Removal of Arsenic from Drinking Water by Hydroxyapatite Nano Particles
Mahsa Mirhosseini2 * , Esmaeil Biazar1 and Keivan Saeb2
DOI: http://dx.doi.org/10.12944/CWE.9.2.13
Arsenic(As) containedin drinking water can cause adverse effect son human health. This study investigated the effect of hydroxyapatite nano particles (nano-HAp) on sorption of As(V) ions in aqueous solution. The amounts of arsenic ion,nano-HAp and pH on removal efficiency were also investigated.Resultsshowed that theremoval ofarsenatefromwater using hydroxyapatite nanoparticles, improved with increasing pH. The optimum amount of nano-HAp for As (V) removal isfound to be 0/6g/L with the removal efficiency of 88 %. The sorption data were then correlated with the Langmuir, Freundlich, adsorption isotherm models. The results indicated that nano-HAp can be used as an effective adsorbent for removal of As(V) from aqueous solution.
Copy the following to cite this article:
Mirhosseini M, Biazar E, Saeb K. Removal of Arsenic from Drinking Water by Hydroxyapatite Nano Particles. Curr World Environ 2014;9 (2) DOI:http://dx.doi.org/10.12944/CWE.9.2.13
Copy the following to cite this URL:
Mirhosseini M, Biazar E, Saeb K. Removal of Arsenic from Drinking Water by Hydroxyapatite Nano Particles. Curr World Environ 2014;9(2). Available from: http://www.cwejournal.org/?p=6566
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2014-01-24 |
|---|---|
| Accepted: | 2014-06-09 |
Introduction
Arsenic compounds are common contaminants in the environment. Because of arsenic toxicity and inducedcarc inogenetic agents(Eblin et al.,2006; Hughes.,2002), higher arsenic concentration in the environment represents serious problems for human health, especially for populations in Bangladesh, Western Bengal, Vietnam,China, Mexico and Chile. The danger of elevated arsenic concentration in waters in these countries was under lined by WHO, which estimated the recommended limit for arsenic concentration in drinking waters up to 10 μg/LArsenic-contaminated drinking water can cause adverse health effects in human beings. Arsenic plays crucial role in making disturbance in RNA and DNA synthesis, which consequently lead to cancer. Increasing birth of exceptional child, low birth weight, malformed child and dead births were reported due to Arsenic compounds (Jain et al., 2000; Kiping et al.,1997; Ng et al.,2001; Bissen et al.,2003; Penrose et al.,2009; Ng et al.,2003; Burkel et al.,1999; Smedley et al.,2002). The conventional technologies for arsenic removal from waters are based on processes of coagulation, sorption, ion-exchange reactions or methods of reverse osmosis. Materials used in these processes are Fe0, Fe (III) oxyhydroxides, Mn (II), Al(III), apatite, silicate sands, carbonates, sulphides, ashor various types of coal(Chmielewská et al.,2008; Daus et al.,2004; DeMarco et al.,2003; Hiller et al.,2007; Lin et al.,2001; Sato et al.,2002; Song et al.,2006). Nowadays, there is a trend to use the alternative and low-cost materials for arsenic removal from the waters in laboratory or medium-scale experiments, too. Effectiveness of chemically modified or native biomass in processes of arsenic removal was evaluated and provedby various authors (Abdel-Ghani et al.,2007; Boddu et al.,2008; Cernan sky et al.,2007; Loukidou et al.,2003; Malakootian et al.,2009; Murugesan et al.,2006; Rahaman et al.,2008; Seki et al.,2005).Calcium hydroxyapatite (HAp), Ca10(PO4)6(OH)2, has also been used for the removal of heavy metals from contaminated soils, waste water and fly ashes (Omar et al.,2003; Takeuchi et al.,1990).Calcium hydroxyapatite (Ca-HAp) is a principal component of hard tissues and has been of interest in industry and medical fields. Its synthetic particles find many applications in bioceramics, chromatographic adsorbents to separate protein and enzyme, catalysts for dehydration and dehydrogenation of alcohols, methane oxidation, and powders for artificial teeth and bones paste germicides (Elliott et al.,1994). These properties relate to various surface characteristics of HAp, e.g., surface functional groups, acidity and alkaline, surface charge, hydrophilicity, and porosity. It has been found that Ca-HAP surface with to P–OH groups acting as sorption sites (Tanaka et al., 2005). The sorption properties of HAp are of great importance for both environmental processes and industrial purposes. Hydroxyapatite is an ideal material for long-term containment of contaminants because of its high sorption capacity for actinides and heavy metals, low water solubility, high stability under reducing and oxidizing conditions, availability, and low cost(Krestou et al.,2004). HAP has been utilized in the stabilization of a wide variety of metals (e.g., Cr, Co, Cu, Cd, Zn, Ni, Pu, Pb, As, Sb, U, and V) by many investigators (Omar et al.,2003; Ramesh et al.,2012; Vega et al.,1999). They have reported the sorption is taking place throughionic exchange reaction, surface complex with phosphate, calcium and hydroxyl groups and/or co-precipitation of new partiallysoluble phases. In this study, the effect of Hap nanoparticl eson removal efficiency of arsenic ions in different conditions investigated.
Materials and Methods
The hydroxyapatite nanoparticles previously have prepared (Montazeri and Biazar., 2011)and characterized by using the different analyses. The pH values of the solution were roughly adjusted from 2 to 12 by adding HNO3 and Na OH respectively. The pH of the solutions was then accurately noted. Hydroxy apatite nanoparticles with different concentrations were added to each flask and securely capped, immediately. The suspension was then manually agitated. The pH values of the supernatant liquid were noted.Metal salt of (HAsNA2O4.H2O)was used to prepare metal ion(As(V)) solution. Sorption studies were carried out by shaking a series of bottles containing different amounts of H Ap-nano in 50 mL of metal ions solution with different concentrations and pH. Suspensions were exposed to ultra sonic waves (50W,20 min), to disperse nanopaticles. The samples were stirred at room temperature at 250 rpm for 1 h (Equilibrium time), then centrifuged for 5 min and the supernatant liquid was analyzed by an atomic absorption spectrometer(S-series, Thermo Scientific; USA).
Results and Discussion
Effect of pH
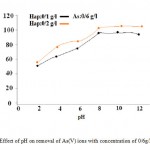
The pH is a significant factor for determining the form ofthe metallic species in aqueous media. It influences the adsorption process of metal ions, as it determines the magnitude and sign of the charge on ions(Gupta et al.,2005). The effect of solution pH on the sorption of As(V)ions from the aqueous solution byHap-nanoin different concentrations was investigated in the pH range of 2–12 with the As(V) concentrations of 0/6g/L. The result is shown in Figure 1. It was found that the adsorption capacity of HAp increases with increase inpH in acidic medium. But in alkaline conditions, the removal efficiency remains constant, approximately. The effects of pH on arsenate removal by HAp-nano suggest that electrostatic attraction is not a major mechanism responsible for the arsenate sorption under our experimental conditions; otherwise the apparent arsenate sorption would decrease with increasing pH. The sorbent dissolution can result in a decrease of sorbent mass and an increase of phosphate concentration in water, both of which can inhibit arsenate sorption. Lowering pH can favor the dissolution HAp-nano, and thus suppress arsenate sorption (Sneddon et al.,2005; Valsami-Jones et al.,1998; Mohan et al.,2007).
|
|
Figure 1: Effect of pH on removal of As(V) |
Effect of Arsenic concentration
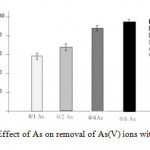
For study the effect of solution arsenate amount on the sorption of As(V)ions from the aqueous solution byHAp-nanoin different concentrations was investigated in the range of (0/1,0/2, 0/4,0/6g/L in pH:8).The result is shown in figure2. increase arsenate absorption occurred with in creasingar senaterate. Arsenate adsorption, indifferent concentrations of arsenate in water, investigated in nano-hydroxyapatite, showed a similar increase approximately,As a result, absorption levels for these different amounts of arsenate are significant.
|
|
Figure 2: Effect of As on removal |
Effect of contact time
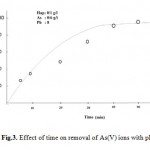
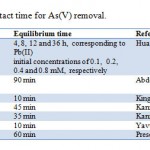
The time-dependent behavior of As(V) dsorption was measured by varying the contact time between adsorbate and adsorbent in the range of 5–120 min. The percentage adsorption of As(V) with different contact time is shown in Fig. 3. From Fig. 3, it can be observed that the rate of removal of As(V) ions was higher at the initial stage, due to the availability of more active sites on the surface of HA and became slower at the later stages of contact time, due to the decreased or lesser number of active sites (Kannan and Karrupasamy 1998). It is apparent from Fig. 3 that until 1 h, the percentage removal of As(V) from aqueous solution increases rapidly and reaches up to 85 %. A further increase in contact time has a negligible effect on the percentage removal. Therefore, a 1 h shaking time was considered as equilibrium time for maximum adsorption. The decrease in rate of removal of As(V) with time may also be due to aggregation of As(V) around the HA particles. This aggregation may hinder the migration of adsorbate, as the adsorption sites become filled up, and also resistance to diffusion of As(V)molecules in the adsorbents increases(Mittal et al. 2010).
|
|
Figure 3: Effect of time on removal |
Effect of mass of adsorbent on As(V) removal
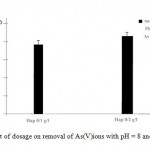
The effect of HA dosage on As(V) removal was analyzed by varying the dosage of HA and the result is shown in Fig. 4. It was observed that the removal efficiency increases with the increase in HA dosage. This reveals that the instantaneous and equilibrium sorption capacities of As(V) are functions of the HA dosage
|
|
Figure 4: Effect of dosage on removal |
Adsorption isotherms
Equilibrium isotherm is described by a sorption isotherm,characterized by certain constants whose values express the surface properties and affinity of the sorbent sorption equilibrium is established when the concentration of sorbentin the bulk solution is in dynamic balance with that at the sorbent interface (Oladoja et al.,2008). The adsorption isotherm study is carried out on well-known isotherms such as Langmuir Langmuir.,1915).
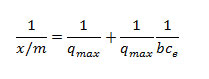
Where b is the constant that increases with increasing molecular size, qmax is the amount adsorbed to form a complete mono layer on the surface (mg/g), X is weight of substance adsorbed (mg), M is weight of adsorbent (g), and Ce is the concentration remaining in solution (mg/L). The essential features of the Langmuir isotherm may be expressed in terms of equilibrium parameter RL, which is a dimension less constant referred to as separation factor or equilibrium parameter (Weber and Chakkravorti, 1974).
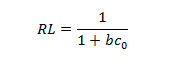
The value of RL indicates the type of the isotherm to be either unfavorable (RL[1]), linear (RL = 1), favorable (0\RL\1) or irreversible (RL = 0). The Freundlich isotherm is expressed as (Freundlich, 1906).
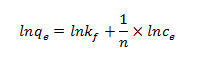
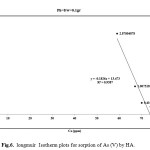
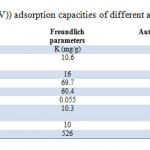
Where Kf and n are the constants depending on temperature An isotherm plot for sorption of As (V) by HAp-nano is shown in Figure 5 and 6 The diagram indicates that the Freundlich isotherm isfavorable for removal of As (V) by HAp-nano. The value of R also indicates that Langmuir isotherm is favorable. It can be concluded that Freundlich isotherm is the best fit langmuir isotherms. The adsorption capacity of HAp-nanofor As (V) adsorption is compared with other adsorbents(Table 3). The value of As (V) uptake by HAp-nano found in this work is significantly higher than that of other adsorbents.
|
|
Figure 5: freundlich Isotherm plots |
|
|
Figure 6: longmuir Isotherm plots |
|
|
Table 1: Comparison of contact |
|
|
Table 2: Isotherm parameters |
|
|
Table 3: Comparison of As (V)) adsorption |
Three types of reactions may control As(V) immobilizationby HAp-nano: surface adsorption, cation substitution orprecipitation. The first mechanism is the adsorption of As(V) ions on the HAp-nano surfaces and following ion exchange reaction between As(V) ions adsorbed and Ca ions of HAp-nano(Suzuki et al.,1984). This ion exchange reaction mechanism (Ma et al.,1994)is expressed as:
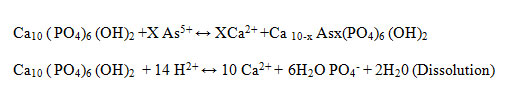
Showed that HAp-nano dissolution and hydroxy pyromorphite (HP) precipitation were the main mechanisms for As(V) immobilization by HAp-nanoin theabsence of other metals. These chemical reactions can bedescribed as follows:
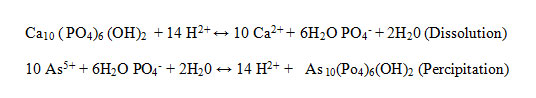
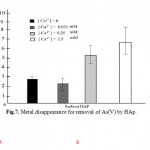
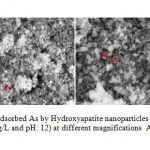
Information about the sorption mechanisms have been inferred by the values of molar ratios (Qs) of cations bound by HAp-nanoto Ca desorbed from HAp-nano (Aklil et al.,2004). Figure 7 presents the effect of calcium concentration on arsenate removal by HAp-nano. For all sorbents tested, increasing calcium level appeared to assist arsenate sorption. When calcium concentration increased from 0 to 2.5 mM, the arsenate removal efficiency increased from 2.4% to 5.4% by using HAp-nano. The calcium effects on arsenate sorption to HAp-nanoare to be due to two reasons. First, according to increasing calcium concentration in water can inhibit HAp-nano, which can inhibit arsenate sorption to the sorbents. Second, Ca2+ in water can complex with phosphate on HAp-nanosurface, resulting in an increase of sorption sites and subsequently an increase of arsenate sorption(Czerniczyniec et al.,2007; Sneddon et al.,2005). SEM image of absorb As(V) by nanoparticles of HAp-nano shown in figure8.
|
|
Figure 7: Metal disappearance for |
|
|
Figure 8: SEM image of adsorbed As by Hydroxyapatite |
Conclusion
The result shows that hydroxy apatite nano particle (HAp-nano)is a powerful adsorbent for removing As(V) from aqueous solution. The optimum dose of HAp-nano for As(V)removal is found to be 0.2 g/L with the removal efficiency of 88 %. Freundlich isotherm had best fit than Langmuir, for experimental data. The adsorption capacity of HAp-nanowas found to be 526 mg/gHAp-nano dissolution and hydroxy pyromorphite precipitation were the main mechanisms for As(V) immobilization byHAp-nano.
Acknowledgment
The authors gratefully acknowledge financial assistance of Iran nanotechnology initiative council.
References
-
Abdel-Ghani N.T., Hefny M. and El-Chagbaby G.A.F. (2007) ‘Removal of lead from aqueous solution using low cost abundantly available adsorbents’ Int. J. Environ. Sci. Tech., Vol.1, pp.67- 73.
-
Aklil M., Mouflih M. and Sebti S. (2004) ‘Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent’ J Hazard Mater., Vol.112, pp.183-190
-
Bissen, M., Frimmel, F.H. (2003) ‘Arsenic- a review. Part I. Occurance, toxicity, speciation, mobility’ Acta Hydrochim. Hydrobiol., Vol. 2, pp.9-18.
-
Boddu V.M., Abburi K., Talbott J.L., Smith E.D. and Haasch R. (2008) ‘Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent’ Water Res., Vol.3, pp.633- 642.
-
Burkel, R.S. and Stoll, R.C. (1999) ‘ Naturally occurring arsenic in sandstone aquifer water supply wells of North Eastern Wisconsin, Ground Water Monit’ Remediation., Vol. 2, pp.114-121.
-
Chmielewská, E., Sabová, L. and Jesenák, K. (2008) ‘Study of adsorption phenomena ongoing onto clinoptilolite with the immobilized interfaces’J. Therm. Anal. Calorim., Vol.2, pp.567-571.
-
Cernansky S., Urík M., Ševc J. and Hiller E. (2007) ‘Biosorption of arsenic and cadmium from aqueous solutions’ Afr. J. Biotechnol., Vol.16, pp.1932- 1934.
-
Czerniczyniec M., Farias S. and Magallanes J. (2007) ‘Arsenic(V) adsorption onto biogenic hydroxyapatite: solution composition effects’ Water Air Soil Pollut., Vol.180, pp.75-82.
-
Daus, B., Wennrich, R. and Weiss, H. (2004) ‘Sorption materials for arsenic removal from water: A comparative study’ Water Res., Vol.12, pp.2948-2954.
-
DeMarco, M.J., SenGupta, A.K. and Greenleaf, J.E. (2003) ‘Arsenic removal using a polymeric/inorganic hybrid sorbent’ Water Res., Vol. 1, pp.164- 176.
-
Eblin, K.E., Bowen, M.E., Cromey, D.W. and Bredfeldt T.G. (2006) ‘Arsenite and monomethylarsonous acid generate oxidative stress response in human bladder cell culture’ Toxicol. Appl. Pharm., Vol.1, pp.7- 14.
-
Elliott J.C. (1994) ‘Structure and Chemistry of the Apatite’s and Other Calcium Orthophosphates’ Elsevier, Amsterdam, Vol.12, pp.371.
-
Freundlich H. (1906) ‘Adsorption in solution’ Z Phys Chem., Vol.57, pp.384- 470.
-
Gupta V.K., Agarwal S. and Saleh T.A. (2011) ‘Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal’ J Hazard Mater., Vol.185, pp.17-23.
-
Hiller E., Veselská V. and Majzlan J. (2007) ‘Arsenic mobility in stream sediments and impoundment material as evaluated by column and batch experiments’ J. Hydrol. Hydromech., Vol.4, pp.223-235.
-
Hughes, M.F. (2002) ‘Arsenic toxicity and potential mechanisms of action’ Toxicol. Lett., Vol. 1, pp.1-16
-
Jain, C.K. and Ali I. (2000) ‘Arsenic occurrence, toxicity and speciation techniques’ Water Res., Vol. 1, pp.4304-4312.
-
Kannan N, Karrupasamy K (1998) Low cost adsorbents for the removal of phenyl acetic acid from aqueous solution. Indian J Environ Protect 18(9):683–690
-
Kiping, M.D., Lenihan, J,, Fletcher, W.W. (1997) ‘Arsenic, the Chemical Environment’ Environment and Man., Vol. 6, pp.93-110.
-
Krestou A., Xenidis A. and Panias D. (2004)‘Mechanism of aqueous uranium(VI) uptake by a natural zeolite’ Tuff. Miner. Eng., Vol. 17, pp.373–381.
-
Langmuir I. (1915) ‘Chemical reactions at low pressures’ J Am Chem Soc ., Vol. 27, pp.1139-1143.
-
Lin T. and Wu J. (2001) ‘Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics’ Water Res., Vol.8, pp.2049- 2057.
-
Loukidou M.X., Matis K.A., Zouboulis A.I. and Liakopoulou- Kyriakido M. (2003) ‘Removal of as (V) from wastewaters by chemically modified fungal biomass’ Water Res., Vol.18, pp.4544- 4552.
-
Ma Q.Y., Traina S.J., Logan T.J. and Ryan J. (1994) ‘ Effect of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb(II) immobilization by hydroxyapatite’ Environ Sci Technol., Vol.28, pp.1219-1228.
-
Maji S.K., Pal M., Pal T. and Adak A. (2007) ‘Adsorption thermodynamic of As on laterite soil’J. Surf. Sci. Technol., Vol.22, pp.161- 176.
-
Malakootian M., Nouri J. and Hossaini H. (2009) ‘Removal of heavy metals from paint industries wastewater using Leca as an available adsorbent’ Int. J. Environ. Sci. Tech., Vol.2, pp.183- 190.
-
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interf Sci 344:497–507
-
Mayo J.T., Yavuz C., Yean S., Cong L., Shipley H., Yu W., et al. (2007) ‘The effect of nanocrystalline magnetite size on arsenic removal’ Sci. Technol. Adv. Mater., Vol.8,pp.71- 75 .
-
Mohan D and Pittman C. (2007) ‘Arsenic removal from water/wastewater using adsorbents-a critical review’ J. Hazard. Mater., Vol.142, pp.1-53.
-
Montazeri, N., Jahandideh, R., and Biazar, E.(2011) ‘Synthesis of fluorapatite–hydroxyapatite nanoparticles and toxicity investigations’ Int.J.Nanomed, Vol.6, pp.197-201.
-
Murugesan G.S., Sathishkumar M. and Swaminathan K. (2006) ‘Arsenic removal from groundwater by pretreated waste tea fungal biomass’ Bioresource Technol., Vol.3, pp.483- 487.
-
Ng, J.G.C., Howe, A., Hughes, P., Kenyon, M., and et al. (2001) ‘Environmenal health criteria for arsenic and arsenic’ world Health Organisation., Vol. 224, pp.508-512.
-
Ng, J.C., Wang, J. and Shraim, A.A. (2003) ‘Global health problem caused by arsenic from natural sources’ Chemosphere ., Vol. 9, pp.1353-1359.
-
Oladoja N.A., Aboluwoye C.O., Oladimeji Y.B. (2008) ‘Kinetics and isotherm studies on methylene blue adsorption onto ground palm kernel coat’ Turkish J Eng Env Sci., Vol.32, pp.303- 312.
-
Omar W and Al-Itawi H. (2003) ‘Removal of Pb+2 Ions from Aqueous Solutions by Adsorption on Kaolinite Clay’ A. J. of Appl.Sci., Vol.7, pp.498-502.
-
Paritam K.D., Ray A.K., Sharma V.K and Millero F.J. (2004) ‘Adsorption of arsenate and arsenite on titanium dioxide suspensions’ J. Colloid Interface Sci., Vol.278, pp.270-275.
-
Penrose, W.R. (2009) ‘Arsenic in the marine and aquatic environments. Analysaism occurrence and significance’ Crit. Rev. Environ Contr., Vol. 1,pp.465-482.
-
Rahaman M.S., Basu A. and Islam M.R. (2008) ‘The removal of As (III) and As (V) from aqueous solutions by waste materials’ Bioresource Technol., Vol.8, pp.2815- 2823.
-
Ramesh S.T., Rameshbabu N., Gandhimathi R., Nidheesh P.V. and Srikanth Kumar M., Kinetics and equilibrium studies for the removal of heavy metals in both single and binary systems using hydroxyapatite, Appl Water Sci., 2, 187- 197( 2012).
-
Sato Y., Kang M., Kamei T. and Magara Y. (2002) ‘Performance of nanofiltration for arsenic removal’ Water Res., Vol.13, pp.3371- 3377.
-
Seki H., Suzuki A. and Maruyama H. (2005) ‘Biosorption of chromium (VI) and arsenic (V) onto methylated yeast biomass’ J. Colloid. Interf. Sci., Vol.2, pp.261- 266.
-
Smedley, P.L., Nicolli, H.B., Macdonald, D.M.J., Barros, A.J. and Tullio, J.O. (2002) ’Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina’ Appl. Geochem., Vol. 3, pp.259-284.
-
Sneddon I., Garelick H. and Valsami-Jones E. (2005) ‘An investigation into arsenic(V) removal from aquesous solutions by hydroxylapatite and bone-char’ Mineral. Mag., Vol.69, pp.769- 780.
-
Song S., Lopez-Valdivieso A.N., Hernandes-Campos D.J., Peng C., Monroy- Fernandez M.G. and Razo-Soto I. (2006) ‘Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite’ Water Res., Vol.2, pp.364-372.
-
Suzuki T., Ishigaki K. and Miyake M. (1984) ‘Synthetic hydroxyapatites as inorganic cation exchangers exchange characteristics of Pb(II) ions’ J Chem Soc Faraday Trans., Vol.80, pp.3157- 3165.
-
Takeuchi Y and Arai H. (1990)‘Removal of coexisting Pb2+, Cu2+,Cd2+ ions from water by addition of hydroxyapatite powder’ J.Chem. Eng. Jpn., Vol. 1, pp.75-80.
-
Tanaka H., Futaoka M., Hino R., Kandori K. and Ishikawa T. (2005) ‘Structure of synthetic calcium hydroxyapatite particles modified with pyrophosphoric acid’ J. Colloid Interf. Sci., Vol.2, pp.283- 609.
-
Thirunavukkarasu O.S., Viraraghavan T. and Subramanian K.S. (2003) ‘Arsenic removal from drinking water using granular ferric hydroxide’ Water S A., Vol.2, pp.1-5.
-
Valsami-Jones E, Ragnarsdottir K., Putnis A., Bosbach D., Kemp A. and Gressey G., (1998) ‘The dissolution of apatite in the presence of aqueous metal cations at pH 2-7’ Chem. Geol., Vol. 151, pp.215- 233.
-
Vega E.D., Pedregosa J.C. and Narda G.E. (1999)‘Interaction of oxovanadium(IV) with crystalline calcium hydroxyapatite: surface mechanism with no structural modification’ J. Phys. Chem.Solids., Vol.60, pp.759-766.
-
Weber T.W and Chakkravorti R.K. (1974) ‘Pore and solid diffusion models for fixed bed adsorbers’ AIChE J., Vol.20, pp.228- 238.
-
Zhang Y., Yang M. and Huang X. (2003) ‘Arsenic(V) removal with a Ce(IV)-doped iron oxide adsorbent’Chemosphere., Vol.51, pp.945-952.