Heavy Metal Runoff Dynamics From Farmland Around Ikpa River Basin, Nigeria
Edem I.Dennis1 * , Rosemary A. Essien2 and Utibe-Abasi H. Udoh3
1
Department of Soil Science and Land Resources Management,
Faculty of Agriculture,
University of Uyo, P.M. B.1017,
Uyo,
Akwa Ibom State
Nigeria
2
Department of Crop Science,
Akwa Ibom State University,
Nigeria
3
Department of Animal Science,
Faculty of Agriculture,
University of Uyo,
Uyo,
Nigeria
DOI: http://dx.doi.org/10.12944/CWE.8.2.09
Copy the following to cite this article:
Dennis E. I, Essien R. A, Udoh U. H. Heavy Metal Runoff Dynamics From Farmland Around Ikpa River Basin, Nigeria. Curr World Environ 2013;8(2) DOI:http://dx.doi.org/10.12944/CWE.8.2.09
Copy the following to cite this URL:
Dennis E. I, Essien R. A, Udoh U. H. Heavy Metal Runoff Dynamics From Farmland Around Ikpa River Basin, Nigeria. Curr World Environ 2013;8(2). Available from: http://www.cwejournal.org/?p=4904
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-07-23 |
|---|---|
| Accepted: | 2013-08-20 |
Soils are not only a medium for plants to grow or a pool to dispose of undesirable materials, but also a transmitter of many pollutants to surface water, atmosphere and food. Therefore accumulated pollutant in surface soil can be transported to different environmental components. Soil pollutant may threaten the human health not only on hygienic quality food, but also on drinking water. In recent years, environmental problems such as heavy metal pollution have become important issues.1 Some heavy metals are necessary to support life, but other heavy metals form toxic chemical compounds can affect the ecosystem.2 The quality of life on earth is linked undeniably to the overall quality of the environment.3 Pollution of the biosphere by heavy metals due to the industrial, agricultural and domestic activities has created a serious problem for safe and rational utilization of soils. Agronomic application of fertilizers and pesticides continue to contribute to metal accumulation in the soil. The pollution of the ecosystem by heavy metals is a real threat to the environment because metals cannot be naturally degraded like organic pollutants and persist in the ecosystem having accumulated in different parts of the food chain.4 Fertilizer toxicity may affect all forms of different organisms. Physical, chemical and biological processes many combine under certain circumstances to concentrate metals rather than dilute them.
Zn, Cu, Cd and Pb are listed in the environmental quality standard for soil and water pollution in Nigeria.5 These heavy metals have not only drawn attention in terms of their listing as part of the environmental standards, but it has also been pointed out that there is some level of toxicity with regard to drinking water and aquatic life. Since lead inhibits the supply of metabolic products that acts as electron acceptors, it tends to inhibit the activity of nitrate reductase.6 They also reported that concentration of arsenic in surface water around agricultural field is high. In this study, water and sediments discharge of Zn, Cu, Cd, and Pb from farm land were examined using field data of 2012 rainy season. In particular, the study focused on the effect of fertilizer applications and rainfall runoff on the discharge of heavy metal from farmland.
Materials and Methods
The research was conducted in a continuous cropped arable experimental plots located at Faculty of Agriculture, University of Uyo Teaching and Research Farm (FAUUTRF), Afaha-Oku, Uyo, Nigeria. The area lies between latitudes 40 52’ and 50 3’N and longitudes 70 51’ and 80 20’ E and altitude 65 m from the sea level. The area is divided into two distinct seasons, the wet and dry seasons. The wet or rainy season begins from April and lasts till October. It is characterized by heavy rainfall of about 2500-4000 mm per annum. The rainfall intensity is very high and there is evidence of high leaching and erosion associated with slope and rainfall factors in the area.7 In the area measuring 0.5ha on a slope of 15 %, were prepared 12 plots; each 60 x 5 m2, separated from each other by foot tracts.
Application of Treatments
Poultry manure (Organic) of 20 t ha-1 and inorganic fertilizer (NPK) 300 kg ha-1.8 were allocated to the experimental plots that were replicated three times to measure heavy metal concentration between fertilizer types. The experimental field was cleared and seed bed was well prepared by ploughing and harrowing during the season. The well-cured poultry manure was applied at two weeks before planting by broadcasting and completely worked into the soil. While the inorganic fertilizer was applied 2 weeks after planting. One Cassava cutting was sown at 1m interval during the cropping seasons giving a total of 270 plants per plot and plant population of 3240 plants per 0.36 hectare planted area. A trench measuring 1.5m high and 1.2m wide were dug across each experimental plot to accommodate runoff collecting tanks that were installed there. There were three collecting tanks (90 cm high and 58cm in diameter) in each of the plots. The collecting tanks were placed directly under the three PVC pipes installed at the silled end of the weirs. At the bottom end of each collecting tank, just below 1cm above the floor of the tank, was a tap installed, through which the runoff water was discharged after taking the measurement.
Sampling Strategy Soil Sampling
A total of 36 soil samples (18 each) of poultry and inorganic fertilizer applied soils respectively from locations labeled P1, P2, P3,P4 P5 and N1, N2, N3, N4, N5 were collected from the soil surface using stainless steel auger. In addition these samples, control soil samples (C1, C2) were collected from the farmland. The collected samples were sieved through a 2 mm sieve and stored for pre-washed polyethene plastic bags for subsequent sample preparation and analysis. Physico-chemical characteristics of the soils; pH, organic matter, and heavy metal contents were determined using the methods described by Gupta9
Discharge Carried by Runoff Water
The discharges from different treatments carried by runoff water into the collecting tanks were sampled from each tank after every storm for determinations of heavy metals concentration from the farm. Before taking the aliquot from the tanks into 50 cl bottle, the runoff water was thoroughly stirred. The samples taken from each of the tanks were taken to the laboratory and allowed to settle for two to three days while the water in the tanks was drained for further use. The settled aliquot was further filtered with Whatman’s filter paper (No 42) and the sediment (residue) was put into a Petri-dish and oven dried. The dried sample was weighed and thereafter multiplied with the total runoff water in the collecting device to obtain the total weight of soil washed by runoff into the respective collecting devices. This amount was then added to the dry weight of the eroded soil collected from the ditch and expressed in milligram per kilogram soil /litres of water (mg*kg-1 andmg/l). The samples were then submitted for multi-element analysis in an accredited laboratory (Lasunnex laboratory Ltd. PHC).
Heavy Metal Determination
Inorder to measure the available contents of zinc, arsenic, cadmium and lead, a single extraction method EDTA leaching test was performed. This method is commonly used by agronomists to evaluate the phyto-availability fraction of metal from soils. Approximately, 0.2 g of the soil sample was weighed and put in a 100 cm3 conical flask. Fifty cubic centimeter 50 cm3 of disodium EDTA (0.05 M at pH 7.0) were added and shaken 10 revolution per minute for an hour on the shaker. The solutions were filtered through Whatman’s 0.45 filter paper and the filtrates were read for zinc, copper, cadmium and lead by Atomic Absorption Spectroscopy (AAS)
It is worthy of note that, the extractable fractions of these metals in soil are the possible content which can be taken up by the plant roots, while the amount in the runoff often give an idea of the size of pool that might be emptied into the water body and caused eutrophication.
Since selected soil properties of pH and organic matter play an important role in the retention and release characteristics of heavy metals, soil pH was measured in suspension (soil/water 1:2.5) and organic matter was analysed by the Walkley-Black method10
Statistical Analysis of Data
The concentrations of Zn, Cd, Cu, Pb and pH and organic matter in agricultural soil were analysed to assess an adequate distinction between different cases. Contents in water and soil were compared using the t-test. A significant difference between any pair of means of sample population is concluded when the probability has a difference by 0.05. Multivariate statistical techniques, ANOVA and discriminant analyses were performed using SPSS for Windows (release Ver. 11. Inc.Chicago).
Results and Discussion
Exploratory Data Analysis
Tables 1 and 2 provides summary statistics for the heavy metal, pH and organic matter contents in soil sample and runoff water from soil amended with poultry droppings and NPK fertilizer. The mean total pH and organic matter and heavy metals levels for both treatments are several orders of magnitude greater than the typical soil samples. The coefficients of skewness and kurtosis of the variables are higher in poultry and NPK amended soils in comparison with the coefficient of normal distribution. Therefore the medians are lower than the arithmetic means which is constant with high skewness, showing that there are some unusual high values. Also the negative skewness which is typical of environmental concentration especially in NPK amended soils indicate that 82 % of samples (soil and runoff) contain heavy metal concentrations which exceed the threshold value (5 mg/kg). The relative high CVs indicate that there is high variability at a local scale and that the variability is not distributed evenly over the area. Therefore, the variables under investigation illustrate two different behavours.
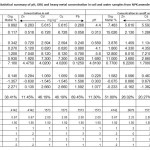 |
Table 1: Statistical summary of pH, ORG and heavy metal concentration in soil and water samples from N PK amended soil Click here to View table |
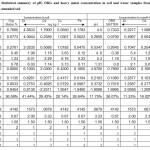 |
Table 2: Statistical summary of pH, ORG and heavy metal concentration in soil and water samples from poultry dropping amended soil Click here to View table |
Figure 1 Show the level of heavy metal concentrations, pH and organic matter levels in water and soil during the end of cropping season in soils amended with fertilizers. The acidity level in runoff water and soils was the same, whereas organic matter content was 300 % higher in the soil than in the runoff water. This implied that the organic matter was not easily removed from the soil during storms that resulted in overland flow. Zinc and cadmium concentration tended to be high in soil, while copper was observed to be more in runoff water washed from the farmland. On the other hand, lead was the only element with least concentration after the application of poultry droppings and NPK fertilizer.
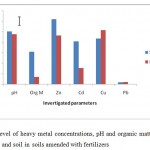 |
Figurre 1: Level of heavy metal concentrations, pH and organic matter levels in water and soil in soils amended with fertilizers Click here to View figure |
Soil pH and Organic Matter
Organic matter and pH have been found to be the most important factors that control the retention mobility of heavy metals in soil. Both parameters also influenced by landuse and agricultural practices. Soil reaction which is given in terms of pH value is a measure of the free hydrogen ion (H+)concentration of soil solution. The value of the free H+ concentration in a soil influences the availability of nutrient elements and biochemical reactions in the soil. Table 3 revealed that the acidity level of this soil significantly increased to slightly acidic when fertilizers were applied in the farm. According to Akinrinde,11 slightly acidity enhances basic cation uptake by plant roots as it does not inhibit the activities of beneficial soil micro organisms which are affected by soil reaction (strong acidity). The pH of the soils ranged from slightly acidic (amended soil) to moderately acidic (control). Soil pH is often considered in terms of the soil capability and suitability to support plant growth. The pH of 4.8 is set as the lower limit for optimum growth of plants, and conversely the pH of 9.5 is regarded as the extreme upper limit of alkalinity at which plants can still grow. Thus, the soils generally can support plant growth.
Soil organic matter usually mixed up with fine clay particles to form soil colloids. It is an important soil fraction due to its binding properties that enhanced most physical and chemical activities in the soil. Thus, there is increased contact with other colloids and with soil solution mostly poultry droppings were added to the soil. Organic matter in poultry droppings amended soils ranged from 0.45 (plot 3) to 1.23 cmol/kg (plot 1) and 0.38 (plot 2) to 1.18 mg/l (Station 1) in the runoff water, with average values of 4.858 cmol/kg. While in the NPK organic matter level in runoff water was higher than soil (12.8 mg/l). This control plots is low in organic matter (0.705 cmol/kg). Already, this has shown in the sparse cassava growth indicating low productivity and unstable soils.
 |
Figure 2: Territorial Map of Canonical Discriminant Functions in heavy metal contaminated farmland due to fertilizer applications. Click here to View figure |
Heavy Metal Contents in the Soil and Water at the end of Farming Season
The mineral element originates from soil and dissolved in water for plant roots’ absorption but those required in small quantity for optimum performance are regarded as trace elements The concentration of these metals can however be increased to become potential pollutants if heavy metals containing in agronomic activities are introduced into the environment.12 Concern over the presence of heavy metals in an environment arises from the fact that they cannot be broken down into non-toxic forms. Thus once aquatic ecosystems are contaminated by heavy metals, they remain a potential threat for many years.
The results obtained are presented in Table 3. Clearly indicate that the two treatments exhibit different concentrations of heavy metals. It is evident that the two amendments had high degree of variation of heavy metals level (canonical correlation is 0.893 and 0.801) between soil and runoff. Whereas the lower the degree of within-group variation (Wilks’ lambda statistics), the greater the within-group variation as a proportion of the total variation. The statistically significant of NPK results also demonstrate that the originally grouped cases have very high percentage (91.2%) of correct classification. For poultry droppings, however, the degree of heavy metal concentration is low both in soil and in runoff water, indicating that this variable is not statistically significant (p > 0.05) for discriminating the heavy metal concentration. Thus only lead and cadmium were found to discriminate in NPK amended soils.
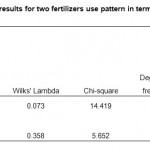 |
Table 3: Discriminant analyses results for two fertilizers use pattern in terms of heavy metal concentration Click here to View table |
All the samples analysed for zinc (Table 4) fell below the 1000 mg/kg level recorded for normal soil content13 This relatively low level of Zn could be the natural background level, and the relatively higher concentration of Zn in soil and water is attributed to the application of Zn containing fertilizers which have the effect of increasing the Zn levels of the soil. Cadmium level was found to be higher in soil and runoff water in both amended soils. Its toxicity level is evident resulting from poultry dropping (PD) and NPK fertilizer application. Surprisingly, Cd level is high when Zn level is low. This contradict the findings of Eggenberger and Waber14 that cadmium is usually present in the environment as minute impurities of Zn. Copper is important for the reproductive growth and root metabolism. A major property affecting the availability is soil pH, a measure of soil acidity or alkalinity. Cu tends to be less available in soils with high pH.15 The mean level of Cu concentration on application of fertilizers was significantly high when compared with the control (C) plot, leading to the conclusion that fertilizer application (organic or inorganic) affects Cu level in a farmland (PD-5.66, NPK-5.82, C- 2.76). Also greater percent of this metal is easily washed off during rainstorm into the stream (PD-5.90, NPK-6.06, C-4.70). Lead concentrations in soil were 0.232, 0.211 and 0.145 mg/kg and in runoff water, 0.12, 0.25, 0.17 mg/kg for Poultry droppings and NPK, amended soils and control respectively.
 |
Table 4: Statistical Estimates of pH, organic matter and heavy metal content among treatments in soil and water samples Click here to View table |
Discriminant Functions Combined with the Structure Matrix
The territorial map (Fig.2) revealed the relationships between the groups and the discriminant functions. Combined with the structure matrix results, it gives a graphical representation of the relationship between predictors (NPK and Poultry fertilizers) and heavy metals. The first function, shown on the horizontal axis, separates heavy metals among the treatments. Since Level of concentration is strongly positively correlated with the first function (NPK), this suggests that NPK amended soil is, in general, the most highly contaminated with heavy metals. The second function separates groups 1 and 3. pH and organic matter tend to have been the determining factor of heavy metal availability in the soil, although the map suggests that they tend to be well concentrated with a moderate amount of fertilizer application. Only two discriminant functions are plotted, but since the third function was found to be rather insignificant, the territorial map offers a comprehensive view of the discriminant model.
Conclusion
The results of the study revealed that the topsoil of the study area is heavily contaminated with heavy metals-; Zn, Cu, Cd and Pb when the farmland is amended with fertilizers either organic or inorganic. This suggests that water bodies in this environments and crops, which absorbed nutrients from the soil have high coefficient of heavy metal concentration particularly Pb and Cd. Moreover, this result point to the need to adopt precision agriculture which can increase yield while decreasing environmental impacts by managing fertilizer applications.
References
- Jaradat, Q.M, A.M. Massadeh, K.A. Momani and M. A.Al Saleem. 2010. The spatial distribution of Pb, Cd, Zn, and Cu in Agricultural Roadside Soils. Soil and sediment contermination. 19:58-71.
- Arizono, K. 1999. Heavy metal toxicity in water in water environment. Nihon Mizukankyo Gakkaishi. J. Japan. Soc. Water Environ., 22, 341-345
- Odu, C.T 2000. Rehabilitation of soils after oil spills. In Akoroda, M. O. (ed), Agronomy in Nigeria. Department of Agronomy, University of Ibadan, Nigeria. P.223-227.
- Hemalatha, S and Francis K. 2000. Lead inhibition of paddy and nitrate reductase. J. Environ. Bio., 21, 355-357
- FEPA 1991. National Interim Guidelines and Standard for industrial effluents, gaseous emission and hazardous waste management in Nigeria. Federal environmental protection agency. Nigeria. Pp. 95-107.
- Nunez-Delgado, B., Morrison L, Gulson, L 2002. Threshold of concern: a technique for evaluating environmental impact and amenity values. Journal of forestry 84-86
- Edem, I. D, U.C. Udoinyang and S.O. Edem 2012. Variability of Soil Physical Conditions along a Slope as Influenced by Bush Burning in Acid Sands. International Journal Of Scientific & Technology Research Volume 1, Issue 6, July 2012. 8-14
- Ibia, T.O. and Udo, E.J. 2011. Guide to fertilizer use for crops in Akwa Ibom state, Nigeria. Ministry of Agriculture and Natural Resources, Uyo, Akwa Ibom State, Nigeria
- Gupta, P.K. 2004. Methods in environmental analysis, water, soil, and air. Updesh Purohil for Agrobios (India)
- Walkey, A. and I. A Black. An Examination of the degtiar off method for determining soil organic matter a proposed modification of the chromic acid titration methods. Soil Sci 1934, 37:29-38
- Akinrinde, E.A. 2006. Issues of optimum nutrient supply for sustainable crop production in tropical developing countries. Pakistan journal of nutrition 5 (4): 377-387
- Cox, P.A.1995. The element of earths. Inorganic chemistry in environment. Oxford University Press, New York.
- National Special Programme for Food Security (NPSP) 2005. Critical values for nutrient elements
- Eggenberger, U. and Waber, H.N., 1998. Cadmium in sewage water of landfills. A statistical and geochemical evaluation, Report of November 20, 1997, for the OECD advisory group on risk management meeting, February 9-10, 1998.
- Aydinapl, C. and Marinowa, S. 2003. Distribution and form of heavy metals in some agricultural soils. Polish J. Environmental studies






