Drinking water assessment of 4 locations from Ghaziabad, Uttar Pradesh
Shikha Bisht1 * , B. A. Patra2 and Monika Malik1
1
Galgotias College of Engineering and Technology, 1,
Knowledge Park-2,
Greater Noida,
201306
India
2
Thermo Fisher Scientific, Thermo Electron LLS India Pvt. Ltd., 102, 104, Delphi “C†wing,
Hiranandani Business Park, Powai,
Mumbai,
400 076
India
DOI: http://dx.doi.org/10.12944/CWE.8.1.11
Copy the following to cite this article:
Bisht S, Patra B. A, Malik M. Drinking water assessment of 4 locations from Ghaziabad, Uttar Pradesh. Curr World Environ 2013;8(1) DOI:http://dx.doi.org/10.12944/CWE.8.1.11
Copy the following to cite this URL:
Bisht S, Patra B. A, Malik M. Drinking water assessment of 4 locations from Ghaziabad, Uttar Pradesh. Curr World Environ 2013;8(1). Curr World Environ 2012;8(1). Available from: http://www.cwejournal.org/?p=3074
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-12-20 |
|---|---|
| Accepted: | 2013-01-19 |
The district of Ghaziabad is situated in the middle of Ganga-Yamuna doab. It lies on the Grand Trunk road about a mile east of the Hindon river in Latitude 280 40' North and Longitude 770 25' East, 19 Kms. east of Delhi.1 Since a few years, drinking water problem has increased in the area. In this study, drinking water samples have been collected from 4 random locations from the area. Different parameters were examined using Indian Standards2 to find out their suitability for drinking purposes. During this examination, mainly the physico chemical parameters and the elemental concentrations were taken into consideration.
Material and Methods
Standard methods of collection, preservation and analysis were adopted. Grab sampling method was used for the collection of 4 drinking water samples. The locations were selected randomly in the district of Ghaziabad. The analysis of physiochemical parameters was done by procedure adopted from standard methods: APHA3 and the elemental analysis were done using ICPMS (Inductively Coupled Plasma Mass Spectrometer, PERKIN ELMER, Elan DRCe).
Results and Discussion
Sample 1, Lohia Nagar
All physiochemical parameters were found to be under the maximum permissible range for drinking purposes. This is with the exception of fluoride which was observed to be 3.723 ppm in the water sample analyzed. Fluoride is released into the ground water through weathering of primary silicates and associated accessory minerals.4 Mineral fluorides are present in underground water structures in the form of leachates from fluorospar, Apatite, Cryolite and fluorosilicates .5 When rain water percolates through the ground, fluoride ions are picked up 9. In arid regions with limited water recharge and with fluoride bearing minerals deposits present, the ground water becomes rich in fluoride.5 It combines with the hydrochloric acid of stomach and leads to the formation of hydrogen fluoride which is highly corrosive.6 Very low doses of fluoride (<0.6 mg/L) in water promote tooth decay. However, when consumed in higher doses (>1.5 mg/L), it leads to dental fluorosis or mottled enamel and excessively high concentration (>3.0 mg/L) of fluoride may lead to skeletal fluorosis.7
Sample 2, Sec-16
All physiochemical parameters were found to be under the maximum permissible range for drinking purposes. Except fluoride, the value for which was found to be 1.66 ppm.
Sample 3, Jatwara
All physiochemical parameters were found to be under the maximum permissible range for drinking purposes except nitrate and fluoride. The values for these were found to be 187.583 and 3.472 ppm respectively for the water sample analyzed. Such high concentration of nitrate in drinking water may be attributed to the leaching of organic material biodegradation products into water sources. Nitrate has long been associated with the occurrence of blue baby disease in infants8 or infantile methaemoglobinaemia, which is caused due to bacterial reduction of nitrate into nitrite in stomach.9
Sample 4, Sahibabad
The values for except Aluminium, Iron and fluoride were found to be high in the sample analyzed. The value for Aluminium was observed to be 228.88 ppb which is higher than the IS prescribed limit of 200 ppb. The values for Iron was recorded to be 4598.76 ppb and for fluoride as 4.68 ppm. All other physiochemical parameters were found to be under the maximum permissible range for drinking purposes. Long-term exposure to such high levels of Aluminum may lead to the occurrence of Alzheimer's disease.10 Aluminium accumulation in the brain is proposed to be associated with neurodegenerative diseases, including Parkinson’s disease, amyotrophic lateral sclerosis and dialysis encephalopathy .11 Aluminium negatively impacts neurotransmission, either by directly inhibiting the enzymes responsible or by affecting the physical properties of synaptic membranes.11 The principal forms of mineralized ferric iron found in soils are amorphous hydrous ferric oxide, maghemite, lepidocrocite, hematite,and goethite.12 High amount of Iron leads to the growth of iron bacteria in the pipelines thereby deteriorating the microbiological quality of drinking water.13 Excessive ingested iron can also cause excessive levels of iron in the blood because high iron levels can damage the cells of the gastrointestinal tract preventing them from regulating iron absorption.14
Conclusion
Drinking water from Lohia Nagar & Sector 16 could be used for drinking after removal of excess of fluoride. Also drinking water from Jatwara could be used for drinking after removal of excess of fluoride and nitrate. Drinking water from Sahibabad could also be used for drinking after removal of excess of fluoride, Aluminium and Iron.
 |
Table 1: List of methods and instruments used for physiochemical and elemental analysis. Click here to View table |
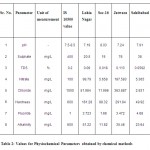 |
Table 2: Values for Physiochemical Parameters obtained by chemical methods Click here to View table |
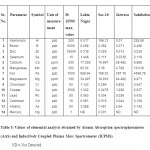 |
Table 3: Values of elemental analysis obtained by Atomic Absorption spectrophotometer (AAS) and Inductively Coupled Plasma Mass Spectrometer (ICPMS). ND = Not Detected Click here to View table |
Acknowledgement
References
- www.ghaziabad.nic.in, downloaded 11 October.
- IS 10500:2004, Drinking Water Specifications.
- APHA. Standard Methods for the examination of water and wastewater, Pg 2:26-2:29, 2:36-2:39, 2:54-2:56, 4:53-4:56, 4:66-4:68, 4:79-4:83, 4:86-4:91, 4:114-4:115, 4:176-4:179. American Public Health Association (1995).
- Apambire W. B., Boyle D. R. and Michel F. A., Geochemistry, genesis and health implications of floriferous ground waters in the upper regions of Ghana. Environ. Geol., 13-24, 33 (1997), http://dx.doi.org/10.1007/s002540050221
- Thakare S. B., Parvate A. V. and Rao M., Analysis of fluoride in the ground water of Akola district, Indian J. Environ. and Ecoplan, Vol. 10 No.3, 657-661 (2005).
- Manjunath D. L., Environmental studies 2ndedition, Pearson Publications, 150 (2008).
- Tiwari K., Dikshit R. P., Tripathi I. P. and Chaturvedi S. K., Fluoride content in drinking water and ground water quality in rural area of tahsil Mau, district Chitrakoot, Indian J. Environmental Protection, Vol. 23, No. 9, 1045-1050 (2003).
- Agrawal K. C., Environmental pollution causes, effects and controls, 56-83 (2001).
- Harrison Roy M., Pollution causes, effects and control 3rd edition, 54-65 (1996).
- Pandian M. Rajasekara, Banu G. Sharmila, Kumar G. and Smila K. H., Physico-chemical characteristics of drinking water in selected areas of Namakkal town (Tamil Nadu), India, Indian J. Environmental Protection, Vol. 10, No. 3, 789-792 (2005).
- Gonclaves P.P., Silva V. S., Does neurotransmission impairment accompany aluminum neurotoxicity? Journal of Inorganic Biochemistry, 1291-1338 (2007), http://dx.doi.org/10.1016/j.jinorgbio.2007.06.002
- Vance David B., Iron - the environmental impact of a universal element, National Environmental Journal, May/June Vol. 4 No. 3, 24-25 (1994).
- Goel P. K., Water pollution Causes, Effects and Control, 1-27, 97-115 (2001).
- El-Harbawi M., Sabidi A.A.B.T., Kamarudin E.B.T., Hamid A.B.A.B.D., Harun S. B., Nazlan A. B, Xi-Yi C., Design of a portable dual purpose water filter system. Journal of Engineering Science and Technology, 165-175 (2010).






