Diversity and Community Composition of Zooplankton in Three Wetlands of Fatehabad, Haryana
Girish Chopra1 * and Pooja Jakhar1
1
Department of Zoology,
Kurukshetra University,
Kurukshetra,
136119
Haryana
India
Corresponding author Email: jakpoo.swift@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.11.3.21
The present study was designed to determine the species diversity and composition of zooplankton of three lentic water bodies from district Fatehabad, Haryana, India. The assessment was done from December, 2012 to November, 2013. A total of 32 species of zooplankton were identified from this study. Rotifera recorded the highest number of species (13) followed by Cladocera (11), which in turn was followed by Copepoda (6), Ostracoda and Insecta (1 species each). Maximum number of zooplankton species (26) were reported from Chilli lake, whereas, Daulatpuria Pond reported the minimum number of species (18). Dominant zooplankton reported at study sites were Nauplius sp., Mesocyclops sp., Phyllodiaptomus sp., Brachionus. falcatus, B. quadridentatus, B. caudatus, Diaphanosoma sp. and Chironomous larva. Presence of a number of pollution tolerant species of zooplankton such as Brachionus quadridentatus, Keratella sp., Ceriodaphnia, Miona sp., Mesocyclops sp., Monostyla sp.and Diaphanosoma sp. indicates the eutrophic nature of the water bodies.
Copy the following to cite this article:
Chopra G, Jakhar P. Diversity and Community Composition of Zooplankton in Three Wetlands of Fatehabad, Haryana. Curr World Environ 2016;11(3). DOI:http://dx.doi.org/10.12944/CWE.11.3.21
Copy the following to cite this URL:
Chopra G, Jakhar P. Diversity and Community Composition of Zooplankton in Three Wetlands of Fatehabad, Haryana. Curr World Environ 2016;11(3). Available from: http://www.cwejournal.org/?p=16567
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2016-09-25 |
|---|---|
| Accepted: | 2016-11-29 |
Introduction
Wetlands are the most productive ecosystem of the world comparable to coral reefs and rainforests.1 However, human activities like leaching of noxious liquids from solid waste deposits or untreated waste discharge reach a climax which has undesirable effects on aquatic environment.2 In addition, aquatic ecosystems are severely affected by anthropogenic activities. The use of various ecological methods is important to know the health status of an aquatic ecosystem. Further, the water quality influences the species composition, abundance, productivity and physiological conditions of the aquatic community and water quality is indicated by the structure and composition of these aquatic communities.3 Zooplankton are the microscopic organisms found in aquatic ecosystems. They are important link in transformation of energy from producers to consumers due to their large density, drifting nature, high species diversity and different tolerance to the stress and formulate the base of food chains and food webs of all aquatic ecosystems. They act as important bio-indicators and eutrophication level of aquatic bodies is characterized by the presence and relative abundance of various zooplankton species.4,5 They also play a major role in recycling nutrients as well as cycling energy in their respective environments.6 The zooplankton in Indian water bodies consists of diverse assemblage of major taxonomic groups. Many of these forms have different environmental and physiological assemblage. The number, type and distribution of these organisms present in any aquatic habitat provide a clue on the environmental condition prevailing in that particular habitat. It is seen that many environmental factors interact to provide conditions for the growth of zooplankton both spatially and seasonally.7 A number of studies have been conducted on freshwater zooplankton in various part of India.8-10 But the ecological studies related to fresh water bodies and the zooplankton diversity were very scanty in Fatehabad district (Haryana, India), the present research, an attempt has been made to study the diversity of zooplankton and to compare the biotic component in the selected water bodies.
Materials and Methods
Study Area
Three wetland bodies, namely, Chilli lake (CL), Bhodia Khera Temple Pond (BP) and Daulatpuria Pond (DP) of district Fatehabad, Haryana (India) were selected for the present study (Plate 1).
Chilli Lake (Urban Lake)
It is situated on the outskirts of city Fatehabad (Haryana, India), along the 500 year old historical fort of Mogul emperor Firoz Shah at geographical coordinates of29ÌŠ.51’N to 75ÌŠ.45’E. Besides the fort, a 250 year old temple of Lord Krishana, a gurdwara and a marhi of goddess are also situated around the lake. Chilli was once a place for recreation and amusement but due to dumping of garbage in the lake its very existence is in peril now. The sewage water of most parts of the town is being allowed to put in this lake
Bhodia Khera Temple Pond (Rural Religious Pond)
It issituated in the village Bhodia Khera at geographical coordinates of 29ÌŠ.49 to 75ÌŠ.42’E. On one side of the pond, there is an ancient temple at which each year thousands of devotees comes to attend the religious ceremony and fare. Effluents from the temple are poured directly into the pond. Villagers also wash clothes at the pond which further causes water pollution due to detergents. The pond is also leased out for fish culture.
Daulatpuria Pond (Rural Pond)
It is present at the border of village Daulatpuria at geographical coordinates of 29ÌŠ.55’N to75ÌŠ.40’E. The pond is affected by anthropogenic activities as domestic animals visit the pond in morning as well as evening for drinking water. Also, the pond is leased out for fish culture.
Methods Used
Plankton samples were collected by filtering 50L water through a plankton net of mesh 50µm. Qualitative and quantitative analyses was carried out following standard methods.11 Evenness and diversity indices were also calculated using standard methodologies.12-14 Zooplankton were identified up to generic level using standard Keys and monographs.11,15-17
 |
Plate 1: Photographs of study sites: Chilli Lake (a, b), Daulatpuria Pond (c, d) and Bhodia Khera Temple Pond (e, f) Click here to View Plate |
Results and Discussion
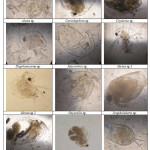
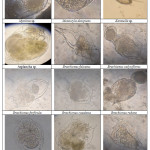
A total of 32 zooplankton taxa were recorded from three study sites (Table 1) of which Rotifera was represented by 13 species followed by Cladocera with 11 species, Copepoda with 6 species, Ostracoda and Insecta with 1 species each (Plate 2 and 3). Per cent contribution of different groups of zooplankton is shown in Fig. 2. In CL, 26 taxa of zooplankton were recorded which included 11 taxa of Cladocera (42.31%), 7 taxa of Rotifera (29.92%), 6 taxa of Copepoda (27.08), 1 taxa of Ostracoda and Insecta (3.87%) each. Species richness in different months ranged from 8-25; being maximum in april and minimum in January (Fig. 1). Species diversity in different months ranged from 1.69-2.76 and Simpson’s diversity index ranged from 0.08-0.26; being maximum in January and minimum in March. Equitability index ranged from 0.86-0.95 (Fig. 3). During the study period, 20 taxa of zooplankton were reported from BP which included 7 taxa from Cladocera and Rotifera (35%) each, 4 taxa of Copepoda (20%) and 1 taxa each from Ostracoda and Insecta (5%). Species richness in various months ranged from 6-20. being maximum in April and minimum in October. Species diversity index ranged from 1.64-2.73, whereas, Simpson’s diversity index ranged from 0.08-0.22. Equitability index ranged from 0.86-0.95 (Fig. 4). At DP, 18 taxa of zooplankton were recorded including 7 taxa of Rotifera (38.89%), 6 taxa of Cladocera (33.33%), 3 taxa of Copepoda (16.67) and like CL and BP, ostracoda and Insecta (5.56%) were represented by 1 taxa each. Species richness in different months ranged from 5-18; being maximum in August and minimum in January. Shannon’s diversity index was found to be maximum in August and minimum in January. Simpson’s diversity index ranged from 0.09-0.23; being maximum in January and minimum in August. Equitability index ranged from 0.87-0.96; being minimum in July and Maximum in February. At CL zooplankton were dominated by Mesocyclops sp., Phyllodiaptomus sp., Nauplius, Brachionus falcatus, B. caudatus B. quadridentatus and Diaphanosoma sp. At BP, Phyllodiaptomus sp., Diaphanosoma sp. and B. calyciflorus were found to be dominant, whereas, at DP, Phyllodiaptomus sp. and B. falcatus were dominant.
Cladocera are designated as bio-indicatorsand represent the eutrophic status of water body due to pollution.18,19 Presence of large number of Cladocerans in the present study supports the view. In the present study, chironomous larvae is present at all the study sites which indicates the degraded water quality as the larvae are pollution tolerant and can occur in low oxgen conditions.20 Occurrence of Brachionusquadridentatus, Keratella sp., Ceriodaphnia, Miona sp., Mesocyclops sp., Monostyla sp.andDiaphanosoma sp. shows the eutrophic nature of water body.21-25 The present study also supports the views and these pollution tolerant species of zooplankton were found to be present at the study sites which indicate the eutrophic nature of the water bodies.
 |
|
 |
|
Table 1: Zooplankton recorded at the study sites- CL, BP and DP during the study period
|
S. No. |
Zooplankton |
CL |
BP |
DP |
|
A CLADOCERA |
||||
|
1 |
Alona sp. |
+ |
- |
- |
|
2 |
Ceriodaphnia sp. |
+ |
+ |
+ |
|
3 |
Chydorus sp. |
+ |
+ |
+ |
|
4 |
Camptocercus |
+ |
- |
+ |
|
5 |
Diaphanosoma sp. |
+ |
+ |
+ |
|
6 |
Macrothrix sp. |
+ |
+ |
+ |
|
7 |
Moina sp. |
+ |
+ |
- |
|
8 |
Moina spp. |
+ |
- |
- |
|
9 |
Moina weismanni |
+ |
+ |
- |
|
10 |
Oxyurella sp. |
+ |
+ |
+ |
|
11 |
Scapholeberis sp. |
+ |
- |
- |
|
B ROTIFERA |
||||
|
12 |
Asplancha sp. |
+ |
- |
- |
|
13 |
Brachionus caudatus |
+ |
+ |
- |
|
14 |
Brachionus forficula |
- |
+ |
+ |
|
15 |
Brachionus calyciflorus |
- |
+ |
+ |
|
16 |
Brachionus quadridentatus |
+ |
+ |
- |
|
17 |
Brachionus sp. |
+ |
+ |
+ |
|
18 |
Brachionus bidentata |
- |
+ |
+ |
|
19 |
Keratella sp. |
- |
+ |
+ |
|
20 |
Monostyla sp. |
- |
- |
+ |
|
21 |
Monostyla spp. |
+ |
- |
- |
|
22 |
Lecane sp. |
- |
- |
+ |
|
23 |
Platyias sp. |
+ |
- |
- |
|
24 |
Testudinella sp. |
+ |
- |
- |
|
C COPEPODA |
||||
|
25 |
Ectocyclops sp. |
+ |
- |
- |
|
26 |
Eucyclops sp. |
+ |
+ |
- |
|
27 |
Mesocyclops sp. |
+ |
+ |
+ |
|
28 |
Nauplius |
+ |
+ |
+ |
|
29 |
Neodiaptomus sp |
+ |
- |
- |
|
30 |
Phyllodiaptomus sp. |
+ |
+ |
+ |
|
D OSTRACODA |
||||
|
31 |
Cypris |
+ |
+ |
+ |
|
E INSECTA |
||||
|
32 |
Chironomous larva |
+ |
+ |
+ |
 |
|
References
- Thomas, M., Deviprasad, A.J. (2007). Phytoplankton diversity in wetlands of Mysore district.Asian J. Microbiol. Biotech. Environ. Sci. 9: 385-392.
- Chapman, D. (1996). Water quality assessments- A guide to use of Biota, Sediments and Water in Environmental Monitoring, Second Edition. UNESCO/WHO/UNEP.
- Ramachandra, T.V., Rishiram, R. and Karthick, B. (2006). Zooplankton as bioindicators: Hydro-Biological investigations in selected Bangalore lakes. The Ministry of Science and Technology, Government of India, Technical Report: 115.
- Park, G.S., Marshall, H.G. (2000). Esturine relationship between zooplankton community structure and trophic gradients. J. Plankton Res. 22: 121-135.
CrossRef - Bhat, N.A., Wanganeo, A. and Raina, R. (2014). Composition and diversity of net zooplankton species in a tropical water body (Bhoj Wetland) of Bhopal, India. J. of Biodiversity and cons. 6(5): 373-381.
- Patel, V., Shukla, S.N., Patel, V.K. (2013).Studies on the diversity of zooplankton and their seasonal variation in Govindgarh lake at Rewa (M.P), India. Ind. J. Appl. Res.3(11): 544 546.
CrossRef - Khanna, D. R., Bhutiani, R., Gagan Matta., Singh, V., Kumar, D., Ahraf, J. (2009). A study of Zooplankton diversity with special reference to their concentration in River Ganga at Haridwar. Con. J. 10(3): 15-20.
- Dakshini, K.M., Gupta, S.K. (1979). Preliminary observations of limnological study of three freshwater lakes around Delhi state, India. Ind. Nat. Sci. Acad. B. 45: 564- 570.
- Mohan, K.S.,1987. Bull. Academia Sinica. 28:13-24.
- Singh, S.P., Pathak, D. and Singh, R. (2002). Hydrobiological studies of two ponds of Satna (M.P.), India. Eco. Environ. Cons. 8: 289-292.
- Roy, U., Shaha, B.K., Mazhabuddin, K.H., Haque, M.F. and Sarower, M.G. (2010). Study on the Diversity and Seasonal Variation of Zooplankton in a Brood Pond, Bangladesh. Res. Aqua. 1(1):30-37.
- APHA (American public health association) (1998). Standard methods for the examination of water and waste water. APHA. AWWA. WPFC, 16th New York.
- Shannon, C. E. and Weaver, W. (1949). The Mathematical Theory of Communication. University of Illinois Press. Urbana, IL., USA.
- Simpson, E. M. (1949). Measurement of diversity. Nature, 163: 688.
CrossRef - Pielou, E. C. (1966). The measurement of diversity in different types of biological collections. Theor. Biol. 13: 131-144.
CrossRef - Ward, H.B. and Whipple, G.C. (1959). Fresh Water Biology. John Wiley and Sons. New York, Wiley. 1248.
- Needham, J.E. and Needham, P.R. (1972). A guide to the study of fresh water biology. Holden Day Inc. San Francisco. California.
- Mellanby, H. (1963). Animal Life in fresh water. 6th Chapman and Hall Ltd., London.
- Khan, M.A. and Rao, I. (1981). Zooplankton in the evaluation of pollution. In: WHO workshop on biological indicators as indices of environmental pollution. 121-133.
- Abrantes, N., Antunes, S.C., Pereira, M.J. and Goncalves, F. (2006). Seasonal succession of Cladocerans and phytoplankton and their interactions in shallow eutrophic lake (Lake Vela, Portugal). Acta Oecologia. 29(1): 54-64.
CrossRef - Abida, S., Mir, M.F., Ifshana, S., Mir, S.A. and Ahangar, I.A. (2012). Macrozoobenthic community as biological indicators of pollution in river Jhelum, Kashmir. J. Environ. Res. Technol. 2(4): 273-279.
- Gannon, J.E. and Stemberger, R.S. (1978). Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Am. Microsc. Soc. 97: 16-35.
CrossRef - Mahajan, C.L. (1981). Zooplankton as indicators for assessment of water pollution. WHO workshop on biological indicators of indices of environmental pollution. Central Board for the Prevention and Control of Water Pollution. 135-148.
- Kumari, P., Dhadse, S., Chaudhari, P.R. and Wate, S.R. (2008). In: Sengupta M, Dalwani R. editors. Proceedings of Taal 2007: The 12th world lake conference. 160-164.
- Rajagopal, T., Thangamani, A., Sevarkodiyone, S.P., Sekar, M. and Archunan, G. (2010b). Environ. Biol. 31: 265-272.
- Shivshankar P. and Venkataramana G.V. (2013). Zooplankton Diversity and their Seasonal Variations of Bhadra Reservoir, Karnataka, India.IRJES. 2(5): 87-91.







