Assessment of the air quality in some Egyptian asphalt laboratories
A.M.M. Saleh1 * , F.H. Ashour2 and Y.M. Moustafa1
1
Egyptian Petroleum Institute (EPRI),
Egypt
2
Chemical Engineering Department,
Faculty of Engineering, Cairo University,
Cairo,
Egypt
DOI: http://dx.doi.org/10.12944/CWE.1.2.04
Indoor air pollution studies have became the focus of increasing regulatory public and research concerns due to the negative impact of their components on the human health and the environment. In the field of road construction, laboratory personnel are exposed to different types of air pollutants; Foremost of which are the asphalt vapours, fumes and dust that have a negative effect on their health. The aim of this research is to monitor the indoor air quality and working conditions in some asphalt laboratories in an attempt to obtain baseline information that would be helpful for future conservation programs; this is because Egypt lacks any database regarding the air quality in the asphalt laboratories. To achieve this objective, the High Performance Liquid Chromatography technique (HPLC), the portable ambient air analyzer as well as the solid particulate and sound intensity meters were all used to assess the air pollution environment in two different road construction laboratories. The study showed that the level of benzene, sulphur dioxide, xylene and solid particulate were above the Egyptian acceptable limits. Furthermore, Polyaromatic hydrocarbons PAHs exceeded the International Standard Limits.
Copy the following to cite this article:
Saleh A.M.M, Ashour F.H, Moustafa Y.M. Assessment of the air quality in some Egyptian asphalt laboratories. Curr World Environ 2006;1(2):117-124 DOI:http://dx.doi.org/10.12944/CWE.1.2.04
Copy the following to cite this URL:
Saleh A.M.M, Ashour F.H, Moustafa Y.M. Assessment of the air quality in some Egyptian asphalt laboratories. Curr World Environ 2006;1(2):117-124. Available from: http://www.cwejournal.org/?p=599
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-09-19 |
|---|---|
| Accepted: | 2006-10-30 |
Introduction
Petroleum Compounds are considered among the most serious environmental threats to human health. Laboratory personnel that deal with cutback asphalt in asphalt paving operations are exposed to a wide range of potentially harmful hazardous substances. Their exposure is caused by either or both inhalation and dermal contact (Burstyn et al., 2000; Reink et al., 2000). These vapours and fumes produced from Bitumen samples, consist essentially of Polyaromatic hydrocarbons (PAHs) and their derivatives; all such compounds have structural similarity to the known carcinogenic or cocarcinogenic substances (Samental et al., 2002). Polynuclear aromatic hydrocarbons are a group of compounds composed of two or more fused aromatic rings that exist in the gaseous and particulate form in the atmosphere. The polyaromatic hydrocarbons are classified into three categories based on their molecular weights. Their chemical structures ranges from 2- to 3-ringed poly aromatics for the low molecular weight (LMW) and reaching up to 6-ringed PAHS for the HMW. In principle, PAHs homologues with high molecular weights are often more carcinogenic than lighter ones (Tsai et al., 2002). Benzo (a) anthracene, chrysene, Benzo (b) fluoranthene, Benzo (ghi) perylene are generally considered as carcinogenic substances (Menzie et al., 1992).
Very limited data could be traced regarding the possible risk from exposure to laboratory generated asphalt vapours and fumes. In the road construction field, workers are generally exposed to different concentrations of bitumen fumes ranging from 0.1 to 0.2 mg/m3 that contain up to 200 ng/ m3 of the carcinogenic benzo (a) pyrene that may affect some immune function in human beings (Karakya et al., 1999). Also, the presence of numerous alkylated PAHs, of low molecular weights, as well as oxygenated and sulfunated poly aromatic compounds are sometimes the essential cause of acute toxicity (Lai et al., 1996; Binet et al., 2002).
The United States Environmental Protection Agency (USEPA) and the International Agency for Research on Cancer (IARC) have actually identified numerous PAHs as known or probable human mutagenic and carcinogenic sources (Mazzera et al., 1999) and (Castellano et al., 2003). In his research (NIOSH 1997) recommended an exposure limit for asphalt fumes of 5 mg/m3, measured as total particulate during any 15-minute period, whereas Australia, Belgium, Denmark, and the United Kingdom have limited exposure to 5 mg/ m3 on the basis of an 8hr TWA (Time Weighted Average). Additionally, Egypt (EEAA1997) and the United Kingdom have both established an exposure limit for asphalt fumes of about 5 mg/m3 for long exposure time and 10 mg/m3 for short periods.
Effects regarding the Human Health
The available Data on possible health risk due to the exposure to bitumen vapours and fumes is scarce. Many epidemiologic studies blame the recorded serious decline in health as due to the variation in local concentration of air pollution (Erbas et al., 2005); other studies also predicted an increased cancer risk of workers exposed to asphalt fumes ( Portanen et al., 1994). Asphalt fumes are generally considered as irritants to the mucus membranes that progress to non malignant lung diseases as bronchitis, emphysema and isotherm (Hansen 1991). Hot asphalt can also cause skin burns, while petroleum distillates and hydrocarbon solvents- like trichloroethylene used in laboratories-lead to skin dryness and weakening of the protective barrier, thus facilitating the ingress of various compounds into the body. In addition, such exposure is able to significantly induce cytogemetic damage in peripheral lymphocytes of the workers (Burgas et al., 1998).
Experimental
Table - 1: Location and characteristics of laboratories selected
| Laboratory Designation | |||
| Aspect | (A) | (B) | |
| • Location in the building | Top floor | Ground floor | |
| • Site Description | 2 large sections separated with a partition | A large area divided into two rooms | |
| • No of Exits | 4 | 1 | |
| • Area (m2) | 324 | 66.6 | |
| • Height (m) | 6 | 5 | |
| • Ventilation | Fair | Poor | |
| • No of workers | 5 | 4 | |
| • Number of engineers | 3 | 1 | |
Areas under Study
Two laboratories, A and B, having the same functions were chosen. Whilst they differ in many aspects such as the location, the dimension, the site description, as well as the number of workers as shown in Table -1. The selected measuring points for determining the air quality in both laboratories are given in Table - 2.
Table - 2: Description of the selected measuring points
| Number | Location |
| 1 | Control point (outdoor air) |
| 2 | Oven [for heating the aggregate and asphalt paving mix at temperatures up to 160°C] |
| 3 | Centrifuge apparatus [used in extraction of asphalt from asphalt paving mix] |
| 4 | Oil bath [used for the Kinematic viscosity measurement for asphalt at 135°C] |
| 5 | Oven [for heating the asphalt cement samples at temperatures up to 150°C] |
| 6 | Air space lying between the distillation and the solvent recovery apparatus |
| 7 | Solvent recovery apparatus |
Methods
Based on the chemical composition of asphalt as well as that of the solvents used in both laboratories, the expected pollutants to be measured in the ambient air at each selected measuring point will be the aromatic family BTX (Benzene, Toluene & Xylene), the carbon oxides (CO2&CO), trichloroethylene (TCE), methyl ethyl ketone (MEK), nitrous oxide (N2O), sulphur dioxide (SO2) and the Poly Aromatic Hydrocarbons (PAHs). The measuring devices used for the experimental work were essentially, a portable Ambient Air Analyzer model [Moran Sapphire 205 A series], a Sound intensity meter, a solid particulate meter as well as a High Performance Liquid Chromatography (HPLC) model (Waters 600E) equipped with auto sampler model (Waters 717 plus) and dual wavelength absorbance detector model (Waters 2487) set at 254 nm.
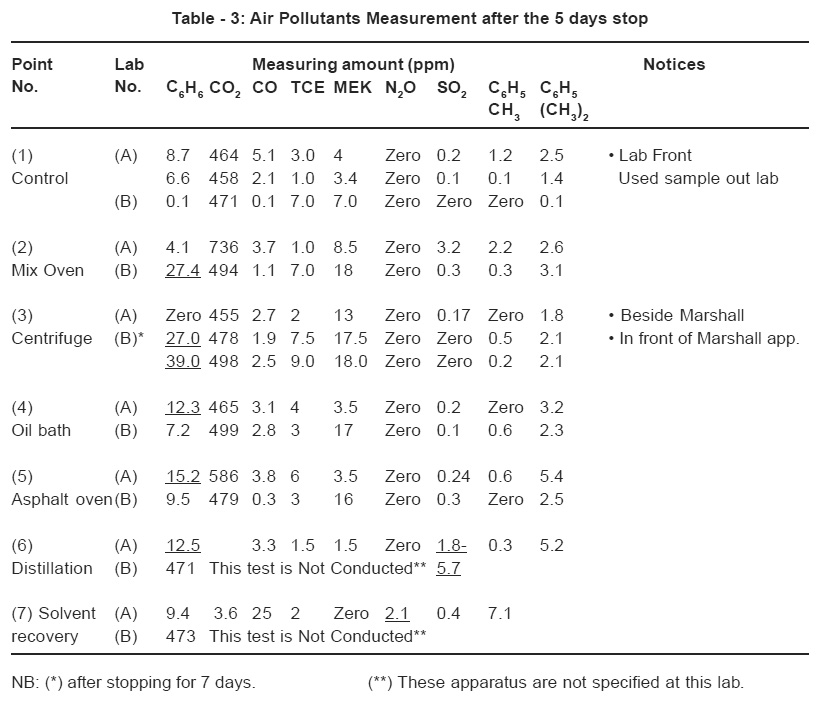 |
Table - 3: Air Pollutants Measurement after the 5 days stop Click here to view table |
Results and Discussion
Results of the Air Analysis
Measurements of the air pollutants that were produced during the different routine analyses, at rest (after 5 days stop) and during work as conducted in the laboratories are shown in Tables -3 and 4 respectively.
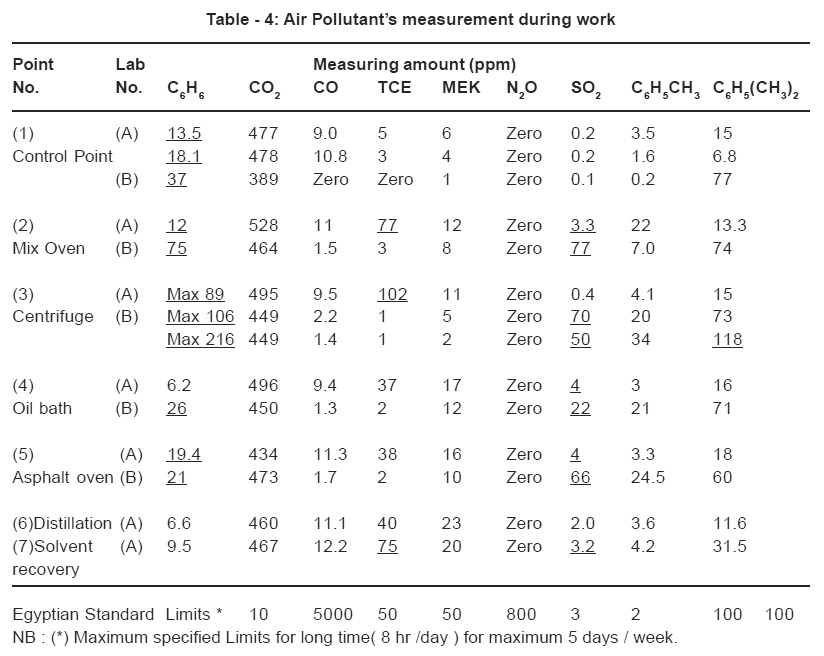 |
Table - 4: Air Pollutant’s measurement during work Click here to view table |
Air pollutants measured after 5 days rest
It is obvious from the data shown in Table 3 that nearly all the compounds measured after the 5-day stop were found in an obvious percentages. This may be attributed to poor ventilation and suction systems in both laboratories as there was an accumulation of some compounds from previous routine work. The two compounds namely C6 H6 and SO2 at some measuring points were found to be in very high percentages ,even that exceeding the allowable standard limits during work. The oil bath, the Asphalt oven heating and the Air space lying between the distillation and the solvent recovery apparatus in lab A gave the highest values of Benzene C6H6 as 12.3, 15.2 & 12.5 ppm respectively. The oven (point 2) and the centrifuge of lab B recorded frightening values of benzene concentrations of 27.4 and 39 ppm. These high values of accumulated benzene are attributed to the use of gasoline (5% by vol. of aromatics) as a cheap solvent in the extraction operation. While, the Trichloroethylene utilized in lab A is the standard solvent stated by the Standard Test Method (ASTMD 2172) for the extraction of asphalt from the asphalt mix. The sulphur dioxide accumulated, ranging from (1.8 to 5.7 ppm) in lab A at points (2&6), may be due to the chemical composition of the asphalt used; this is because it is known that the heavier the molecular weight of the petroleum fraction the higher the probability of the presence of sulphur. Also, the high number of samples tested in this lab A adds additional amounts of gaseous sulphur dioxide produced.
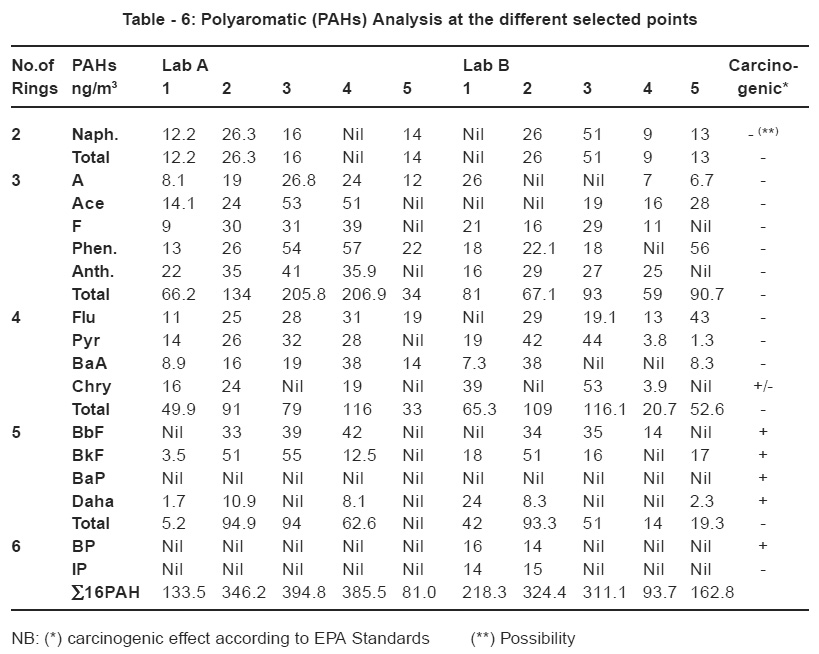 |
Table - 6: Polyaromatic (PAHs) Analysis at the different selected points Click here to view table |
Air Pollutants Emitted during the Experimental Work
It is worth mentioning that, the results obtained were based on the analysis of three asphalt samples in lab A as compared to only one sample tested in lab B. It is to be noted that the majority of the compounds measured were within the standard limits as seen in
Table - 4.
Analysis of Laboratory A Results
- Nearly all the selected measuring points showed concentrations of the benzene emitted above the permissible levels. This may be attributed to the fact that, the heating operations of asphalt specimen up to 145°C asphalt. This process causes the release of and of asphalt paving mix to 160°C release the already existing benzene as a constituent of asphalt.
- Also, the benzene emissions may be caused by the heating of the asphalt mix up to 160°C that was followed by a total immersion in cold trichloroethylene used in solvating the asphalt. This process causes the release of large amounts of benzene from the samples.
- The increase in exposure limits to trichloroethylene at the mixing oven and at the centrifuge measuring points reached values of 77 and 102 respectively. This may be attributed as mentioned previously to the use of this solvent in the test. The drained mixture of asphalt and solvent is received directly in an open pan in order to collect the fine portion of the mix hence causing the release of large amounts of solvent to the oven surrounding area.
- The increase in the exposure limit to the sulphur dioxide SO2 at the measuring points 2, 4 and 5 can be related to the presence of sulphur compounds in the asphalt sample itself. The fact that the oil bath (point 4) lies beside the asphalt-heating oven is responsible for the rise in the exposure limits measured at this point.
 |
Table - 7: Sound intensity and solid particulate measurement Click here to view table |
Analysis of Laboratory B results
- The use of gasoline rich in aromatics as a cheap solvent instead of the standard trichloroethylene increases the benzene emissions. The highest measured value was 216 ppm next to the centrifuge.
- The gasoline used contains a certain amount of sulphur compounds as allowed by the Egyptian specifications. The heating of asphalt paving mix at temperatures up to 160°C produces SO2 that adds to those released from the gasoline. The poor ventilation as well as the small area of this laboratory in addition to the previously mentioned causes led to the increase in exposure limit to SO2 in nearly all the measuring points.
- The increase in exposure limit of xylene in point 3 (centrifuge) may be attributed to the presence of the 5% by volume of aromatics in the chemical composition of the gasoline.
 |
Table - 8: Egyptian Specification Limit for Sound Intensity Click here to view table |
C- Polyaromatic PAHs Study
The Environmental Protection Agency (EPA) has identified 16 PAH compounds as priority pollutants. Some of these PAHs, as previously mentioned, are considered to be human carcinogens and hence became the focus of much attention and monitoring. The collected air samples from the different selected measuring points in both laboratories were analyzed using a High Performance Liquid Chromatographic analysis (HPLC).
The Conditions of the Operation were as follows:
Column:
Supelcosil LC-PAH, 15 cm x 4.6 mm ID, 5µm particle size.
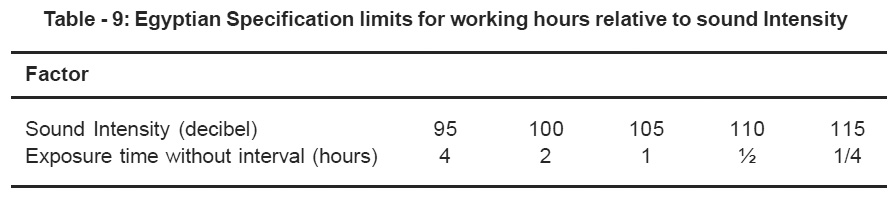 |
Table - 9: Egyptian Specification limits for working hours relative to sound Intensity Click here to view table |
Mobile Phase
Acetonitrile: water HLPC grades, gradient ranging from 50% to 100% of acetonitrile. (Lai et al., 1999).
Flow Rate
Gradient program: 0.2 ml/min for retention time ranging from zero to 2 minutes. 1.0 ml /min for retention time ranging from 2 to 45 minutes.
The result of the investigation conducted on the concentrations of the PAHs present in the ambient air in both asphalt laboratories is given in Table 6. The individual mean concentrations of PAHs over the selected sites are expressed in ng/ m3. The total sum of 16 PAHs at the same selected points in both laboratories was ranged from 81.00 to 394.80 ng / m3 in lab A and 93.7 and 324.4 ng / m3 in lab B. Worldwide studies have been made recently on the concentrations of PAHs in the atmospheric particulate matters in different cities. The reported levels in the urban atmosphere ranged from 22 to 400 ng /m3 in Barcelona, Spain (Rosell et al.,1991) and 10 to160 ng/m3 in Los Angeles, USA (Eisenreich et al., 2002). Indian cities showed recorded PAHs levels 10 to 50 times higher than the reported international limits of 23 to 190 ng/m3(Vascancellos et al., 2003).
The total sum of the concentrations of 16 PAHs measured at both Egyptian laboratories exceeded twice the allowed international levels. Unfortunately, the Egyptian Environmental law 4- 94 did not set standard limits for this type of hazardous substances.
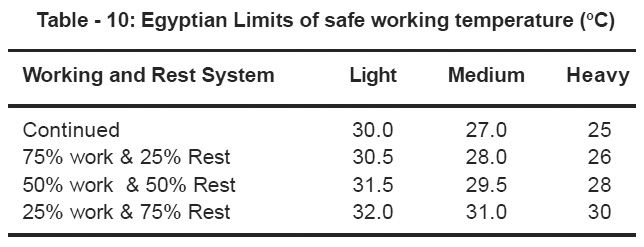 |
Table - 10: Egyptian Limits of safe working temperature (oC) Click here to view table |
D- Sound Intensity (noise) and Solid Particulate Concentration
The sound intensity (noise) was measured at both laboratories in the area surrounding the Marshall test sieve analysis & Los Angeles apparatus, Knowing that the arrangement of equipment in both labs was as follows: the Los Angeles machines and two big rectangular sieve analysis apparatus are placed In lab A in an isolated room -having a total area of 30m2 - inside the material laboratory with one suction blowers exhausting the resulting dust to the outside corridor. On the other hand the Marshall instrument and the small sieve analysis apparatus are put in a corner in the paving asphalt laboratory. Where as in lab B: The Los Angeles apparatus, a small circular sieve and a bigger rectangular sieve are placed in a small partition of the inside laboratory having an area of 3.2 m2. The resulting dust is exhausted to the air through a small window.
The sound intensity for each apparatus was measured separately and the obtained results were in the following order: 80 85, 90 and 95 decibel for the small circular sieve apparatus, Los Angeles machine, Marshall and big rectangular sieve apparatus. Sound intensity and solid particulates measurement values are illustrated in Table -7, while the Egyptian standard limit for sound intensity (noise) and the standard working hours relative to sound intensity (noise) were illustrated in Tables -8 and 9) respectively. From Tables -7, 8 and 9, it was found that the sound intensity values were within the standard levels taking into consideration that the real working hours did not exceed the allowable limits.
The Los Angeles test measures the abrasion resistance of solid aggregates as an indication of the hardness of the rock by using a standard number of steel balls. A large increase in the amount of solids produced of 25.3 mg/m3 was noticed in lab A during the draining operation of aggregate from the Los Angeles machines and the uncovered rectangular sieve. This solid particulate increase is attributed to the presence of fines at the end of the test also the amount of solids produced depend on the route of Los Angeles analysis itself. The Egyptian standard exposure limit for the (not harmful) type of dust should not exceed 10 mg/m3. The noise and the particulate solid measurements were found to be within the standard Egyptian limits in Lab B except for the period of aggregate discharge from the Los Angeles machine.
E-Working Temperature.
The measured indoor temperatures were found to be 35°C in lab A, 32°C in the other one. The poor ventilation and the absence of air conditioning in the asphalt analysis partition and the oven discharge cause the rise in temperature in Lab A. The hot asphalt vapours produced as well as the number of asphalt samples required to be tested are responsible for the temperature increase. The indoor temperature in lab B did not rise in the outer room due to the air conditioning system while it have increased in the inside room due to the presence of the oven placed at the corner.
Conclusions
Two Egyptian asphalt laboratories were thoroughly investigated for the suitability of their working environment. Analysis of the air quality and polyaromatic were conducted. The solid particulate and noise intensity have been measured. The following conclusions were drawn:
- The increase in the emissions of benzene, xylene, sulphur dioxide and trichloro - ethylene in both laboratories was due to different reasons among which :
a- Laboratory design (Location in the building, laboratory area, ventilation, arrangement of equipment).
b- Use of low price non- standard solvent as is the case in lab B.
The authors suggest that the use of other solvents rather than harmful chlorinated one. - The rise of solid particulate emission in both laboratories is caused by the standard procedure used in the Los Angeles test. Poor ventilation and loss of the cover of the sieve analysis apparatus produce additional solid particulates in lab A.
- The sound intensity is considered to be within range according to the number of working hours. Still, the authors suggest that all the noise producing apparatus be placed in a sound proofed compartment to improve the working conditions.
- The high values of PAHs obtained are alarming as they exceeded the allowable International Standard. The Egyptian Environmental law 4-94 did not set a standard limit for this type of hazardous substances. We recommend that the PAHs allowable International levels be added to the law in the near future.
- The authors suggest that the laboratory personnel in working places have to enlist in safety courses. It has to be mandatory by law to wear the appropriate uniforms, goggles, shoes, gloves, ear plugs and dust masks. Also, periodical health check- up are recommended.
References
- Binet S; Leszkowicz A P; Brandt H; Lafontaine M; Castengaro M. The science of the total environment. (2002) 300(1-3): 37-49.
- Burgaz S; Erdmen O; Karahalil B; Karakia AE. Mutation Research / Genetic Toxicology and environmental mutagenesis. (1998) 419(1-3): 123-130.
- Burstyn I; Kromhout H; Kauppinen T; Heikkilä P; Boffetta P. Annual Occupational Hygiene (2000) 44(1): 43-56.
- Castellano A V; Cancio J L; Alemán PS; Rodriguez JS. Enviroment International, (2003) 29, 475.
- Egyptian Environmental Affair Agency (EEAA). Law No 4. Egypt (1994).
- Erbas B; Kelly AM; Physick B; Code C; Edwards M. International Journal of Environmental Health Research. (2005) 15(10): 11-20.
- Halsall CJ; Coleman P J; Davis BJ; Burnett V; Water house K S; Harding- Jones P; Jones KC. Environmental Science and Technology. (1994) 28, 2380.
- Hansen E.N. Scandinavian Journal Work Environmental Health. (1991) 17: 20-24.
- Karakya A; Yücesoy B; Turhan A ; Erdem O ; Burgaz S. ; Karakya A E. Toxicology. (1999) 135(1): 43-47.
- Lai B; Khanna S. J. Appli. Bacterol. (1996) 181. 355-362.
- Mazzera D; Hayes T; Lowenthal D; Barbara Z. The science of the total environment. (1999) 220(1-2): 65-71.
- Menzie C A; Potock BB; Santodonato J. Environmental Science and Technology. (1992) 26, 7.
- NIOSH. National Toxicology program (1997).
- Partanen T ; Boffetta P. Am J Ind. Med. (1994) 26(6): 721-740.
- Reinke G; Swanson M; Paustenback D; Beach J. Mutat Res. (2000) 429(1): 41-50.
- Rosell A; Grimalt JO; Rosell MG; Guardino X; Albaigés J. Fresenius’ Journal of Analytical Chemistry. (1991) 339, 689.
- Samenta S; Singh O.V; Jaim R.K. 2002. Polyaromatic hydrocarbons environmental pollution and bioremedation. TRENDS in Biotechnology.20: 243-248
- Tsai PJ; Shieh HY; Lee W J; Lai SO. Journal of Hazardous Materials. (2002) A91, 25.
- Vascancellos PC; Zacarias D; Pires MAF; Pool C; Carvalho LRF. Atmospheric Environment. (2003) 37, 3009.​​​​​​​







