Rice Husk Ash Derived Zeolite Blended with Water Hyacinth Ash for Enhanced Adsorption of Cadmium Ions
G. W. Mbugua1 * , H. M. Mbuvi1 and J. W. Muthengia2
1
Department of Chemistry,
Kenyatta University,
43844-00100
Nairobi
Kenya
2
Department of Chemistry,
Embu University College,
6-60100
Embu
Kenya
DOI: http://dx.doi.org/10.12944/CWE.9.2.08
In order to helpcurtail or imposesustained control to the offensive water hyacinth plant,it is essential to explore ways of generatingwater remediation materials from it. In the current study, the capacity and efficacy of water hyacinth ash (WHA),its insoluble residue (WHAR) and rice husk ash (RHA)to remove cadmium ionsand methylene blue from contaminated water was investigated. Mixtures of the two ashes were used to formulatezeolitic materialsby hydrothermal reactions. Material A, ZMA was prepared by using rice husk ash and the soluble portion of WHA while for material B, ZMB a mixture of equal amounts the two ashes including the insoluble fraction of WHA were used. Batch experiments was carried out to determine the effect of metal ion concentration, initial PH, contact time (t), temperature (T), shaking speed and adsorbent dose on percentage removal of Cd2+ and methylene blue by the ashes and their zeolitic products. The data obtained for adsorption of Cd2+ on RHA, ZMA, and ZMB was found to best fit in the Langmuir isotherm model while WHA and WHAR data best fitted in theFreundlich model. Adsorption capacities for cadmium on RHA, WHA, WHAR, ZMA and ZMB adsorbents ions were 3.745, 52.00, 56.89, 11.24 and 22.22mg/g respectively.The findings showed that incorporating the WHAR during synthesis of the zeolitic material enhanced its adsorption capacity and efficiency for Cd (II) ions and methylene blue.
Copy the following to cite this article:
Mbugua G. W, Mbuvi H. M, Muthengia J. W. Rice Husk Ash Derived Zeolite Blended with Water Hyacinth Ash for Enhanced Adsorption of Cadmium Ions. Curr World Environ 2014;9 (2) DOI:http://dx.doi.org/10.12944/CWE.9.2.08
Copy the following to cite this URL:
Mbugua G. W, Mbuvi H. M, Muthengia J. W. Rice Husk Ash Derived Zeolite Blended with Water Hyacinth Ash for Enhanced Adsorption of Cadmium Ions. Curr World Environ 2014;9(2). Available from: http://www.cwejournal.org/?p=6262
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-02-28 |
|---|---|
| Accepted: | 2014-05-23 |
Introduction
17.2 million People in Kenya who constitute about 43 percent of the population have no access clean water. The major factors contributing to this water crisis include rapid population growth, droughts, forest degradation, poor management of water supply and water contamination. This water crisis may worsen as industrial development and population grows as projected if drastic measures are not taken immediately. The water hyacinth menace has further complicated the issue by not only contaminating our water bodies but also rendering many Kenya lakes idle with minimal economic activities taking place. The rapid population growth both in rural and urban areas has stressed the existing water supply systems.1 This accompanied by unsuitable water supply infrastructure has hindered access to water by the poor in many developing countries.2 There has been remarkable growth in light and informal (jua kali) industrieslike textiles, leather, paper, plastics, electroplating, cement, metal processing, wood preservatives, paints, pigments and steel fabricating industries.3 These industries discharge large quantities of toxic wastes.4 Recent studies in Kenya have reported that the open-air mechanical workshops are significant sources of mobile and bioavailable heavy metal contaminants.5,6 Several processes exist for removing dissolved heavy metals, including ion exchange, precipitation, ultrafiltration, reverse osmosis, electrodialysis and adsorption.7 Many of these approaches demand high energy, advanced operational requirement or difficult to treat and do not enable recovery of metals or material. It is therefore most desirable to explore possibilities of developing cheap water treatment materials and curbing or putting water hyacinth under maintained control in tandem.The findings herein suggest that rice husk and water hyacinth can be used as raw materials for synthesizing adsorbent materials for heavy metal removal from contaminated water.
|
|
Figure 1: High resolution picture of a |
Materials and Methods
Chemicals
A stock solution of cadmium with a concentration of 1000mg/L was prepared by dissolving 2.745g of cadmium nitrate in 1000mL distilled water in volumetric flask. Thesolution was then diluted to obtain standard solutions containing 0.1, 0.2, 0.4, 0.8, 1.6 and 3.2ppm.Concentrated Nitric acid and sodium hydroxide solutions were used throughout experiments to adjust the pH of the solution.
|
|
Figure 2: High resolution picture |
Rice husk ash, water hyacinth ash and water hyacinth ash residue preparation
Water hyacinth plants were obtained from Nairobi dam. They were then transported to the laboratory where they were extensively washed with tap water to remove soil and dust, sliced into pieces and then air dried for one week using a procedure already documented by.1 Ashing was done using an oven at a temperature of 950o for five hours. The ash was mixed to obtain a composite sample. Samples were sieved to obtain particles of the same size. Fig I shows a sample of water hyacinth ash, WHA. Water hyacinth ash residue (WHAR) was prepared by dissolving 50g of water hyacinth ash in 250cm3 of distilled water. The mixture was shaken for five minutes and allowed to settle. Filtration was done using whats man No. filter paper. The residue was then sun dried for five hours.Rice husks were provided by Euros rice millers in Kirinyaga County, Mwea west district. They were then washed several times with distilled water to remove soil and dust, followed by filtration and then dried at 100°. The clean and dry rice husks were burnt in an oven at 500° for 3hrs to obtain ash.Figure II shows a picture of a sample of rice husk ash, RHA.
Potassium hydroxide solution
1M potassium hydroxide was generated from water hyacinth ash by dissolving 50g of WHA and filtering off the residue. Titration experiments were done to determine the concentration of the alkaline solution using 0.1M hydrochloric acid.
Synthesis of zeolitic material, ZMA
25g of rice husk ash was conditioned into three stainless steel digestion bomb. 250mL of potassium hydroxide solution was added on this ash; the bomb was closed and introduced into a pre-heated oven at 200° for a period of 24hrs. The contents were then allowed to cool andfiltered. The solid residue was washed with distilled water to remove the excess alkali and dried at 105º for 12hrs. The solid residue was designated as ZMA and is shown in Fig 3.
|
|
Figure 3: High resolution picture |
Preparation of zeolitic material B, ZMB
For the preparation of ZMB, 50g of rice husk ash and 50g of water hyacinth ash were put in a reaction bomb. 250mL of distilled water was added to the mixture. The bomb was put in a preheated oven at 200º for 12hrs. The formed solid was washed with hot distilled water to remove the excess alkali. It was then dried at 105º for 12hrs. The samples were designated as ZMB and shown in fig 4.
|
|
Figure 4: High resolution picture |
Preparation of standard and test solution of Cd2+
A known mass, 2.745g of analytical grade cadmium nitrate Cd (NO3)2) was dissolved in 200 mL of distilled water. The resulting solution was diluted to 1000mL mark using distilled water. This was the 1000ppm stock solutionof cadmium. Standard solutions were prepared by successive dilution of the stock solution.
|
|
|
Instrumentation
The Cd2+ ionconcentrations in the various solutions were determined using atomic absorption spectrophotometer model AAS 4141, ECIL, India at wave length 283.3nm in flame mode using air-acetylene flame. The pH meter, model PHEP, Hanna instrument, Italy, was used in this study between pH ranges 2-12 at a temperature of 22.7o and UV-visible spectrometer. The concentrations were determined in triplicates. A standard and blank sample was run after every seven samples to check instrumental drift.Calibration curve method was used to quantify the heavy metal concentration.
|
|
|
Batch experiments
Atemperature-controlled water-bath shaker (DKZ-1 NO.1007827) was used for the batch adsorption experiments. The experiments were performed at the same shaking speed. For each experimental run, 50mL aqueous solution of known concentrations of Cd2+ ion wereput in 120mL plastic bottles that contained known masses of RHA, ZMA, ZMB, WHA and WHAR. These bottles were agitated at a constant shaking rate of 150 rpm and temperature of 25º, centrifuged and filtered.The concentration of Cd2+ ions in the filtrates obtained were measured using flame atomic adsorption spectrometry. Amount of Cd2+ ions adsorbed per unit mass of adsorbed and the percentage of Cd2+ ions removedwere calculated using the equations 1 and 2 respectively Formula 1,2

Where, qe = Amount of Cd2+ ions adsorbed per unit mass of adsorbed at equilibrium. Co = Initial concentration of sorbate. Ce = Concentration of sorbate at equilibrium. m = mass of sorbate (atomic mass). V = volume of solution,
|
|
|
Effect of the various parameters on the percentage of Cd2+ ions adsorbed
The effects of various parameters (adsorbent dose, contact time, initial concentrations, pH and temperature) on the percentage of Cd2+ ions adsorbed were investigated by varying the parameter of interest while keeping all the others constant. The effect of initial concentration was investigated by varying initial concentration from 10 to 500ppm at same conditions of: 0.1g of adsorbents, temperature of 25º, agitation speed of speed of 150rpm, pH 5, and contact time of 24hrs. The effect of the adsorbent dosage was investigated by varying the doses from 0.02 to 2.5g at same conditions of: 10ppmCd2+ ion solutions for WHA, RHA and ZMA and 100ppm for WHAR and ZMB,pH 5, agitation speed of speed of 150rpm, temperature of 25º and contact time of2hrs. The effect of contact time was investigated by varying contact time from 1 to 14400min at same conditions of: 0.1g of adsorbents, 10ppm Cd2+ ion solutions for WHA, RHA and ZMA and 100ppm for WHAR and ZMB, temperature of 25º, agitation speed of speed of 150rpm, pH 5. The effect of pH was investigated by varying pH from 2 to 12 at same conditions of: 0.1g of adsorbents, 10ppm Cd2+ ion solutions for WHA, RHA and ZMA and 100ppm for WHAR and ZMB, temperature of 25º, agitation speed of speed of 150rpm, contact time 2hrs.
Table 1: XRF analysis for Rice husk ash, RHA
|
Compound |
Al2O3 |
SiO2 |
K2O |
CaO |
TiO2 |
MnO |
Fe2O3 |
|
% oxide |
12% |
77% |
1.5% |
1.3% |
0.97% |
0.21% |
7.21% |
Removal of methylene blue dye from water
A stock solution of methylene blue of concentration 1000ppm was prepared by dissolving 1g of methylene blue in a 100ml volumentric flask using distilled water. The solution was shaken to obtain homogeneity. Solutions of various concentrations were obtained by dilution.The effect of the amount of methylene blue adsorbed was studied by agitating different concentrations of 50 ml of coloured water with0.1, 0.2, 0.3, 0.4, and 0.5g of WHA, WHAR, RHA, ZMA and ZMB for two hours. The solution was then filtered using whatman No. 1 filter paper and the colour in water was determined using UV Spectrophotometer. All these studies were conducted at 25º and agitation speed of 150rpm.
Table 2: XRF analysis for Water hyacinth ash, WHA
|
Compound |
Al2O3 |
P2O5 |
Cl |
K2O |
CaO |
MnO |
Fe2O3 |
ZnO |
|
% oxide |
1% |
6.0% |
21% |
35.8% |
27% |
7.1% |
1.8% |
0.2% |
Results and Discussions
Samples Characterization
The chemical compositions of RHA, WHA, WHAR, ZMA, and ZMB were determined by XRF and are shown in tables 1, 2, 3, 4 and 5 respectively. As shown in table 1, RHA is a good source of SiO2 and Al2O3 as it contained 77% and 12% respectively. WHA had 35.8% of K2O followed by 27% of CaO and Cl at 21% hence serving as a good source of KOH base.
Table 3: XRF analysis for Water hyacinth ash residual, WHAR
|
Compound |
Al2O3 |
P2O5 |
K2O |
CaO |
MnO |
Fe203 |
|
% oxide |
4 % |
9.9 % |
9.9 % |
54.8 % |
15.9 % |
5.9 % |
Effect of theinitial concentrations of Cd2+ ions
The percentage of Cd2+ions adsorbed by ZMA, ZMB, WHA, RHA and WHARwas significantly influenced by the initial concentration of Cd2+ ions in aqueous solutions. The initial Cd2+ concentration was varied from 10ppm to 500ppm while maintaining the adsorbent dosage at 0.1g. Figure 5 shows the effect of initial concentration on percentage removal of Cd2+ ions. The percentage removal of Cd2+ increased from 86.2% to 99.75% for RHA, 85.45% to 99.93% for ZMA, 99.0% to 98.1% WHAR, 50.5% to 99.5% for WHAand 97.8% to 99.4% for ZMB at the same contact time and adsorption temperature. Further increase in concentration lead to a decrease in percentage removal of cadmium ions. It is be due to an increase in the number of Cd2+ ions for the fixed amount of adsorbent.
Table 4: XRF analysis for Zeolitic material A, ZMA
|
Compound |
Al2O3 |
SiO2 |
K2O |
CaO |
TiO2 |
MnO |
Fe2O3 |
|
% oxide |
7.8% |
75% |
3.3% |
1.1% |
1.3% |
0.45% |
10.45% |
Effects of temperature on the adsorption of cadmium ions
The effect of temperature on the removal of Cd2+ ions by WHA, RHA, WHAR, ZMA and ZMB at initial solution concentration of 10 ppm for RHA, WHA and ZMA and 100ppm for WHAR and ZMB, using 0.1 g and contact time of 120min and agitation speed of 150rpm are shown in figure 6. From the results, it was observed that the percentage removal of cadmium ions by WHA, WHAR and ZMB remained constant with increase in temperature. There was a rapid decrease in percentage removal of cadmium ion by RHA and ZMA when the temperature was increased from 298k to 374k. The observed initial decrease in cadmium removal with increasing temperature suggests weak binding interaction between the active sites and cadmium ions which support physisorption which are exothermic and therefore favoured by low temperature.9
Table 5: XRF analysis for Zeolitic material B, ZMB
|
Compound |
Al2O3 |
SiO2 |
K2O |
CaO |
TiO2 |
MnO |
Fe2O3 |
P2O5 |
|
% oxide |
10.52% |
76.22% |
2.7% |
1.76% |
0.04% |
2.08% |
2.01% |
4.63% |
Effect of adsorbent dosage on adsorption of Cadmium ions
Experiments were conducted with the adsorbent dose of 0.02, 0.04, 0.06, 0.1, 0.2, 0.3, 0.4, 0.5, 1.0, 1.5, 2.0 and 2.5g per 50mL of test solution. When the addition of the adsorbent dose increased, the percentage removal of metal ions also increased as shown in fig 7. Adsorption of cadmium ions increased due to increase in number of binding sites as the adsorbent dose increased. A maximum removal of 99.99% at 2.0g ZMA, 99.40% at 2.5g RHA, 99.12% at 0.1g ZMB, 100% at 1.5g WHA and WHAR was observed. A further increase in adsorbent dose did not have any significant effect on the removal of cadmium ions from the solution.
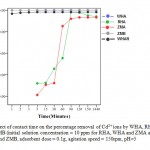
Effect of contact time on percentage removal of cadmium ion
Experiments were conducted by, varying contact time from 1 to 1440 min while maintaining all other experimental conditions constant. The results obtained are shown in Fig. 8. As shown the adsorption process occurred rapidly when WHA, WHAR and ZMB were used with almost 100% of the cadmium present adsorbed after 1min.There was an overlap between ZMB and WHA. ZMA achieved66% removalafter 30min, 95% after 90min and reached equilibrium within 2hrswhile RHAachieved 72% removal by 30 min, 99% by 90min and reachedequilibrium within 2hrs. This suggests that the bonding of the cadmium ion to active sites occurs preferably on the solid surface.10
|
|
|
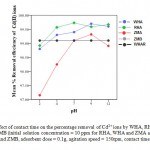
Effects of PH on percentage removal of cadmium ion
The effect of pH on the removal efficiency of cadmium ions is studied from pH 2 to 12. The percentage removal was found to be 99.73% for RHAat pH 4, 99. 32% for ZMA at PH 9, 99.7% for water hyacinth ash at PH 9, 99.12% forWHA and ZMB at PH 7 and 9as shown in Figure 9. At low pH, carboxylic and amino functional groups of adsorbents are protonated,11 thus active sites are less available for metal ion binding and thus cadmium ions were less absorbed. Thereafter, the percentage removal of cadmium ions decreased with increase in PH. This is as a result of increase in OH− ions cause a decrease in adsorption of metal ions at adsorbent adsorbate interface.12
|
|
|
Langmuir and Freundlich isotherm constants for cadmium
As shown in Table 6, adsorption data for WHA and WHAR best fitted inFreundlich model with R2 = 0.979 and 0.992 respectively while RHA ZMA and ZMB best fitted to the Langmuir model with R2 = 0.999, 1 and 0.998. WHAR and WHA had the highest affinity for Cd2+ions with adsorption capacity of 56.89 and 52.00mg/g respectively. They also had a1/n value greater than one indicating the adsorption process was favourable in Freundlich isotherm model. ZMA and ZMB had adsorption capacity of 11.24 and 22.22mg/g respectively indicating that incoperating the insoluble WHAR in the zeolitic synthesis enhances the materials adsorption for Cd2+ ions. The study showed that ZMA, ZMB, WHA, WHAR and RHA could effectively remove cadmium ions and that adsorption was enhanced in WHAR blended ZMB.
Table 6: Langmuir and Freundlich isotherm constants for cadmium
|
|
Langmuir |
Freundlich |
||||
|
sample |
qmaxmg/g |
b dm3/g |
R2 |
1/n |
Kfmg/g |
R2 |
|
RHA |
3.745 |
5.03 |
0.999 |
0.785 |
7.59 |
0.674 |
|
WHA |
200 |
0.208 |
0.934 |
1.43 |
52.00 |
0.979 |
|
WHAR |
250 |
0.018 |
0.842 |
1.486 |
56.89 |
0.992 |
|
ZMA |
11.24 |
22.25 |
1 |
0.385 |
15 |
0.908 |
|
ZMB |
22.22 |
0.303 |
0.998 |
5.103 |
5.103 |
0.987 |
Acknowledgement
We are grateful to Kenyatta University and Chemistry Department for supporting our project
References
2. Cline, S., Rosegrant , M., Cai X. Global water outlook to 2025. International Food Policy Research Institute (2002).
3. Chen, C., Dong, C., Kao, C. Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere, (8), 1431–1440 (2007).
4. Cheng, S. Heavy metal pollution in China: origin, pattern and control. Environmental Science and Pollution Research, (3), 192– 198 (2003).
5. Chengo K., Murungi J., Mbuvi H.M. Speciation of Zinc and Copper in Open-Air Automobile Mechanic Workshop Soils in Ngara AreaNairobi Kenya, Resources and Environment, (3), 145-154 (2013).
6. Chengo K., Murungi J., Mbuvi H.M. Speciation of Chromium and Nickel in Open-Air Automobile Mechanic Workshop Soils in Ngara Area-Nairobi Kenya, World Environment, (3), 143-154 (2013).
7. Burton, F. and Tchobanoglous, G. wastewater engineering treatment, disposal and reuse (Metcalf and Eddy, Inc,). McGraw-Hill, NewYork (1991).
8. Kruatrachue, M., Lu, X., Pokethitiyook, P., Homyok, K. Removal of cadmium and zinc by water hyacinth, Eichhorniacrassipes.Science Asia, 30, 93–103 (2004).
9. Mataka LM, Salidu SM, Masamba WRL, Mwatseteza JF. Cadmium sorption by Moingastenopetala and Moringaoleiferaseed powder. Int. J. Environ. Sci. Technol 3(2):131- 139 (2010).
10. Blazquez, G. Hernainz, F. Calero, M. RuizNuìnÞez, L.F. Removal of cadmium ions with olive stones: the effect of some parameters, Process Biochemistry, 40: 2649–2654 (2005).
11. Krishnan, K. A., and Anirudhan, T. S. Removal of cadmium (II) from aqueous solutions by steam-activated sulphurised carbon prepared from sugar-cane bagasse pith: Kinetics and equilibrium studies.Water Sa, 29(2), 147–156 (2003). 12. Periasamy, K., &Namasivayam, C. Removal of nickel (II) from aqueous solution and nickel plating industry wastewater using an agricultural waste: peanut hulls. Waste management, 15(1), 63–68 (1995).