Cadmium Induced Histopathological Changes in the Intestine of Indian Flying Barb, Esomus danricus
Suchismita Das1 * and Abhik Gupta2
1
Department of Life science and Bioinformatics,
Assam University,
Silchar,
788011
India
2
Department of Ecology and Environmental Science,
Assam University,
Silchar,
788011
India
DOI: http://dx.doi.org/10.12944/CWE.8.2.17
Copy the following to cite this article:
Das S, Gupta A. Cadmium Induced Histopathological Changes in the Intestine of Indian Flying Barb, Esomus danricus. Curr World Environ 2013;8(2) DOI:http://dx.doi.org/10.12944/CWE.8.2.17
Copy the following to cite this URL:
Das S, Gupta A. Cadmium Induced Histopathological Changes in the Intestine of Indian Flying Barb, Esomus danricus. Curr World Environ 2013;8(2). Curr World Environ 2013;8(2). Available from: http://www.cwejournal.org/?p=4846
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-07-04 |
|---|---|
| Accepted: | 2013-08-13 |
Cd is a non essential heavy metal, mainly used for rechargeable nickel-cadmium batteries, pigments, coatings and plating, and as stabilizers for plastics.1 Cadmium naturally occurs in the aquatic environment, but is of no known biological use and is considered one of the most toxic metals.2 Inhalation of cadmium-containing fumes can result initially in metal fume fever but may progress to chemical pnemonitis, pulmonary edema,3 Itai-itai disease, renal abnormalities, including proteinuria and glucosuria and finally death.4 Cd has been shown to inhibit enzyme integrity, interfere with RNA and protein synthesis and to complex with DNA. In aquatic systems, cadmium quickly partitions to sediment, but is readily remobilized through a variety of chemical and biological processes.4,5 The Indian flying barb, Esomus danricus (Hamilton-Buchanan) is an economically important cyprinid fish which mostly inhabits shallow water bodies of Northern India. This fish, owing to its particular habitat, runs the risk of being exposed to water borne toxicants including, Cd. It is very much essential to devise a rapid method to detect the effects oftoxicants in various organs of fish and histopathology is one such effective tool.6 Amongst various organs, very little is known about the effects of Cd on the fish intestine which is believed to be the first organ that come into contact with food-borne contaminants.7 Also, the intestine is one of the most important sites where food enters and is assimilated. Thus, toxic substances, that enter the intestine, directly affect the vitality of the organism.8 Therefore intestine can serve as a potent indicator for water borne Cd. The present study was thus, aimed to determine the histopathological effects of chronic doses of Cd to Indian flying barb intestine.
Materials and Methods
Fishes of similar length (46.77 ± 4.30 mm) and weight (0.86 ± 0.16 g) were collected from unpolluted, freshwater ponds near Assam University campus, Barak valley, South Assam, India.9 They were acclimatized under laboratory conditions seven days prior to experimentation. Temperature, pH, hardness and dissolved oxygen under laboratory condition were 29°C, 6.8, 30 mg l-1 and 5.5 mg l-1 respectively. A stock solution of actual concentration of Cd was prepared using double distilled water. Serial dilutions of stock solutions were prepared using tap water as per dilution techniques.10 Three sub-lethal test concentrations viz., 636.3, 63.6 and 6.3 µgl-1 Cd were selected for inducing histological changes in fish intestine. Ten fish for each concentration of test chemical were kept separately in three litres of toxicant treated media for 28 days. Food was given during the study period. Test water was renewed every 24 hrs. After 28 days of exposure, fish were sacrificed and intestine was removed immediately and kept in 10% Formalin, as fixative, for 24 h, dehydrated, embedded in paraffin and sections cut at 5 µm thickness and stained with Harris Haematoxylin and Eosin. Changes induced by treatment in the intestinal tissues were photographed and analyzed by light microscope at 10X eye piece magnification and 40X objective magnification {Olympus (model U-CMAD3) with Camera attachment of Samsung (model SDC-313B)}. The work was done as per the Assam University Ethical guidelines on laboratory animal care.
Results
The intestine of Esomus danricus shows four layers of tissues namely serosa, muscularis, submucosa and mucosa. The outermost serosa is followed by a well developed muscularis (longitudinal and circular muscle) embedded in loose connective tissue richly supplied with blood capillaries. It merges with tunica propria of the underlying mucosal coat. The mucosa is raised into several longitudinal folds (Fig.1). In 636.3 µg l-1 of Cd administered E. danricus intestine after 28 days of exposure, superficial erosion of the mucosa, vacuolation and chronic inflammatory cell infiltration were seen. Lamina propria became dense (Fig. 2). For similar exposure duration, 63.6 µg l-1 of Cd exposed intestine showed mild mucosal lesion and infiltration of lymphocytes (Fig. 3). In 6.36 µg l-1 of Cd administered intestine, infiltrations of inflammatory cells (lymphocytes) were observed (Fig. 4).
 |
Figure 1: T.S of control Intestine of Esomus danricus: (a) epithelium (b) lamina propria (c) muscularis (d) serosa H&E, 400×. Click here to View figure |
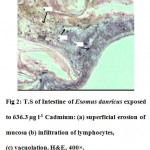 |
Figure 2: T.S of Intestine of Esomus danricus exposed to 636.3 µg l-1 Cadmium: (a) superficial erosion of mucosa (b) infiltration of lymphocytes, (c) vacuolation. H&E, 400×. Click here to View figure |
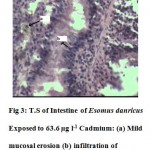 |
Figure 3: T.S of Intestine of Esomus danricus Exposed to 63.6 µg l-1 Cadmium: (a) Mild mucosal erosion (b) infiltration of lymphocytes. H&E, 400×. Click here to View figure |
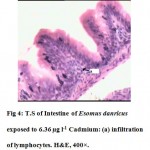 |
Figure 4: T.S of Intestine of Esomus danricus exposed to 6.36 µg l-1 Cadmium: (a) infiltration of lymphocytes. H&E, 400×. Click here to View figure |
Discussion
In the present study, the result of the effect of Cd on the gastrointestinal system of Esomus danricus clearly show that this heavy metal exert toxic effects on the different layers of intestine. The alterations in the intestine of the flying barb were more severe in higher doses. Toxic lesions most common in the intestine of fishes exposed to cadmium chloride include hyperemia, degenerative changes in the tips of villi, loss of structural integrity of mucosal folds, degenerative mucosal epithelium (hypertrophy, vacuolation, hyper-chromasia) necrosis, desquamation of mucosal epithelium, cellular debris, excessive mucus in gut of lumen, necrosis of submucosa and inflammatory infiltration of submucosa.11-13 Histopathological change due to Cd, like hypertrophy may lead to increased serum glucose in the intestine, as seen in estuarine teleost fish, and is possibly due to the fulfilment of extra energy requirement under stress condition.11 In another study, cadmium exposure caused degenerative changes in the tips of villi like hydropic degeneration, cloudy swelling and necrosis of intestine of Ophiocephalus striatus.14 In the intestine of Channa punctatus exposed to mercuric chloride, the cytoplasmic hyperchromasia, edema, loss of pepsinogen granules from chief cells, disintegration of glandular epithelium, desquamation of gastric mucosa in the stomach, hyperemia, degenerative changes in the tips of mucosal folds, hypertrophy and necrosis were observed.15 Similarly, intestinal toxic lesions, includes hyperemia, loss of structural integrity of mucosal folds, necrosis, cellular debris, vacuolation in intestine of Mugil auratus exposed to inorganic and organic mercury were also observed in another study.16 On exposure to another heavy metal, lead, hypersecretion of pepsin, leading to the degradation of tissue protein and increased ammonia and urea excretion by Channa punctutus was observed.17 Such conditions are possibly due to the extremely adverse effects on stomach of fish due to lead nitrate toxicity. Although, the present study did not include these findings, yet we observed severe erosion of mucosa at highest sublethal exposure dose of Cd, which might hamper the normal gastrointestinal physiology. Besides, profuse infiltration of lymphocytes were observed in flying barb intestine, which was a sign of chronic inflammation. Thus, Indian flying barb intestine was adversely affected by Cd exposure which in turn might have impaired the growth rate of fishes. Hence, it is suggested that this heavy metal in higher dosage will be lethal to fish population in general.
Acknowledgement
We wish to thank Prof Arabinda Das, former HOD, Dept. of Pathology, Silchar Medical College, Assam for providing microscope facility.
References
- Butterworth, R.G., Metal Toxicology. Springer, New York, p 118. (1995).
- De Conto Cinier, C. Petit Ramel, M. Faure, R. and Bortolato, M., Cadmium accumulation and metallothionein biosynthesis in Cyprinus carpio tissues. Bull Environ Contam Toxicol, 61: 793–799 (1998).
- Hayes, A.W., Principles and Methods of Toxicology. Taylor and Francis Publishing Inc. Philadelphia, fourth edition, (2001).
- Nogowa, K. Kobayashi, E. Okubo, Y. and Suwazono, Y., Environmental cadmium exposure, adverse effects, and preventative measures in Japan. Biometals, 17: 581-587, (2004).
- Nordberg, G.F., Cadmium and health in 21st century-historical remarks and trends for the future. BioMetals 17: 485–489, (2004).
- Johnson, L.L. Stehr, C.M. Olson, O.P. Myers, M.S. Pierce, S.M. Wigren, C.A. McCain, B.B. and Varanasi, U., Chemical contaminants and hepatic lesions in winter flounder (Pleuronectes americanus) from the Northeast Coast of the United States. Environ Sci Technol. 27: 2759-2771, (1993).
- Braunbeck, T. and Appelbaum, S., Ultrastructural alterations in the liver and intestine of carp Cyprinus carpio induced orally by ultra-low doses of endosulfan. Dis. Aquat. Organ. 36: 183–200, (1999).
- Hinton, D.E. and Lauren, D.J., Integrative histopathological approaches to detecting effects of environmental stressors on fishes in Biological Indicators of Stress in Fish (Adams, S.M. (Ed.). American Fisheries Symposium 8.American Fisheries Society, Bethesda, Maryland, pp 51–66. (1990).
- Das, S. and Gupta, A., Biometrics and growth features of Esomus danricus (Hamilton-Buchanan), from Barak Valley, South Assam. J. Inland Fish. Soc. India. 41: 81-83, (2009).
- APHA. Standard methods for the examination of water and wastewater. 21st Edn., Washington, DC: American Public Health Association, AWWA, WPCP, (2005).
- Gardner, G.R. and Yevich, P.P., Histological and haematological responses of an estuarine fish to cadmium. J. Fish Res. Board, Canada, 27: 2185-2196, (1970).
- Gutierrez, M. Establier, R. and Arias, A., Accumulation and histopathological effects of cadmium on the sapo Halobatracus didactylus. Invest. Pesq. 42: 141-154, (1978).
- Newman, M.W. and MacLean, S.A., Physiological response of the cunner, Tautogolabrus adspersus to cadmium.VI. Histopathology. National Oceanographic and Atmospheric Administration Technical Report. NMFS SSRF 681: 27, (1974).
- Bais, U.E. and Lokhande, M.V., Effect of Cadmium Chloride on Histopathological Changes in the Freshwater Fish Ophiocephalus striatus (Channa). Intl. J. Zoological Research, 8: 23-32, (2012).
- Sastry, K.V. and Gupta, P.K., Effects of merucuric chloride on the digestive system of Channa punctutus: A histopathological study. Environ. Res, 16: 270-278, (1978).
- Establier, R. Gutierrez, M. and Arias, A., Accumalation and histopathological effect of inorganic and organic mercury in the lisa Mugil auratus. Invest. Pesq. 42: 65-80, (1978).
- Sastry, K.V. and Gupta, P.K., Histopathological and enzymological studies on the effects of chronic lead nitrate intoxication in the digestive system of fresh water teleost Channa punctatus. Environ. Res. 17: 472-479, (1978).







