Leaf Litter Decomposition of two Central Himalayan Oaks
Kirtika Padalia1 * , Rajendra Singh Parihaar1 , Nidhi Bhakuni 1 and Bhawana Kapkoti1
1
Department of Botany,
DSB Campus,
Kumaun University,
Nainital,
263002
India
DOI: http://dx.doi.org/10.12944/CWE.10.2.16
The study was conducted in two natural oak forest of Nainital (Uttarakhand) India, during 2012-2013 to determine the weight loss pattern in leaf litter of two Central Himalayan Oaks (i.e., Quercus leucotrichophora A. Camus. and Quercus floribunda Lindl.) with the help of litter bag technique. The present study concluded that weight loss proceeded throughout the study period and relatively higher within 60 days after the placement of litter bags into the soil. Among these two species, higher weight loss observed in Q. floribunda as compared to Q. leucotrichophora across both the sites. Within 365 days, average weight loss observed about 60% in Q. leucotrichophora and 62% in Q. floribunda. Decay rate coefficient rate ranged from 0.0596- 0.0014 for Q. leucotrichophora while it varies from 0.0558 to 0.0013 for Q. floribunda. The monthly relative decomposition rate (RDR) ranged between 0.0598-0.0014 g/g/day and 0.0208-0.0050 g/g/day for Q. leucotrichophora and Q. floribunda, respectively. Climatic factors (rainfall, temperature and relative humidity) also influenced the rate of decomposition.
Copy the following to cite this article:
Padalia K, Parihaar R. S, Bhakuni N, Kapkoti B. Leaf Litter Decomposition of two Central Himalayan Oaks. Curr World Environ 2015;10(2) DOI:http://dx.doi.org/10.12944/CWE.10.2.16
Copy the following to cite this URL:
Padalia K, Parihaar R. S, Bhakuni N, Kapkoti B. Leaf Litter Decomposition of two Central Himalayan Oaks. Curr World Environ 2015;10(2). Available from: http://www.cwejournal.org/?p=12627
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-07-14 |
|---|---|
| Accepted: | 2015-08-16 |
Introduction
Decomposition refers to a biological process in which organically bound nutrients released in the form of free ions into the soil solution. Plants take up these nutrients from the soil, use them for metabolic process and return the nutrients through litter fall. Litter decomposition influence the nutrient dynamics of an ecosystem and serve as one of the input of nutrients in the forest ecosystem. It is estimated in a study that about 69-87% of the total annual requirement of essential elements in forest plants is fulfilled by the litter decomposition. Therefore, litter decomposition contributes a significant role in the nutrient budgeting of forest as well as in agroecosystem where vegetation depends primarily on the recycling of plant detritus for nutrients requirment.1 Physical factors (temperature and soil moisture etc) influence the rate of decomposition significantly. The process of decomposition slows down or nearly restricted in a very dry soil due to the low population of microbes (because the fungi and bacteria dry out).
Other than this, the food source (i.e. decomposing leaves) of microbial decomposers is one of the controlling factor which influence the rate of decomposition,2,3 basically nutrient-rich leaves (high quality leaves: alder) decomposed faster than nutrient deficit leaves(low quality leaves: conifer needle). Decomposition process plays an important role in soil fertility in terms of nutrient cycling and formation of soil organic matter.4,5,6 Other than this, litter cover also act as a protective layer by buffering changes in soil water content,7 temperature,8 hindering erosion9 and soil compaction.10
Oak species presume significant conservation in the Himalayan region as provided numerous ecosystem services.11,12 Oaks, particularly Q. leucotrichophora and Q. floribunda associated not only with agro-ecosystems but serve as the life support systems of inhabitants in hills of the Himalaya.13,14 Oak forests are the source of fuel, timber and can be interrelated with natural springs and wildlife.14,15 In view of this, as oak is a key stone species and serve as the multipurpose tree in the Central Himalayan forest, present study was carried out with the major objectives (i) to evaluate the weight loss pattern of ecomposing leaf litter of two Himalayan Oak species (i.e. Q. leucotrichophora A. Camus.ex Bohadur and Q. floribunda Lindl. ex Rehder) and (ii) to correlate weight loss pattern with different parameters.
Materials and Methods
Study Site, Climate and Soil
The present study conducted in Nainital The present study conducted in Nainital (29°23’N 79°27’E29.38°N 79.45°E) at an altitude of 2,084 m (6,837 ft) asl. Study area was dominated by natural oak forest as in the Indian Central Himalaya, generally one and two species of oak dominated forest, hence dominant types are easily recognizable.16 These are Q. leucotrichophora forest which covers extensive areas in lower elevation (1,000-2100 m), Q. floribunda forest are distributed in the limited areas between 1,800-2,400 m.
The site is further divided into two sub sites; the first site situated near to Botany department in DSB Campus (altitude 2,084 m), Nainital, considered as site-1, dominated with Quercus leucotrichophora A. Camus., Quercus. floribunda Lindl., Cedrus deodara (Roxb. ex D. Don) G. Don, Acer oblongum Wall., Cornus capitala wall. and Prunus ceresoides D. Don while the other site-2 was at the forest of Kailakhan (altitude 1800-1950 m) (Nainital) and was dominated with Quercus leucotrichophora A. Camus., Quercus. floribunda Lindl, Cedrus deodara (Roxb. ex D. Don) G. Don, Lyonia ovalifolia (Wallichi.) Drude, Myrica esculenta Buch.-Ham., Cupressus torulosa D. Don and Rhododendron arboreum Smith.
Climate is monsoonal temperate and characterized by discernible seasonality. The whole year can be divided into three marked seasons: summer (Mid-March-mid June), rainy (mid June-September) and winter (November-February). October compose the intermediary between rainy and winter seasons. Average maximum temperature fluctuated between 15oC in January to 27oC in May and mean monthly minimum from 2oC in January to 15oC in June. Total rainfall was about 2315 mm of which nearly 83% occurs during rainy season from monsoon. Mean monthly relative humidity ranged from 92% and was maximum in September and minimum in December. The months of June to September constitute for the yearly showers and from December to February Nainital engulfed by snow. Meteorological data of the study year is given in figure 1.
Soil of the study sites was black in color, loam in texture, moderate in nutrients and slightly acidic in nature. The average soil temperature of the site was 15°C-17°C in summer and in winter it varies from 5°C to 8°C.
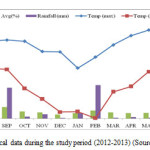 |
|
Experimental Design
Characteristics of Experimental Leaf
All the Himalayan oaks are evergreen with lifespan of about one year, sclerophyllous with specific leaf mass exceeding 130 g/m2 and their size class varies from microphyll to misophyll.17 Among the Himalayan oaks, the leaf of Q. floribunda has spinose leaves having green on both dorsal and ventral surface while Q. leucotrichophora have dentate margin in the upper half and entire in the lower half, contain silvery white colour in the ventral surface.18 The colour of the leaves also changed with the age, for example, the leaves of Q. leucotrichophora appears reddish brown when young and turn green when mature. In contrast, the young leaves of Q. floribunda do not contain any extra coloured pigments other than the chlorophyll. The young leaf of Q. leucotrichophora has a thick wax layer to check the transpiration because stomata control is not yet developed.17
Experimental Setup
First of all, mature, nearly senesced but attached leaves of Q. leucotrichophora and Q. floribunda from the study site were collected after the leaf fall and brought to the laboratory. Flatten the leaves in newspaper and dry them in at room temperature for 24-48 hours. Litter bag (Nylon litter bags) technique was used to measure decomposition rates. Litter bags were prepared having the size of 20 x 20 cm with 1 mm mesh size in order to minimize artificial litter loss from the bags. 10 gm (initial weight) of air dried leaf of each species were kept in litter bag, separately. Mesh size of 1 mm was sufficient to permit movement of micro-organisms, which are the predominant litter feeders.19 Oven drying method was used to determine the moisture content of the litter. A total of 36 litter bags (3 sub-sample bags for a month) for each species were placed in each forest site. Litters bags were buried into the soil separately for each species in such a way that bags were kept in contact with soil and not to be disturbed as much as possible.
Three litter bags of each species recovered randomly at every 30 days intervals for 12 months at each location. Rebuff all external impurities and slide the sample bags into a separate bag for bring back to the laboratory. The recovered residual materials were washed carefully with tap water to remove all adhere surface impurities and also the soil particles, then dried the residual at 60oC-80 oC in oven to constant and weighed.
Calculation
The decomposition rate computed through the 6 replicates (3 replicate for each site) of each species. The average value was used for the further analysis.
Litter Mass Loss and Decay Rate Coefficient
Litter mass loss was expressed as the % remaining after in a given time and was calculated as:
%R= W (tx) / W (ti) x100
Where W (tx) is the dry weight (g) of the leaf material remaining after time (tx), and W (ti) is the initial weight (Peterson).20
Decay Rate Coefficient (k)
was calculated as:-k= ln (%R/ 100) /t
Where, t is the time in days (Peterson).20
Results and Discussion
Weight Loss Pattern
Loss of dry matter in decomposition was measured at monthly interval during one year (July 2012-June 2013) of the study period. Q. leucotrichophora had shown an average of 60.15% weight loss within 365 days on both the sites. The maximum (16.7% of the original litter mass) decomposition of the litter has been observed after the first month of the study period and the minimum was observed in the month of December-January (1.85%). The relationship between days elapsed and % weight loss for this species is given in figure 2.
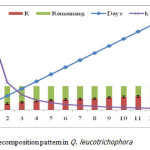 |
|
During entire course of study, the average rate of litter decomposition in Q. leucotrichophora was obtained 60.15% at both the sites. Decomposition rate of leaf litter gradually decreased with the increase of time. As the time move on the process of decomposition was also slow down at both the sites. The statement is supported by a study proclaimed that two phases recognized in the process of decomposition: Decomposition during the first phase proceed rapidly because molecules of starch and amino acids easily break down and transfer the more soluble compounds to the soil as C and N which progressively mineralized or immobilized while the second stage follow relatively slower because humification of lignin and cellulose is complex and time taking21.
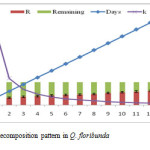 |
|
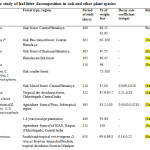 |
Table 1: Comparative study of leaf litter decomposition in oak and other plant species Click here to View table |
The decay rate coefficient and relative decomposition rate for both species of oak is given in the table 2. Decay rate coefficient rate ranged from 0.0596- 0.0014 for Q. leucotrichophora while it varies from 0.0558 to 0.0013 for Q. floribunda. The monthly relative decomposition rate (RDR) ranged between 0.0598-0.0014 g/g/day and 0.0208-0.0050 g/g/day for Q. leucotrichophora and Q. floribunda, respectively. The decomposition rate was greater during the rainy season, possibly due to the pronounced activities of microbes under the favorable conditions of temperature, moisture and rainfall.22 Several studies shown highest disappearance rate of 36-53% during rainy season and 15-26% in winter.23 The Correlation between decomposition rate and different parameters is given in table 3 and showed negative correlation with the days elapsed and significant positive correlation with the rainfall and the soil temperature.
Table 2: Decay rate coefficient (k) and Relative decomposition rate (g/g/d) of oak species
|
Day elapsed |
Q. leucotrichophora |
Q. floribunda |
||
|
|
k (day-1) |
RDR (g/g/d) |
k (day-1) |
RDR (g/g/d) |
|
30 |
0.0597 |
0.0171 |
0.0559 |
0.0209 |
|
60 |
0.0225 |
0.0159 |
0.0194 |
0.0190 |
|
90 |
0.0124 |
0.0132 |
0.0114 |
0.0142 |
|
120 |
0.0081 |
0.0111 |
0.0080 |
0.0112 |
|
150 |
0.0060 |
0.0094 |
0.0058 |
0.0096 |
|
180 |
0.0046 |
0.0082 |
0.0046 |
0.0099 |
|
210 |
0.0037 |
0.0073 |
0.0037 |
0.0072 |
|
240 |
0.0031 |
0.0065 |
0.0031 |
0.0065 |
|
270 |
0.0024 |
0.0061 |
0.0024 |
0.0062 |
|
300 |
0.0020 |
0.0057 |
0.0020 |
0.0057 |
|
330 |
0.0017 |
0.0053 |
0.0016 |
0.0054 |
|
360 |
0.0014 |
0.0050 |
0.0013 |
0.0051 |
Table 3: Pearson correlation between decomposition rate and different parameters
|
|
D |
LR |
R |
T |
RF |
RH |
|
D |
1 |
|||||
|
LR |
-967** |
1 |
||||
|
R |
0.967** |
-1** |
1 |
|||
|
T |
0.047 |
-0.23 |
0.023 |
1 |
||
|
RF |
-1 |
0.24 |
-0.24 |
0.224 |
1 |
|
|
RH |
-660** |
0.702** |
-0.702 |
0.285* |
0.520** |
1 |
Conclusions
This study concluded that the weight loss continued throughout the study period and relatively higher weight loss occurred within 60 days after the placement of litter bags. The rate of decomposition at monthly interval showed the different pattern for the different species. Among these two species, higher weight loss observed in Q. floribunda as compared to instead of in Q. leucotrichophora across both the sites. It was also concluded that the rate of decomposition also influenced by the physical factors and climatic conditions. Comparatively faster weight loss occurred during the rainy season (33-36%) while reached in its minimum during winter (11-9%).
Further, the natural forests are enrich in diversity of litter and the litter of many species intermingled with each other on the ground in natural way. So a detail study is needed to know the decomposition rate of all those species and their role in soil fertility to determine the nutrient dynamics in the forest.
Acknowledgment
The authors are highly thankful to Prof. S.S. Bargali, Department of botany, DSB Campus, Nainital, for review this paper. We are also thankful to University Grant Commission (Basic Science and Research) fellowship, New Delhi, for financial support.
References
- Bargali, S. S., Shukla, K., Singh, L., Ghosh, L., and Lakhera, M. L., Leaf litter decomposition and nutrients dynamics in four tree species of dry deciduous forest. Tropical Ecology. 2015;56(2):191-200.
- La Caro F., Rudd R. L. Leaf disappearance rates in Puerto Rican Montane rain forest. Biotropica. 1985;17:269-276.
- Bargali S. S., Singh S. P., Singh R. P. Pattern of weight loss and nutrients release from decomposing leaf litter in age series of eucalypt plantations. Soil Biology and Biochemistry. 1993;25:1731-1738.
- Usman S., Singh S. P., Rawat Y. S., Bargali S. S. Fine root decomposition and nitrogen mineralization pattern in Quercus leucotrichophora and Pinus roxburghii forest in Central Himalaya. Forest Ecology & Management. 2000;131:191-199.
- Rottmann N., Dyckmans J., Joergense R. G. Microbial use and decomposition of maize leaf straw incubated in packed soil columns at different depths. European Journal of Soil Biology. 2010;46:27-33.
- Bargali S. S., Singh S. P., Singh R. P. Patterns of weight loss and nutrients release from decomposing leaf litter in an age series of Eucalypt plantation. Soil Biology and Biochemistry. 1993;25(12):1731-1738.
- Ginter D. L., McLeod K. W., C. Sherrod Jr. Water stress in long leaf pine induced by litter removal. Forest Ecology and Management. 1979;2:13-20.
- MacKinney, A. L., Effects of forest litter on soil temperature and soil freezing in autumn and winter. Ecology. 1929;10:312-321.
- Bargali, S.S., Weight loss and nitrogen release in decomposing wood litter in an age series of eucalypt plantation. Soil Biology and Biochemistry, 28: 699-702 (1996)
- Benkobi, L., Trlica, M. J., and Smith, J. L., Soil loss as affected by different combination of surface litter and rock. Journal of Environmental Quality. 22:657-661 (1993)
- Singh, J. S., and Singh, S. P., Structure and function of the Central Himalayan Oak forests. Proceedings of Indian National Science Academy, 96(3):156-189 (1986)
- Singh, J.S., and Singh, S. P., Forest Vegetation of the Himalaya. Botanical Review. 1987;53(1):80-192.
- Bargali K., Joshi B., Bargali S. S., Singh S. P. Oaks and the Biodiversity They Sustain. International Oaks. 2015;26:65-76.
- Singh G., Rawat G. S. Quantitative analysis of tree species diversity in different oak (Quercus spp.) dominated forest in Garhwal Himalaya, India. Notulae Scientia Biologicae. 2012;4(4):132-140.
- Singh J. S. Rural ecosystems and development in the Himalaya, 74-87 p. In: Singh JS, Singh SP, Shastri C (Eds.). Science and Rural Development in Mountains. Gyanodaya Prakashan, Nainital, India. 1981
- Bargali, K., Bisht, P., Khan, A., and Rawat, Y. S., Diversity and regeneration status of tree species at Nainital catchment, Uttarakhand, India. International Journal of Biological Conservation. 2013;5(5):270-280.
- Bargali K., Joshi B., Bargali S. S., Singh S. P. Diversity within oaks. International oaks. 2014;25:57-70.
- Bargali K., Bargali S. S. Nutrient utilization efficiencies of two Central Himalayan species. Journal of Tropical Forest Science. 2000;12(3):450-458.
- Sharma A. P., Bidhan R. P., Bisht J. S. Micro-arthropods in litter bags. In an Integrated Ecological study of Eastern Kumaun Himalaya with emphasis on natural resources (J.S. Singh and S.P. Singh, eds.), 299-304. Department of Science & Technology, New Delhi. 1984.
- Petersen, R. C., and Cummins, K.W., Leaf processing in a woodland stream. Fresh Water Biology. 1974;4:243-368.
- Couteaux M. M., Bottner P., Berg B. Litter decomposition, climate and litter quality. Trends in Ecology and Evolution. 1995;10:63-66.
- Singh S. P., Pande K., Upadhyay V. P., Singh J. S. Fungal communities associated with the decomposition of a common leaf litter (Quercus leucotrichophora A Camus) along an elevation transect in the Central Himalaya. Biology and fertility of soil. 1990;9:245-51.
- Gupta S. R., Singh J. S. Decomposition of litter in a tropical grassland. Pedobiologia. 1977;17:234-237.
- Usman S. Nitrogen release pattern in decomposition leaf litter of banj oak and chir pine seedling leaf under glass house condition. Journal of Environmental Biology. 2013;34:135-138.
- Kumar S., Tewari L. M. Pattern of litter fall and litter decomposition in a Quercus leucotrichophora Camus forest in Kumaun Himalaya. International Journal of Biodiversity and Conservation. 2014;6(1):108-114.
- Singh J. S., Singh S. P. An Integrated Ecological study of Eastern Kumaun Himalaya with Emphasis on Natural Resources. Final report (HCS/DST/76) Kumaun University, Nainital.1984;1-3.
- Pandey U., Singh J. S. Leaf litter decomposition in an oak conifer forest in Himalaya: the effects of climate and chemical composition. Forestry. 1982;55:47-59.
- Bargali K., Manral V., Bargali S. S. Weight loss pattern in decomposing litter of Coriaria nepalensis, an actinorhizal shrub from degraded land. Indian journal of Agricultural Science. 2015;85(2):270-273.
- Das D. K., Chaturvedi O. P. Litter quality effects on decomposition rates of forestry plantations. Tropical Ecology. 2003;44(2):261-264.
- Pandey C. B., Sharma D. K., Bargali S. S. Decomposition and nitrogen release from Leucaena leucocephala in Central India. Tropical ecology. 2006;47(1):149-151.
- Kumar R., Tapwal A., Baruah D. M. Leaf litter decomposition pattern in Dipterocarpus tuberculatus and Dipterocarpus retusus forest of North East India. Research journal of Forestry. 2012;6(1):24-31.






