The level of ash indicates the efficiency of the demineralization (DM) step for removal of minerals. The ash content of the shells was more than 20% initial stage. It has reduced to 0.48, 0.96 and 0.76 after demineralisation step. The mineral content of crab shells showed more than other two shells because of hard structure due to strong bond between chitin and minerals. The ash content of demineralised crab shell was higher than that of shrimp and squilla. It elucidate that the acid concentration and duration for demineralisation is not sufficient for crab shell waste. Because the crab shells have more mineral contents than the other two shells. Chitosan with less than 1% ash content considered as a high quality grade.20 In the present study, the shrimp chitosan showed 0.36% considered to be the high quality chitosan followed by squilla (0.58%) and crab (0.76%). The ash content of crab shell was less than 1% has been reported by No and Meyers.20 The residual ash content is considered as an important parameter affects the solubility, consequently reducing the viscosity.15
Degree of Deacetylation
No and Meyers [20] shown the range of degree of deacetylation (DD) of chitosan from 56% to 99% with an average of 80% considered as high quality chitosan. The shrimp chitosan showed superior quality with DD value 71.58% than squilla (65.54%) and crab (63.53%). The lower DD values of chitosan obtained from crab and squilla was agreed with the findings of No and Meyers20 and Toan28 respectively.
Viscosity
The viscosity range of 60 to 5,110 cPs reported by various researcher, which is depends on the source of chitosan.2,4,8 These ranges of viscosity have also been supported by the Cho et al.11 with five commercially available chitosans. Bough et al.8 reported the viscosity of shrimp and krill chitosan as 5,110 cPs and 5,074 cPs, respectively. It is agreed with the experimental shrimp chitosan as 5300 cPs. The viscosity of shrimp chitosan is of superior quality than squilla (2600 cPs) followed by crab (465 cPs). The presence of residual ash content and lower molecular weight of the chitosan were decreasing the viscosity of the crab chitosan.21 Sometime, the deacetylation process with concentrated sodium hydroxide (50%) usually at 100° C for 30 min is a harsh treatment. It may revert the demineralization (DM) and deproteinization (DP) process while production of chitosan.28 The parameters such as concentration, molecular weight, degree of deacetylation, temperature, pH, Ionic strength are affecting viscosity of chitosan.8 Moorjani et al.18 stated that frequent bleaching steps significantly reduces the viscosity of the chitosan so it is not advisable to bleach the final product.
Solubility
The presence of residual ash content reduces the solubility of the chitosan; subsequently affect the viscosity.15 The shrimp chitosan showed 97% solubility in 1% acetic acid with good viscosity of 5300cPs. The squilla chitosan showed only 89% solubility with viscosity of 2600cps. The poor solubility and viscosity of crab chitosan related with high ash content. The results were agreed with Toan28 experiment on shrimp chitosan.
Yield
The yield of shell wastes from the animal were around 50% on wet weight basis, but the chitin yield was 17% for shrimp, 14.5 % for crab and 13% for squilla. These results were concurred with shrimp shell chitin estimated by Trung and Phuong.29 The chitosan yield from the shrimp showed better result of 15.40 % after reduction of 2 % yield from chitin by the deacetylation process. The shrimp shells yielded significantly more chitosan. David et. al.12 explained the economic comparison of the yield of chitin and chitosan by experimental lab-scale and commercial plant process. The result showed the same yield for these two processes.
 |
|
Conclusions
Shrimp, crab and squilla shell wastes were treated with Toan28 chemical extraction procedure to produce chitin and chitosan. As per the research output of the present study, the chitosan obtained from shrimp shell waste appeared to be the highest degree of deacetylation, solubility and viscosity along with yield. Hence it can be concluded that shrimp chitosan appeared of superior quality than crab and squilla chitosan. Utilisation of shrimp shell waste for the production of chitin and chitosan will give more economical and biological value along with reduction of environmental pollution.
Acknowledgment
The authors would like to express their sincere thanks to the Tamil Nadu Fisheries University, Tamil Nadu, India for generous financial support to carry out the experiment.
References
- Abdulkarim, A., Isa, M.T., Abdulsalam, S., Muhammad, A., J., and Ameh, A. O., Extraction and Characterisation of Chitin and Chitosan from Mussel Shell, Civil and Environmental Research, 3, 2, 108-114(2013)
- Alimuniar, A., and Zainuddin, R. An economical technique for producing chitosan. In Advances in Chitin and Chitosan, C.J. Brine, P.A. Sanford, and J.P. Zikakis (Ed.), p.627. Elsevier Applied Science, Essex, UK (1992)
- AMEC. Management of wastes from Atlantic seafood processing operations. AMEC Earth and Environment Limited, Dartmouth, Nova Scotia, Canada (2003)
- Anderson, C.G., De Pablo, N., and Romo, C.R. Antarctic Krill (Euphausia superba) as a source of chitin and chitosan. In Proceeding of the First International Conference on Chitin /Chitosan, R.A.A. Muzarrelli and E.R. Pariser (Ed.), p.54. MIT Sea Grant Program, Cambridge, MA (1978)
- AOAC (Association of Official Analytical Chemist). Official Methods Of Analysis, Association of Official Analytical Chemists. 1th Ed. Gaithersburg, USA: AOAC Press (1990)
- Batista, I. and Roberts, G.A.F. A novel, facile technique for deacetylating chitin. Marcro mol. Chem. 191: 429-434 (1990)
- Bligh and Dye, Neutral Lipid Extraction by the Method of Bligh-Dyer, Can. Journal of Biochemistry Physiology. 37, 922 (1959)
- Bough, W.A., Salter, W.L., Wu, A.C.M., and Perkins, B.E. Influence of manufacturing variables on the characteristics and effectiveness of chitosan products. 1. Chemical composition, viscosity, and molecular weight distribution of chitosan products. Biotechnol. Bioeng. 20. p.1931 (1978)
- Bozzano A., and Sarda, F. Fishery discards consumption rate and scavenging activity in the north western Mediterranean Sea. ICES J. Marine Science, 59:15 – 28 (2002)
- Brine, C.J., and Austin, P.R. Chitin variability with species and method of preparation.Comp.Biochem. Physiol. 1981a 69B: 283-286 (1981)
- Cho, Y.I., No, H.K., and Meyers, S.P., Physicochemical Characteristics and Functional Properties of various Commercial Chitin and Chitosan Products. Journal of Agricultural and Food Chemistry. 1998. 46(9). P. 3839-3843 (1998)
- David G. R., Rolando B. Z. and Rigoberto R. E., Comparison of process technologies for chitosan production from shrimp shell waste: A techno-economic approach using Aspen Plus. Journal of Food and Bioproducts Processing (103) 49–57 (2017)
- Domard A., Roberts G.A.F., and Varum K.M. Eds. Chitosan production routes and their role in determining the structure and properties of the product. Lyon, France; Jacquers Andre Publisher (1997)
- FAO. Code of conduct for responsible fisheries ,Food and Agriculture Organization of the United Nations Rome (1995)
- Hossain, M. S. and Iqbal A. Production and characterization of chitosan from shrimp waste, J. Bangladesh Agril. Univ. 12(1): 153–160 (2014)
- Islama, M.M., Shah, M.D., Masumb, M., Mahbubur R., Ashraful Islam Mollab M.D., Shaikhc, A.A., and Roya, S.K. 2011, Preparation of Chitosan from Shrimp Shell and Investigation of Its Properties. International Journal of Basic & Applied Sciences. 11(1) 77-80
- Marwa H., Amal H., Sawssen H., Mourad J., Moncef N. and Rim N. Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. International Journal of Biological Macromolecules (101) 455–463(2017)
- Moorjani, M.N., Achutha, V., and Khasim, D.I. Parameterss affecting the viscosity of chitosan from prawn waste. J. Food Sci. Technol. 12. p.187-189 (1975)
- MPEDA, 2016, www.mpeda.gov.in
- No, H.K., and Lee, M.Y. Isolation of Chitin from Crab Shell Waste. Journal Korean Soc. Food Nutrition. 24(1). p. 105-113 (1995)
- No, H.K., Cho,Y.I., Kim, H.R., and Meyers, S.P. Effective Deacetylation of Chitin under Conditions of 15 psi/121ºC. Journal of Agricultural and Food Chemistry, 48 (6). p.2625-2627 (2000)
- No, H.K., Meyers, S.P. Crawfish Chitosan as a Coagulant in Recovery of Organic Compounds from Seafood Processing Streams. J. Agric. Food Chem. 37(3): 580-583 (1989)
- Rao M.S., Yu P., Stevens W.F., Chandrkrachang S., Kungsuwan A., and Hall G.M. The Proceedinggs of the Second Asia Pacific Chitin and Chitosan Symposium; November 1996; Bangkok, Thailand (1996)
- Rout, S. K. Physicochemical, Functional, and Spectroscopic analysis of crawfish chitin and chitosan as affected by process modification. A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College (2001)
- Rutherford, F.A., and Austin, P.R. Marine chitin properties and solvents. In Proceedings of the First International Conference on Chitin /Chitosan; Muzzarrelli, R.A.A., Austin, P.R., Eds.; MIT Sea Grant Program, Cambridge, MA. p.182-192 (1978)
- Suchiva K., Chandrkrachang S., Methacanon P., Peter M.G. Proceedinggs of the 5th Asia Pacific Chitin and Chitosan Symposium & Exhibition. Bangkok, Thailand (2002)
- Sukumaran, K. K. Squilla (Mantis shrimp) fishery of Karnataka state. R & D Series for Marine Fishery Resources Management, 18. pp. 1-3 (1987)
- Toan, N.V. Production of Chitin and Chitosan from Partially Autolyzed Shrimp Shell Materials, The Open Biomaterials Journa,l 1, 21-24 (2009)
- Trung, T.S. and Phuong P.T. D. Bioactive Compounds from By-Products of Shrimp Processing Industry in Vietnam Journal of Food and Drug Analysis, 20, Suppl. 1, 2012, Pages 194-197 (2012)







