Chlorpyrifos toxicity in fish: A Review
Nobonita Deb1 and Suchismita Das1 *
1
Department of Life Science and Bioinformatics,
Assam University,
Silchar,
788 011
India
DOI: http://dx.doi.org/10.12944/CWE.8.1.17
Copy the following to cite this article:
Deb N, Das S. Chlorpyrifos toxicity in fish: A Review. Curr World Environ 2013;8(1) DOI:http://dx.doi.org/10.12944/CWE.8.1.17
Copy the following to cite this URL:
Deb N, Das S. Chlorpyrifos toxicity in fish: A Review. Curr World Environ 2013;8(1). Available from: http://www.cwejournal.org/?p=3107
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-01-24 |
|---|---|
| Accepted: | 2013-02-14 |
Chlorpyrifos (O,O-diethyl-O-3,5,6-trichlor-2-pyridyl phosphorothioate; CPF) is a broad spectrum organophosphate insecticide (OP) that is commercially used to control foliar insects that affect agricultural crops1 and subterranean termites2 CPF, since it was first introduced into the marketplace in 1965, has been used globally as an insecticide to control pests agriculturally and in the home. It is the second largest selling OP and found to be more toxic to fish than organochlorine compounds.3 Earlier reports revealed that fish kill incidents in association with chlorpyrifos in water reaching several hundred parts per billion.4 CPF has an average soil half-life of 30 days and two months in less alkaline soils. It also can persist indoors for weeks to months.5 CPF passes via air drift or surface runoff into surrounding waters and gets accumulated in different aquatic organisms, particularly fish, adversely affecting them.6 This OP insecticide is known to inhibit acetylcholinesterase, which plays an important role in neurotransmission at cholinergic synapses by rapid hydrolysis of neurotransmitter acetylcholine to choline and acetate.7 The inhibitory effects of CPF insecticide is dependent on its binding capacity to the enzyme active site and by its rate of phosphorylation in relation to the behaviour and age8,9 and is widely used for rapid detection to predict early warning of pesticide toxicity.10 It is reported to be activated by contact, ingestion and vapour action, causing convulsions and paralysis. CPF can enter the body either by inhalation of air containing CPF, ingestion of contaminated food or by dermal contact with CPF. It can cause acute poisoning and well known symptoms include myosis, increased urination, diarrhoea, diaphoresis, lacrimation and salivation.11 It is also reported to be involved in multiple mechanisms like causing hepatic dysfunction,12 genotoxicity,13 neurobehavioral and neurochemical changes.14 CPF intoxication is shown to cause a significant decrease in the reduced glutathione (GSH), catalase (CAT) and glutathione S-transferase (GST) activities.15 Although the United States Environmental Protection Agency (USEPA) has terminated indoor residential use since 2000, CPF is still one of the most widely used insecticides, and more than 8 million pounds of CPF are used each year for agricultural purposes in the United States.16,17 CPF is also widely used in agriculture as the substitutes for methamidophos and parathion in China, and has become one of the major pesticides detected in farm products.18,19 Since agricultural uses on orchards and row crops persist, CPF has been frequently detected in air, food and water. Although various standards exist to minimize its exposure in food and water, CPF is frequently used and bio-accumulates in certain scenarios.6,20
Fish are probably the most important non-target victims of pesticide over exposure as they have an important role in food chain. The present study thus, aims at reviewing existing literatures on the probable adverse effects of pesticide chlorpyrifos in fish.
Structure of Chlorpyrifos
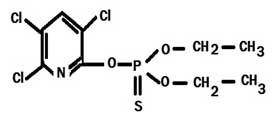
Common Name
CPF is also known by its trade names Dursban and Lorsban
Acute Toxicity
96 h LC50 values for technical grade CPF in Bluegill sun fish, Lepomis macrochirus, is 3.3 ppb, in rainbow trout, Oncorhynchus mykiss is 3ppb and for channel catfish, Ictalurus punctatus is13.4 ppb. 96 h LC50 values for 97.0% CPF (active ingredient, a.i.) in lake trout, Salvelinus namaycush at pH 6.0 is 140ppb, at pH 7.5 is 98 ppb and at pH 9.0 is 205 ppb while for cutthroat trout, Salmo clarki, at pH 7.5 96 h LC50 is 18.4 while at pH 9.9 it is 5.4 ppb. 96 h LC50 values for 99.9% CPF (a.i.) in fathead minnow, Pimephales promelas is 203ppb, while 96 h LC50 values for 99.0% CPF (a.i) in golden shiner, Notemigonus crysoleucas is 35ppb.21 The 96 h LC50 values of CPF in juvenile and adult of Oreochromis niloticus were determined to be 98.67 µgL−1 and 154.01 µgL−1, respectively, which reveals that CPF can be rated as highly toxic to fish.22 The 96 h LC50 value of CPF to Poecila reticulata was found to be 0.176 ppm/L.23 LC50 for 96 h in mosquito fish, Gambusia affinis was found to be 297mg/ L24 and LC50 value of chlorpyrifos in common carp was found to be 580 µg/L.25 In addition, during toxic stress of CPF, several behavioural anomalies like gulping, increased opercular movement, erractic swimming and subsequent lethargy can be observed.23
Developmental Effects
A variety of studies have shown that exposure to CPF during development can cause persisting neurobehavioural dysfunction, even with low doses that do not elicit acute toxicity. CPF exposure during early development in zebrafish caused long lasting neurobehavioral deficits. Effects of CPF on zebrafish hatchling’s swimming behaviour were studied.26 Results show that a persistent behavioural impairment was caused that lasts into adulthood. This early behavioural effect can be used to help determine which critical molecular mechanisms of CPF underlie the behavioural impairment.26 Zebrafish, with their clear chorion and extensive developmental information base, provide an excellent model for assessment of molecular processes of toxicant-impacted neurodevelopment.26 Recently, it was found that embryonic exposure of zebrafish to CPF causes significant impairment in discrimination learning and swimming speed27 The results of the study indicate that the administration of sub-chronic dose of 1 µM CPF to zebrafish larvae from 0 to 7 day post fertilization significantly impacted body morphology.28 The results also show that even extremely low sub-chronic doses of CPF administered induced specific behavioural defects or morphological deformities.
Neurotoxic Effects
CPF can produce neurotoxic effects. Several studies have assessed the cognitive alterations after acute or chronic exposure to CPF in mammalian models29,30 but in fish the data available are sparse. CPF causes persistent neurobehavioral impairment in zebrafish, where tests of sensorimotor response (tap startle response and habituation), stress response (novel tank diving test) and learning (3-chamber tank spatial discrimination) were conducted with adult zebra fish after early developmental CPF exposure. The study demonstrated that CPF caused selective long-term neurobehavioral alterations in zebrafish.31 In another study, a significant decrease in whole brain activity of zebrafish after exposure to CPF has been found.32
AChE Activity
Currently, reports on the toxicity of CPF on AChE activity in aquatic species focused on acute toxicity, but there are few reports on subchronic toxicity of this pesticides on AChE activity in aquatic species. CPF is well known as an AChE inhibitor.33 Symptoms of high level exposure to OPs include muscle twitching, hyperactivity, paralysis, loss of equilibrium and eventually death34 whereas low level exposures have been implicated in various behavioural and physiological impairments.34 Acetylcholinesterase (AChE) and carboxylesterase (CbE) have been used as a specific biomarker for pesticides.24,35 AChE in fish is mainly cholinergic and its activity is essential for normal behaviour and muscular function.36 The acute systemic toxicity of CPF is caused by inhibition of cholinesterase through the active metabolite chlorpyrifos oxon, chlorpyrifos is more toxic in immature animals despite their ability to recover rapidly from cholinesterase inhibition. Chlorpyrifos itself, rather than chlorpyrifos oxon is stated, directly targets events that are specific to the developing brain and that are not necessarily related to inhibition of cholinesterase. Acetylcholinesterase is critical to the normal development of the zebrafish nervous system,37 therefore zebrafish studies of the neurobehavioral teratology of acetylcholinesterase inhibitors like CPF are particularly relevant. CPF was a potent inhibitor of AChE activity in fingerling channel catfish.38 In a study with Gambusia affinis exposed to lethal concentration of CPF for 96 h, an inhibition of AChE activity was observed.24 The inhibition of AChE leading to the accumulation of ACh at synaptic junctions might have been altered the locomotor behaviour of exposed fish. A positive correlation was found between the recovery pattern of AChE activity and locomotor behaviour. The current findings clearly illustrated that the locomotor behaviour of test organism as a promising tool in ecotoxicology, to assess the recovery status of test organism after adverse affects. In a study in Poecila reticulate, brain AChE showed dose-dependent inhibition in fish. Exposure to the higher concentrations of CPF showed upto 66% inhibition of AChE.23
Likewise, CbE is widely distributed in living organisms and its physiological role is still unclear, but it might be related to lipid metabolism and steroidogenesis.39 Some environmental pollutants found to inhibit AChE activity may also inhibit CbE activity40-42 demonstrated that muscle ChE of mosquito fish was more sensitive than brain to chlorpyrifos exposure. In a study on carp, CbE activity exhibited a dose–response relationship, with activity decreasing with increasing CPF. Effects of CPF on carp suggest toxic action of low dose CPF on fish recovered after a period of time and toxic action of high dose CPF on fish which was not easily recovered, but the relevant mechanism need further study.43
Behavioural Toxicity
Behaviour is also considered a promising tool in ecotoxicology behaviour44 is an integrated result of endogenous and exogenous processes and low level of exposures have been implicated in various behavioural and physiological impairments.34 In a study in zebrafish, persisting behavioral dysfunction after developmental CPF exposure was observed.31,26 Swimming behaviour of fish is frequently observed as a response in toxicity investigations because altered locomotor activity can indicate effects to the nervous system. In a study, the exposure of Poecila reticulata to CPF resulted in the exhibition of aggressive behaviour, rapid gulping of water, increased opercular movement and abnormal and erractic swimming movements. Fish was stressed progressively with time before death. They were lethargic and at the time of death exhibited transient hyperactivity before collapsing.23
Oxidative Stress
For the last decade pesticide induced oxidative stress has also become a very popular of toxicological research as a possible mechanism of toxicity.45,46 An antioxidant defense system (ADS) is needed to protect biomolecules from the harmful effects of reactive oxygen species (ROS). Fish are endowed with defensive mechanisms to neutralize the impact of reactive oxygen species (ROS) resulting from the metabolism of various chemicals. These include various antioxidant defense enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPOx), glutathione S-transferase (GST), and glutathione reductase (GR). Low molecular weight antioxidants such as glutathione (GSH), ascorbate (vitamin C), and vitamin A are also reported to contribute in the quenching of oxyradicals.47 ROS which is not neutralized by this antioxidant defense system damages all biomolecules. One of the most important targets of ROS is the membrane lipids which undergo peroxidation (LPO). Thus, LPO estimation has also been successfully employed to signify oxidative stress induced in aquatic animals by such chemicals.48,49 Pesticides may cause generation of reactive oxygen species (ROS), which may lead to oxidative stress, indicating the role of ROS in pesticide toxicity.50 Pesticide induced oxidative stress has been also a focus of toxicological research for the last decade as a possible mechanism of toxicity.51,45 The antioxidants in fish could be used as biomarkers of exposure to aquatic pollutants.52 Lipid peroxidation (LPO) is one of the molecular mechanisms involved in pesticide toxicity.53 In Gambusia affinis exposed to lethal concentration of CPF for 96 h, an elevated lipid peroxidation level was observed.24 The same study found decreased levels of antioxidant enzyme (SOD, CAT and GR) activities in the exposed fish can also be effectively used for better assessment of chlorpyrifos toxicity in biomonitoring of aquatic environment. The treatment of Oreochromis niloticus with chlorpyrifos results in an increase in GST activity.22 Inductive effects on GST activity have been observed in studies with Salmo trutta after propiconazole exposure.54 GST-mediated conjugation may be an important mechanism for detoxifying peroxidized lipid breakdown products which have a number of adverse biological effects when it presents in higher amounts.55 Induced GST activity indicates the role of this enzyme in protection against the toxicity of xenobiotic-induced lipid peroxidation. Elevated GST activity may reflect the possibility of better protection against pesticide toxicity. In guppy, Poecila reticulate exposed for 96 h to different sublethal concentrations of CPF,estimated the oxidative stress-induction potential in brain, liver and gill tissues. MDA content is induced in all tissues but maximum rise was observed in gills (153% for CPF). In the same study, with regard to antioxidant defense system (ADS), GSH level showed increase in brain and gills CPF treated (23% and 21% respectively). CAT, GST, GR and SOD levels fluctuated in all treatment groups relative to the control. Collective findings demonstrated that pesticide exposure of fish induced an increase in MDA and fluctuated ADS along with inhibited AChE activity.
Endocrine Function
Chlorpyrifos can interfere with steroid hormone production. CPF is suspected as triggers for harmful effects on the reproductive system in fish. In Tilapia, Oreochromis niloticus, CPF exposure decreased serum estrogen and testosterone levels. Estradiol level after 15 days of exposure decreased by 60.45%, 48.65%, 56.93% after 5, 10, 15 ppb chlorpyrifos treatments.22 Cortisol, a corticosteroid hormone, is considered to be an important physiological effector of homeostasis in all vertebrates, through its effects on metabolism and immune function.56 Cortisol level in Oreochromis niloticus was found to be lower than that of control level after 10 ppb (59.97%) and 15 ppb (39.41) chlorpyrifos treatments.22
Genotoxicity and Mutagenic Effect
The genotoxic properties of CPF have been studied in a variety of assays in the past, but the results were contradictory.57,58 Since there is growing a concern over the presence of genotoxins in the aquatic environment, the development of sensitive biomarkers for detection of genotoxic effects in aquatic organisms has gained importance.59 It has been reported to be genotoxic in Channa punctatus.60 The exposure to 0.08 lg/l of CPF caused reproductive impairment in Daphnia magna.61 In a study on Channa punctatus, it was observed that CPF produced a concentration- dependent increase in DNA single-strand breaks in the form of comet induction and a time-dependent decrease in the damage, due to the DNA repair.62 The decrease in DNA damage has been observed in the tissues of fishes exposed to different concentrations of CPF, although the decrease was non-linear, which may indicate repair of damaged DNA, loss of heavily damaged cells, or both.63 This inverse relationship between time of exposure and DNA damage may be due to toxicity of contaminants that could disturb the enzymatic processes in the formation of DNA damage.64 Another possible explanation could be the gene activation of metabolizing enzymes such as cytochrome P450 in various tissues that provides a defensive mechanism against the persistent organic pollutants.65
Histopathological Changes
Morphological alterations in fish livers and gills are useful biomarkers to indicate prior exposure to environmental stressors or toxicants. Although the liver is the main organ of detoxification, due to their lipophilicity, CPF have a high rate of gill absorption; this could be a contributing factor in the sensitivity of the fish to this pesticide exposure.62 The aberrant hepatocytes could disturb the normal metabolism of the organisms, thereby inducing diseases or even death. Gills, on the other hand, are extremely important in respiration, osmoregulation, acid–base balance and excretion of nitrogenous wastes in fish, and they are the first area of contact of the animal with the external environment. Therefore, gill morphology is considered a useful indicator in environmental monitoring.
In a study on common carp, CPF altered the structure of the gills and liver. The liver tissue of common carp revealed different degree of hydropic degeneration, vacuolisation, pyknotic nuclei, and fatty infiltration while the gills of common carp displayed varied degrees of epithelial hypertrophy, telangiectasis, oedema with epithelial separation from basement membranes, general necrosis, and epithelial desquamation.66
Conclusion
The present paper reviews the works on effects of CPF in fish. All the workers were of the opinion that widespread use of this pesticide for agricultural as well as domestic purposes can adversely affect the non-target organisms like fish. Investigations on the effects of pesticide on fish have diagnostic significance as the results obtained can be used to predict probable mechanisms of toxicity in human. Besides, fish have proven to be useful experimental models for the evaluation of the health of aquatic ecosystems exposed to environmental pollution and the associated biochemical changes. It was found that the primary toxicity associated with acute exposure to CPF pesticides is acetylcholinesterase inhibition in cholinergic synapses and at neuromuscular junctions. Besides, oxidative stress, disruption of endocrine system, behavioural, neuro and developmental toxicity are some of the probable manifestations of CPF toxicity in fish.
References
- Rusyniak, D.E. and Nanagas, K.A. (2004) Organophosphate poisoning. Semen. Neurol. 24: 197–204, http://dx.doi.org/10.1055/s-2004-830907
- Venkateswara Rao, J. Parvati, K. Kavitha, P. Jakka, N.M. and Pallela, R. (2005) Effect of chlorpyrifos and monocrotophos on locomotor behaviour and acetylcholinesterase activity of subterranean termites, Odontotermes obesus. Pest. Manage. Sci. 61: 417–421, http://dx.doi.org/10.1002/ps.986
- Tilak, K.S. Veeraiah, K. and Ramanakumari, G.V. (2001) Toxicity and effect of chloropyriphos to the freshwater fish Labeo rohita (Hamilton). Neurol Research 20: 438–445.
- AbdelHalim, K.Y. Salama, A.K. Elkhateeb, E.N. and Barky, N.M. (2006) Organophosphorus pollutants (OPP) in aquatic environment at Damietta Governorate, Egypt: implications for monitoring and biomarker responses. Chemosphere 63: 1491–1498, http://dx.doi.org/10.1016/j.chemosphere.2005.09.019
- Arcury, T.A. Grzywacz, J.G. Barr, D.B. Tapia, J. Chen, H. and Quandt, S.A. (2007) Pesticide urinary metabolite levels of children in eastern North Carolina farm worker households. Environ Health Perspect.115:1254–60, http://dx.doi.org/10.1289/ehp.9975
- Varó, I. Serrano, R. Pitarch, E. Amat, F. López, F.J. and Navarro, J.C. (2002) Bioaccumulation of chlorpyrifos through an experimental food chain: study of protein HSP70 as biomarker of sublethal stress in fish. Arch Environ Contam Toxicol. 42:229–235, http://dx.doi.org/10.1007/s00244-001-0013-6
- Kwong, T.C. (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther. Drug. Monit. 24: 144–149, http://dx.doi.org/10.1097/00007691-200202000-00022
- Richmonds, C.R. and Dutta, H.M. (1992) Effect of malathion on the brain acetylcholinesterase activity of bluegill sunfish Lepomis macrochirus. Bull. Environ. Contam. Toxicol. 49: 431- 435, http://dx.doi.org/10.1007/BF01239648
- Dutta , H.M. Munshi, J.S.D. Dutta, G.R. Singh, N.K. Adhikari, S. and Richmonds, C.R. (1995) Age related differences in the inhibition of brain acetylcholinesterase activity of Heteropneustes fossillis (Bloch) by malathion. Comp. Biochem. Physiol. C 111: 331–334, http://dx.doi.org/10.1016/0300-9629(94)00166-Q
- Dutta, H.M. and Arends, D.A. (2003) Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environ. Res. 91: 157–162, http://dx.doi.org/10.1016/S0013-9351(02)00062-2
- Samsum, T.E. Hunter, D.L. and Bushnell, P.J. (2005) Effect of chronic dietary and repeated acute exposure to chlorpyrifos on learning and sustained attention in rats, Toxicological Sciences 87: 460–468, http://dx.doi.org/10.1093/toxsci/kfi264
- Poet, T.S. Wu, H. Kousba, A.A. and Timchalk, C. (2003) In vitro rat hepatic and intestinal metabolism of the organophsophate pesticides chlorpyrifos and diazinon. Toxicological Sciences 72: 193–200, http://dx.doi.org/10.1093/toxsci/kfg035
- Mehta, A. Verma, R.S. and Srivastava, N. (2008) Oxidative DNA damage induced by chlorpyrifos in rat tissues. Environmental and Molecular Mutagenesis 49: 426–433, http://dx.doi.org/10.1002/em.20397
- Slotkin, T.A. Olivier, C.A. and Seidler, F.J. (2005) Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Brain Research Development 157: 172–180, http://dx.doi.org/10.1016/j.devbrainres.2005.04.001
- Goel, A. Danni, V. and Dhawan, D.K. (2005) Protective effects of zinc on lipid peroxidation Antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chemico-Biological Interactions 156: 131–140, http://dx.doi.org/10.1016/j.cbi.2005.08.004
- USEPA (2011a). Chlorpyrifos: Preliminary Human Health Risk Assessment for Registration Review. DP No. D388070.
- USEPA (2011b). Revised Chlorpyrifos Preliminary Reg Review Drinking Water Assessment. DP No. D368388,389480.
- Chen, C. Li, Y. Chen, M. Chen, Z. And Qian, Y. (2009) Organophosphorus pesticide residues in milled rice (Oryza sativa) on the Chinese market and dietary risk assessment. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 26:340–7, http://dx.doi.org/10.1080/02652030802524516
- Sun, F. and Chen, H.S. (2008) Monitoring of pesticide chlorpyrifos residue in farmed !sh: investigation of possible sources. Chemosphere.71:1866–9, http://dx.doi.org/10.1016/j.chemosphere.2008.01.034
- Jantunen, A.P. Tuikka, A. Akkanen, J. and Kukkonen, J.V. (2008) Bioaccumulation of atrazine and chlorpyrifos to Lumbriculus variegatus from lake sediments. Ecotoxicol Environ Saf. 71: 860–8, http://dx.doi.org/10.1016/j.ecoenv.2008.01.025
- Barron, M.G. and Woodburn, K.B. (1995). Ecotoxicology of chlorpyrifos. Rev. Environ. Contam.Toxicol. 144:1-93, http://dx.doi.org/10.1007/978-1-4612-2550-8_1
- Oruç, E.O. (2010) Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pesticide Biochemistry and Physiology 96:160–166, http://dx.doi.org/10.1016/j.pestbp.2009.11.005
- Sharbidre, A.A. Metkari,V. and Patode, P. (2011) Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish, Poecilia reticulate. Pesticide Biochemistry and Physiology 10: 132–141, http://dx.doi.org/10.1016/j.pestbp.2011.09.002
- Kavithaa, P. and Venkateswara Rao, J. (2008). Toxic effects of chlorpyrifos on antioxidant enzymes and target enzyme acetylcholinesterase interaction in mosquito fish, Gambusia affinis. Environ. Toxicol. Pharmacol. 26: 192–198, http://dx.doi.org/10.1016/j.etap.2008.03.010
- Xing, H. Wang, X. Sun, G. Gao, X. Xu, S. and Wang, X. (2011) Effects of atrazine and chlorpyrifos on activity and transcription of glutathione S-transferase in common carp (Cyprinus carpio L.). Environ. Toxicol. Pharmacol. 33: 233–244, http://dx.doi.org/10.1016/j.etap.2011.12.014
- Levin, E.D. Swain, H.A. Donerly, S. and Linney, E. (2004) Developmental chlorpyrifos effects on hatchling zebrafish swimming behaviour. Neurotoxicology and Teratology 26: 719–723, http://dx.doi.org/10.1016/j.ntt.2004.06.013
- Levin, E. Crysthansis, E. Yacisin, K. and Linney, E. (2003) Chlorpyrifos exposure of developing zebrafish: Effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicology and Teratology 25: 51– 57, http://dx.doi.org/10.1016/S0892-0362(02)00322-7
- Richendrfer, H. Pelkowski, S.D. Colwill, R.M. and Créton, R. (2012) Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicology and Teratology 34: 458–465, http://dx.doi.org/10.1016/j.ntt.2012.04.010
- Braquenier, J.B. Quertemont, E. Tirelli, E. and Plumier, J.C. (2010) Anxiety in adult female mice following perinatal exposure to chlorpyrifos. Neurotoxicol Teratol. 32: 234–9, http://dx.doi.org/10.1016/j.ntt.2009.08.008
- Middlemore-Risher, M.L. Buccafusco, J.J. Terry, Jr A.V. (2010) Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol Teratol 32: 415–24, http://dx.doi.org/10.1016/j.ntt.2010.03.008
- Sledge, D. Yen, J. Morton, T. Dishaw, L. Petro, A. Donerly, S. Linney, E. and Levin, E.D. (2011) Critical duration of exposure for developmental chlorpyrifos-induced neurobehavioral toxicity. Neurotoxicology and Teratology 33: 742–751, http://dx.doi.org/10.1016/j.ntt.2011.06.005
- Eddins, D. Cerutti, D. Williams, P. Linney, E. and Levin, E.D. (2010) Developmental chlorpyrifos causes behavioral and neurochemical defects in zebrafish. Neurotoxicol Teratol. 32: 99–108, http://dx.doi.org/10.1016/j.ntt.2009.02.005
- Taylor, B. and Brown, J.H. (1999). Acetylcholine. In: Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D. (Eds.), Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. LippincottRaven, Philadelphia, PA, pp. 213–242.
- Sandahl, J.F. Baldwin, D.H. Jenkins, J.J. and Scholz, N.L. (2005). Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ. Toxicol. Chem. 24: 136–145, http://dx.doi.org/10.1897/04-195R.1
- Leticia, A.G. and Derardo, G.B. (2008). Determination of esterase activity and characterization of cholinesterases in the reef fish Haemulon plumieri. Ecotoxicol. Environ. Saf. 71: 787–797, http://dx.doi.org/10.1016/j.ecoenv.2008.01.024
- Kirby, M.F. Morris, S. Hurst, M. Kirby, S.J. Neall, P. Tylor, T. And Fagg, A. (2000) The use of cholinesterase activity in flounder (Platichthys flesus) muscle tissue as a biomarker of neurotoxic contamination in UK estuaries. Mar. Pollut. Bull. 40: 780–791, http://dx.doi.org/10.1016/S0025-326X(00)00069-2
- Behra, M. Cousin, X. Bertrand, C. Vonesch, J. Biellmann, D. Chatonnet, A. and Strahle, U. (2002) Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo, Nature Neuroscience 5: 111– 118, http://dx.doi.org/10.1038/nn788
- Straus, D.L. and Chambers, J.E. (1995) Inhibition of acetylcholinesterase and aliesterases of fingerling channel catfish by chlorpyrifos, parathion, and S,S,Stributyl phosphorotrithioate (DEF). Aquat. Toxicol. 33: 311–324, http://dx.doi.org/10.1016/0166-445X(95)00024-X
- Li, M.H. (2008). Effects of nonylphenol on cholinesterase and carboxylesterase activities in male guppies (Poecilia reticulata). Ecotoxicol. Environ. Saf. 71: 781-786, http://dx.doi.org/10.1016/j.ecoenv.2008.02.014
- Küster, E. (2005) Cholin and carboxylesterase activities in developing zebrafish embryos (Danio rerio) and their potential use for insecticide hazard assessment. Aquat. Toxicol. 75: 76–85, http://dx.doi.org/10.1016/j.aquatox.2005.07.005
- Boone, J.S. and Chambers, J.E. (1997) Biochemical factors contributing to toxicity differences among chlorpyrifos, parathion, and methyl parathion in mosquitofish (Gambusia affinis). Aquat. Toxicol. 39: 333, http://dx.doi.org/10.1016/S0166-445X(97)00019-2
- Carr, R.L. Ho, L.L. and Chambers, J.E. (1997) Selective toxicity of chlorpyrifos to several species of fish during an environmental exposure: biochemical mechanisms. Environ. Toxicol. Chem. 16: 2369, http://dx.doi.org/10.1002/etc.5620161124
- Xing, H. Wang, J. Li, J. Fan, Z. Wang, M. and Xu, S. (2010) Effects of atrazine and chlorpyrifos on acetylcholinesterase and Carboxylesterasein brain and muscle of common carp. Environmental Toxicology and Pharmacology 30: 26–30, http://dx.doi.org/10.1016/j.etap.2010.03.009
- Cohn, J. and MacPhail, R.C. (1997) Chlorpyrifos produces selective learning defecits in rats working under a schedule of repeated acquisition and performance. J Pharmacol Exp Ther. 283: 312–20.
- Abdollahi, M. Ranjbar, A. Shadnia, S. Nikfar, S. and Rezaie, O.E. (2004) Pesticides and oxidative stress: A review. Medical Science Monitoring 10:141– 147.
- Sharma, Y. Bashir, S. Irshad, M. Gupta, S.D. and Dogra, T.D. (2005) Effects of acute dimethoate administration on antioxidant status of liver and brain of experimental rats, Toxicol. 206: 49–57, http://dx.doi.org/10.1016/j.tox.2004.06.062
- Orbea, A. Ortiz Zarragoitia, M. Sole, M. Porte, C. and Cajaraville, M.P. (2002) Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish form the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat. Toxicol. 58: 75–98, http://dx.doi.org/10.1016/S0166-445X(01)00226-0
- Favari, L. Lopez, E. Martinez-Tabche, L. and Diaz-Pardo, E. (2002) Effect of insecticides on plankton and fish of Ignacio Ramirez reservoir (Mexico): a biochemical and biomagnification study. Ecotoxicology and Environmental Safety 51:177–186, http://dx.doi.org/10.1006/eesa.2002.2142
- Monserrat, J.M. Geracitano, L.A. Pinho, G.L.L. Vinagre, T.M. Falerios, M. Alciati, J.C.and Bianchini, A. (2003)Determination of lipid peroxides in invertebrates tissues using the Fe(III) xylenol orange complex formation, Archives of Environmental Contamination and Toxicology 45: 177–183, http://dx.doi.org/10.1007/s00244-003-0073-x
- Sayeed, I. Parvez, S. Pandey, S. BinHafeez, B. Haque, R. and Raisuddin, S. (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish Channa punctatus Bloch. Ecotoxicol. Environ. Saf. 56: 295–301, http://dx.doi.org/10.1016/S0147-6513(03)00009-5
- Akhgari, M. Abdollahi, M. Kebryaeezadeh, A. Hosseini, R. and Sabzevari, O. (2003) Biochemical evidence for free radicalinduced lipid peroxidation as a mechanism for subchronic toxicity ofmalathion in blood and liver of rats. Hum. Exp. Toxicol. 22: 205–211, http://dx.doi.org/10.1191/0960327103ht346oa
- Ahmad, I. Hamid, T. Fatima, M. Chand, H.S. Jain, S.K. Athar, M. and Raisuddin, S. (2000). Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim. Biophys. Acta 1523: 37–48.
- Kehrer, J.P. (1993). Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 23: 21–48, http://dx.doi.org/10.3109/10408449309104073
- Egaas, E. Sandvik, M. Fleld, E. Kallqvist, T. Goksoyr, A. and Svensen, A. (1999) Some effects of the fungicide propiconazole on cytochrome P450 and glutathione S-transferase in brown trout (Salmo trutta). Comp. Biochem. Physiol. 122: 337–344.
- Leaver, M.J. and George, S.G. (1998) A piscidine glutathione S-transferase which efficiently conjugates the end products of lipid peroxidation, Mar. Environ. Res. 46: 71–74, http://dx.doi.org/10.1016/S0141-1136(97)00071-8
- Hontela, A. Daniel, C. and Rasmussen, J.B. (1997) Structural and functional impairment of the hypothalomo–pituitary–interrenal axis in fish exposed to bleached kraft mill effluent in the St. Maurice River, Quebec. Ecotoxicology 6: 1–12, http://dx.doi.org/10.1023/A:1018699405158
- Patnaik, K. and Tripathy, N.K. (1992) Farm grade chloropyrifos (Dermot) is genotoxic in somatic and germ-line cells of Drosophila. Mutat. Res. 279: 15–20, http://dx.doi.org/10.1016/0165-1218(92)90261-W
- Gollapudi, B.B. Mendrala, A.L. and Linscombe, V.A. (1995) Evaluation of the genetic toxicity of the organophosphate insecticide chloropyrifos. Mutat. Res. 342: 25–36, http://dx.doi.org/10.1016/0165-1218(95)90087-X
- Hayashi, M. Ueda, T. Uyeno, L. Wada, K. Kinae, N. Saotome, K. Tanaka, N. Takai, A. Sasaki, Y.F. Asano, N. Sifuni, Y. and Ojimma, T. (1998) Development of genotoxicity assay systems that use aquatic organisms. Mutat. Res. 399: 125–133, http://dx.doi.org/10.1016/S0027-5107(97)00251-0
- Porichha, S.K. Sarangi, P.K. and Prasad, R. (1998) Genotoxic effect of chlorpyrifos in Channa punctatus. Pres. Cytol. Genet. 9: 631–638.
- EPA (1985). Disciplinary review ecological effects profile. Available from US Environ. Protection Agency, Office of Pesticide Programs, 401 M St. S.W., Washington DC 20460, 10p.
- Ali, D. Nagpure, N.S. Kumar, S. Kumar, R. Kushwaha, B. and Lakra, W.S. (2009) Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem. Toxicol. 47: 650–656, http://dx.doi.org/10.1016/j.fct.2008.12.021
- Banu, B.S. Danadevi, K. Rahman, M.F. Ahuja, Y.R. and Kaiser, J. (2001) Genotoxic effect of monocrotophos to sentinel species using comet assay. Food Chem. Toxicol. 39: 361–366, http://dx.doi.org/10.1016/S0278-6915(00)00141-1
- Rank, J. and Jensen, K. (2003) Comet assay on gill and hemocytes from the blue mussel Mytilus edulis. Ecotoxicol. Environ. Saf. 54: 323–329, http://dx.doi.org/10.1016/S0147-6513(02)00006-4
- Wong, C.K.C. Yeung, H.Y. Woo, P.S. and Wong, M.H. (2001) Specific expression of cytochrome P4501A1 gene in gill, intestine and liver of Tilapia exposed to coastal sediments. Aquatic Toxicol. 54: 69–80, http://dx.doi.org/10.1016/S0166-445X(00)00173-9
- Xing, H. Li, S. Wang, S. Gao, X. Xu, S. and Wang, X. (2012) Oxidative stress response and histopathological changes due to atrazine and chlorpyrifos exposure in common carp. Pesticide Biochemistry and Physiology 103: 74–80, http://dx.doi.org/10.1016/j.pestbp.2012.03.007







