Adsorption of Formaldehyde on Treated Activated Carbon and Activated Alumina
Madhu Agarwal1 * , Manan Dave1 and Sushant Upadhayaya1
1
Department of Chemical Engineering,
MNIT,
Jaipur,
India
DOI: http://dx.doi.org/10.12944/CWE.6.1.06
Formaldehyde is extensively used for disinfection and antisepsis in medical facilities and especially in textile and paint industries as a solvent. Owing to the higher concentration of formaldehyde, both in waste effluents and local environment of medical facilities and industries, it has been reported to cause cancer and as an irritant to eyes and skin. The removal efficiency and the adsorption mechanism of formaldehyde onto the treated activated carbons and activated alumina were studied to analyze the effect of modified surface chemistry of adsorbents. The adsorption capacities of all the selected adsorbents were investigated in batch study at varying contact time and initial concentration of formaldehyde at STP. It was found that commercially implemented activated alumina, treated with effective nucleophile, showed significant formaldehyde adsorption with less variation in adsorption capacity even with decrease in initial concentration of adsorbate. These set of experiments indicate that acidity of formaldehyde can be exploited for its removal from waste effluent and air. The optimization studies were carried out to find out the optimum conditions for this adsorption process with adsorption model fitting and kinetic studies. The investigation results were evaluated on per gram of adsorbent basis to predict feasibility of scaling up at commercial level.
Copy the following to cite this article:
Agarwal M, Dave M, Upadhayaya S. Adsorption of Formaldehyde on Treated Activated Carbon and Activated Alumina. Curr World Environ 2011:6(1);53-59. DOI:http://dx.doi.org/10.12944/CWE.6.1.06
Copy the following to cite this URL:
Agarwal M, Dave M, Upadhayaya S. Adsorption of Formaldehyde on Treated Activated Carbon and Activated Alumina. Curr World Environ 2011:6(1);53-59. Available from: http://www.cwejournal.org/?p=1272
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2011-03-09 |
|---|---|
| Accepted: | 2011-04-29 |
Introduction
Formaldehyde, identified as one of the substances causing sick house syndrome, has been used as preservative, fungicide, germicide and disinfectant in hospitals and industries due to its bactericidal action. Formaldehyde is also found in other products such as chemicals, particle board, household products, glues, permanent press fabrics, paper product coatings, fiberboard, and plywood. Use of formaldehyde has raised concern due to its intensive ability to polymerize owing to inadequate dispensing techniques and lack of air circulation.1 In view of its widespread use, toxicity and volatility, exposure to formaldehyde is of significant consideration for human health (IARC, France). It is also a suspected human carcinogen that is linked to nasal cancer and lung cancer.2,3 The irritation in the upper respiratory tract starts at 120 g/m3 of HCHO and becomes more common at 240 g/m3, while Symptoms in the lower airways such as cough, chest tightness and wheezing have been observed at higher levels (>6,000 g/m3) but may occur at lower concentrations (about 80 g/m3) in the presence of fine particles.4
Due to higher vapor pressure at standard atmospheric condition formaldehyde is found as vapor in higher quantity. Hence, majority of the research is carried out in removal of formaldehyde vapors by designing probes from alkali metals or combination with the rare earth supported on quartz or silica to detect the concentration of formaldehyde.5 The liquid effluent from textile, paint, paper and pulp and furniture industries also contains high levels of formaldehyde owing to its properties to be used as a solvent. Formaldehyde removal had been attempted through chemical treatment of waste effluent by urea resulting into a solid product removed by filtration and centrifugation.6 Adsorbent slike activated carbon, wood bark of plant species, carbon prepared from natural sources like rice husk and coffee residue have been studied for formaldehyde removal economically.7-10 The influence of the thermal activation of celites with various contents of klinoptilotit, implementation of Al-doped graphene, activated carbon fiber on the sorption of gaseous formaldehyde from its atmospheric mixtures with aqueous vapor showed an increase in the interaction of the formaldehyde carbonyl and aqueous hydroxyl groups in celite channels.11-13
In this work, batch removal of formaldehyde has been carried out at room temperature from liquid system by adsorption on to the commercially available adsorbent like activated carbon and alumina. The acidity of commercial formalin is often ascribed to the presence of carboxylic acids, particularly formic acid, as impurities.14 Therefore, the adsorbents had been treated with nucleophile to enhance the activity of the selected adsorbent which could exploit the acidity of formaldehyde. Kinetic and isotherm study of only the selected adsorbent has been emphasized.
Material and Methods
Activated carbon powder was procured from Merck Chemicals. Fresh activated alumina was collected from the sewage treatment plant around Jaipur city dedicated to remove industrial effluents. Formalin was used as the source for formaldehyde. Methanol was used as a solvent to dissolve formaldehyde to avoid polymerization of the formaldehyde during severe agitation for adsorption. Since the estimation of adsorbed formaldehyde was aimed at industrial effluent level which usually has high amount of formaldehyde ranging to 600 ppm, spectroscopy analysis assumes drawback at such high levels of formaldehyde concentration detection. Hence the analysis method used was OSHA’s titration analytical laboratory method for determination of formaldehyde which is based on titration of formaldehyde against sodium sulfite with thymopthalein as indicator.
HCl was standardized using double end point method using sodium carbonate with phenolphthalein and methyl orange as indicator.
Steps involved in preparation of treated adsorbents of from carbon and alumina is as follows:
Amino Group Introduction in Activated Carbon Through Acid Treatment (AC-1)7
The concentrated sulfuric acid and nitric acid treatment introduced nitro groups onto the surface of the activated carbon. The nitro groups were reduced by the reaction of powdered iron and hydrochloric acid to the amino groups.
Amino Group Introduction in Activated Carbon (AC-2) and Alumina (NAl) Through Ammonia Liquor Treatment
To modify the adsorbent for higher removal of formaldehyde, adsorbent like activated carbon and activated alumina were treated with basic compounds like ammonia liquor to provide basic entities to remove formaldehyde. The adsorbents were weighed and taken in the flask to add sufficient amount of 25% ammonia water. The adsorbent were allowed to stand by for 24hrs and then dried in hot air oven to remove excess of ammonia water.
Adsorption Experiment
Sample preparation for 50ml formaldehyde stock for each adsorbent and the concentration determination was carried out by OSHA’s titerimetric analysis method for formaldehyde. 5 g of each adsorbent was weighed in 125 ml beaker to which 45ml of formaldehyde stock was added and the systems were agitated for 5 to 12 hrs on magnetic stirrer to attain adsorption equilibrium. After contact time period filtrates were recovered for each batch using Whatmann filter paper Grade 42. Equilibrium concentration of formaldehyde for each batch of adsorbent was determined by OSHA’s titerimetric method. Same protocol was used to collect the data points for time study and dosage study to be used in establishing the kinetics of adsorption and isotherm.
Results and Discussion
For the surface of the AC-1, which was treated with both concentrated sulfuric acid and nitric acid and then reduced with powdered iron and hydrochloric acid, to produce the amino groups, it is considered that the amount of formaldehyde adsorbed onto the activated carbon increased with the introduced amino groups because of the increasing interaction between the surface of the activated carbon and the formaldehyde, and the adsorption of formaldehyde onto the activated carbon depended on the chemical, that is, the interaction between formaldehyde and the amino groups of the activated carbon [7]. AC-2 and NAl enabled the ammonia ions to adhere to their surface which was supposed to be a surface interaction. Figure 1 shows the standard curve prepared for formaldehyde using referred titration method. This standard curve was used to find out the concentration of formaldehyde stocks prepared in various experiments.
Time Study of Unmodified and Modified Adsorbent
The time study of activated carbon for formaldehdye adsorption reflected in figure 2 defines that when initially the process started, the develpoment of monolayer was very quick. As the contact time increased saturation level was achieved after time period of 12hrs. The dips in the graph as it approaches equilibrium after a sudden rise in the adsorption, directs that after 3hrs of contact time the monolayer is disturbed for futher adsorption.
The smooth flow of the curve of figure 3 depicted that there was a uniform decrease in the adsoprtion of formaldehyde because the amino groups, uniformly introduced on the surface of the AC-2 were getting depleted by adsorption of formaldehyde. The equilibrium was achieved very distinctly with low contact time as compared to activated carbon.
Figure 4 shows the time study of NAl. The notable feature of the curve obtained was thought the adsorption was comparatively lower than ammonia liquor treated activated carbon, the adsorption during fixed interval of time was uniform and hence the treatment with ammonia liquor can be improved to enhance the adsoprtion capacity of activated alumina
Time study of the unmodified and modified adsorbent helped to determine suitable adsorbent with uniform adsorption pattern and predictably feasible kinetic study. Ammonia liquor treated activated alumina (NAl) showed very high improvement in adsorption capacity w.r.t untreated activated alumina as compared to the unmodified and modified activated carbon. Based on the improved adsorption capacity characteristic, kinetic study as well as equilibrium study for ammonia treated activated alumina was carried out to determine the rate constants which would further help to model the kinetics.
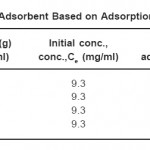 |
Table 1: Selection of Adsorbent Based on Adsorption per g of Adsorbent Click here to View table |
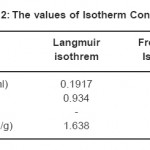 |
Table 2: The values of Isotherm Constants Click here to View table |
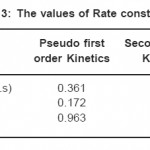 |
Table 3: The values of Rate constants Click here to View table |
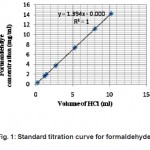 |
Figure 1: Standard titration curve for formaldehyde Click here to View figure |
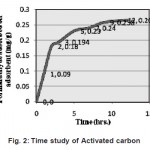 |
Figurre 2: Time study of Activated carbon Click here to View figure |
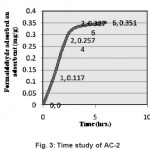 |
Figure 3: Time study of AC-2 Click here to View figure |
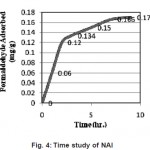 |
Figure 4: Time study of NAl Click here to View figure |
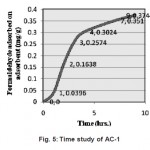 |
Figure 5: Time study of AC-1 Click here to View figure |
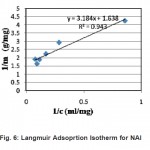 |
Figure 6: Langmuir Adsoprtion Isotherm for NAl Click here to View figure |
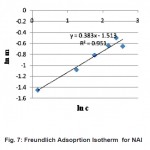 |
Figure 7: Freundlich Adsoprtion Isotherm for NAl Click here to View figure |
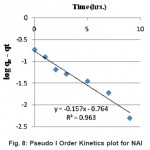 |
Figure 8: Pseudo I Order Kinetics plot for NAl Click here to View figure |
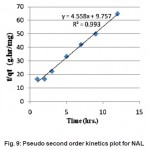 |
Figure 9: Pseudo second order kinetics plot for NAL Click here to View figure |
Isotherm Study
The fitting of Experimental data obtained were studied using two well known adsorption isotherms, Langmuir and Freundlich.
Data fitting using Langmuir Isotherm
Langmuir isotherm was used to fit the experimental data. The Langmuir isotherm equation is given below (equation 1).
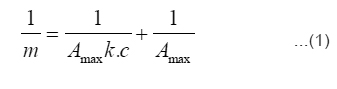
In the above equation m and c represents the equilibrium mass of formaldehyde adsorbed on adsorbent and in the solution respectively. A graph (Fig.6) was plotted against 1/m and 1/c to calculate the value of Langmuir adsorption constants (Amax and k) and the values obtained are reported in Table-2.
Data Fitting Using Freundlich Isotherm
Freundlich isotherm was also used to fit the experimental data. The Freundlich isotherm equation is given below (equation 2), where m and c represents the equilibrium mass of formaldehyde adsorbed on adsorbent and in the solution respectively. The value of Freundlich adsorption constants (k and n) were calculated by plotting a graph (Fig.7) between ln (m) and ln (c) and same are reported in Table-2.
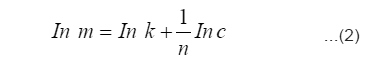
Kinetic Study
The adsorption kinetic study was done to find the rate constant for adsorption assuming pseudo first and second order reaction.
Pseudo First Order Reaction Kinetics
Pseudo first order reaction kinetics follows a straight line equation mentioned below,
log(qeq -qt ) = log(qeq )-k1/2.303xt ...(3)
where qeq, qt and t are equilibrium concentration, concentration at any time of formaldehyde in solution and time respectively. The above equation is a straight line equation provides the estimate of rate constant k1 by plotting ( Fig. 8) experimental data and the value obtained is reported in Table-3.
Second Order Reaction Kinetics
The adsorption kinetics was also checked by assuming a Second order reaction. The integrated linearized form of second order equation is given below,
where, qeq, q and t are equilibrium concentration, concentration at any time of formaldehyde in solution and time respectively. k2 is the second order rate constant of formaldehyde adsorption. The Fig.-9 shows the plot of t/q versus t from which the value of k2 was calculated and reported in Table 3.
For the surface of AC-1, it is considered that the amount of formaldehyde adsorbed onto the activated carbon increased with the introduced amino groups because of the increasing interaction between the surface of the activated carbon and the formaldehyde. NAl developed more impregnation of amino group when subjected to high temperature drying resulting in more introduction of amino group as compared to AC-1 which might have lost surface available nitro group during washing to remove ferrous sulphate. The adsorption of formaldehyde onto the heterogeneous activated carbon was principally based on chemisorption, that is, the interaction between formaldehyde and the amino groups of the activated carbon.
Adsorption constant k values range in the one demonstrated by Tanada et al., (1999) and also the RMS for the Freundlich isotherm is approximately near to 1 and more justified than the one obtained from Langmuir isotherm. Hence it can be deduced that the adsorption of formaldehyde
| 1 | 1 | 1 | ||
| __ | = | _________ | + | ________ |
| q | k2q2eq | q2eq |
onto the surface of modified alumina follows Freundlich Isotherm and pseudo second order kinetics with improved surface interaction and adsorption capacity than the one achieved by activated carbon modified by both concentrated sulphuric acid and nitric acid.7
References
- Braswell, J. R., Spiner, D. R., Hoffman, R. K., Adsorption of Formaldehyde by Various Surface During Gaseous Decontamination, Applied Microbiology, 20: 765-769 (1970).
- Registry of Toxic Effects of Chemistry Substances; National Institute of Occupational Safety and Health, Washington, DC (1976).
- Cockcroft, D.W., Hoeppner, V.H., Dolovich, J., Occupational Asthma Caused by Cedar Urea Formaldehyde Particle Board, Chest 82(49) (1982).
- Liu, K. S., Huang, F. Y., Hayward, S. B., Weslovski, J. and Sexton, K., Irritant Effects of Formaldehdye. (1991).
- Lee, Y., Oh, C., Kim, D., Jun, Y., Oh, S., Preparation of composite silica particles for the removal of formaldehyde at room temperature, Journal of Ceramic Processing Research, 9(3): 302-306 (2008).
- Bednarik, V., Vondruska, M., Removal of Formaldehyde from Acrylic Acid Production Wastewater, Environmental Engineering Science, 20(6): 703-707 (2003).
- Tanada, S., Kawasaki, N., Nakamura, T., Araki, M., Isomura, M., Removal of Formaldehyde by Activated Carbons Containing Amino Groups., Journal of Colloid and Interface Science 214: 106-108 (1999).
- Kumagai, S., Sasaki, K., Shimizu, Y., Takeda, k., Formaldehyde and acetaldehyde adsorption properties of heat-treated rice husks, Separation and Purification Technology, 61: 398-403 (2008).
- Takano, T., Murakami, T., Kamitakahara, H., Nakatsubo, F., Formaldehyde adsorption by karamatsu (Larix leptolepis) bark, Journal of Wood Science, 54: 332-336 (2008).
- Boonamnuayvitayaa, V., Sae-ungb, S., Tanthapanichakoonc, W., Preparation of activated carbons from coffee residue for the adsorption of formaldehyde, Separation and Purification Technology 42: 159-168 (2005).
- Khodosova, N. A., Belchinskaya, L. I., Petukhova, G. A., Voishcheva, O. V., Adsorption of Formaldehyde from Gaseous Phase by Thermally Activated Nanoporous Celite., Protection of Metals and Physical Chemistry of Surfaces, 46 (1): 90–95, 2010.
- Chi, M., Zhao, Y., Adsorption of formaldehyde molecule on the intrinsic and Al-doped graphene: A first principle study, Computational Material Science 46: 1085-1090 (2009).
- Song, Y., Qiao, W., Yoon, S., Mochida, I., Guo, Q., Liu, L., Removal of formaldehyde at low concentration using various activated carbon fiber, Journal of Applied Polymer Science 106(4) : 2151-2157 (2007).
- Euler, H., and Euler, A., Ber. them. Ges., 38: 2551 (1905).







