Extraction and Characterization of Chitin and Chitosan from Penaeus Monodon and its Application for Water Purification: An Approach to Utilize Waste
1
Department of Zoology and Research Centre,
Scott Christian College (Autonomous),
Affiliated to Manonmanium Sundaranar University,
Tirunelveli,
Tamil Nadu
India
Corresponding author Email: ashaberlin3030@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.3.24
Copy the following to cite this article:
Berlin M. A, Leena R. Extraction and Characterization of Chitin and Chitosan from Penaeus Monodon and its Application for Water Purification: An Approach to Utilize Waste. Curr World Environ 2022;17(3). DOI:http://dx.doi.org/10.12944/CWE.17.3.24
Copy the following to cite this URL:
Berlin M. A, Leena R. Extraction and Characterization of Chitin and Chitosan from Penaeus Monodon and its Application for Water Purification: An Approach to Utilize Waste. Curr World Environ 2022;17(3).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-08-30 |
|---|---|
| Accepted: | 2022-11-08 |
| Reviewed by: | 
 Vijaya Kumara
Vijaya Kumara
|
| Second Review by: |

 Medhat Ali
Medhat Ali
|
| Final Approval by: | Dr. Saravanan Pichiah |
Introduction
In marine Arthropods, the largest group of Crustaceans contains about 30,000 species. Shells of marine crustaceans along with shrimp, crabs, and lobsters are biowastes that make contributions to the pollution of coastal areas. The crustacean shell wastes contain 8-10% chitin, 30-65% protein, and 10-20% calcium1. The preparation of chitin, chitosan, and their components from shrimp shells takes place in three stages: demineralization, deproteinization, and deacetylation 2.
Chitin is white, hard, elastic, natural polysaccharide compound, and insoluble in water. It is the second most excessive organic polysaccharide compound. It is commonly present in fungi, yeasts, arthropods, molluscs, and annelids3. Chitin is a biopolymer that contains repeating units of ?-(1?4 linked residues)- 2-acetamido - 2-deoxy- D-glucopyranose semi-crystalline components. Chitin is composed of linear chains of acetylglucosamine groups. The differentiation between chitin and chitosan is the presence of acetyl groups4. Chitin has excellent biocompatibility, adsorption capacity, nontoxicity, antibacterial, immunological activity, drug delivery, and chelating properties.
In the enzymatic method, chitosan is obtained by cleaving the N-acetyl units of the polymer to form D-glucosamine units. Chitosan is non-toxic, insoluble in water, and dissolves after mixing with acids6,7,8. Chitosan is characterized by biocompatibility, biodegradability, and, adsorption activity 9. Chitin, chitosan, and its residues are mainly used in the food industry, pharmacy, cosmetics products, biochemistry, biotechnology, and polluted water treatment10.
Chitin, chitosan, and its derivatives are organic coagulants and do not create secondary pollutants. The functional groups of –NH2 and -OH can induce the adsorbing capacity of dyes11. They could be used as coagulating or flocculating agents for polluted textile wastewater12. The shrimp shells chitosan can be used as a bio adsorbent for the removal of colour from textile wastewater13. In the present study, the shrimp shell, chitin, chitosan, and membrane were prepared, and their Physico-chemical and functional properties were elucidated. The chitosan applied in the field of water purification had also been worked.
Materials and Methods
Collection of samples
The Penaeus monodon exoskeletons were collected from the local fish market in Colachel, Tamil Nadu during December. The shrimp shells were thoroughly washed with tap water and cleaned. Then, the shrimp exoskeleton shells were dried under sunlight for 2-3 days.
Preparation of chitin and chitosan
Shrimp exoskeleton obtained from Penaeus monodon were soaked in 0.05M acetic acid solution at 30°C for 24 hours. The soaked exoskeletons were thoroughly cleaned with water and air-dried. The particles were deacetylated using HCl (0.68 M) at 30°C for 6 h. The deacetylated particle was separated and cleaned with water and pH was adjusted at 6.5-7.5. Protein contents were removed from the dried materials using 0.62M NaOH solution at 30°C for about 16 h. The deproteinized residues were then separated and cleaned with distilled water until a pH was maintained at 6.5 - 7.5. The particles were dried, ground, and sieved through a 150 ?m sieve (mesh). Chitin became deacetylated using NaOH solution (25 M) at 65°C for approximately 20 h. Afterward, the residues were separated, and the pH was maintained at 6.5-7.5.
Preparation of chitosan membrane
Five grams of dried chitosan powder was dissolved in a 3% acetic acid. The chitosan solution was spread on a Petri dish and was heated at 60°C. Afterward, with a 50% reduction, the dish was kept at 30°C for 24h. Then, the dried membrane was soaked in NaOH solution for 24h. The membrane was cleaned with double distilled water and dried by natural drying.
Estimation of protein
The protein content was determined by the Foltch method14. Sample (0.1 g) was added with 1 ml of distilled water and centrifuged at 5000 rpm for 10 min. Solution (A)was prepared by adding 75 ml of NaOH (0.1 N) solution and adding 2 g of sodium carbonate. Solution (B) was prepared by adding 0.05 g of copper sulphate and dissolved in 5 ml double distilled water, to which 0.1 g sodium potassium tartrate was added. Solution (C) was prepared by taking 49 ml solution (A) to which 1 ml solution (B) was added. Three test tubes were taken and labelled as blank, test, and standard. 2.5 ml of solution (C) was added to all test tubes. The blank tube was kept in 0.1 ml double distilled water. In test one, 0.1 ml of sample was taken. The standard solution of 0.1 ml of bovine serum albumin was poured into the standard tube. Finally, 0.6 ml folin reagent was taken to each tube and thoroughly mixed. All tubes were maintained at 30°C for 20 min and the OD (optical density) was determined at 625 nm14.
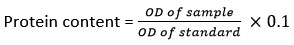
Estimation of lipids
Total lipids were extracted by a chloroform-methanol method15. The dried sample powder was homogenized with chloroform (2 ml) and methanol (1 ml). Then, the residues were mixed in a vortex at 2800 rpm. The extract was equilibrated with about 20 % of sodium chloride solution. The extracted contents were weighed and calculated12.
Lipid content = Petri dish with sample – Petri dish without sample × 100
Estimation of carbohydrates
The carbohydrate content was extracted by the Dubois method16. One milliliter of the sample was dissolved with 1 ml phenol (5 %), and H2SO4 (5 ml) and maintained at 30°C for 20 min. Finally, the OD of sample was recorded at 490 nm.
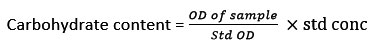
Ash content
The contents of ash were measured by the laboratory muffle furnace method17. The 1 g sample was sealed with a lid, kept in a furnace, and maintained at 575°C for 6 h. After chilling, the weight was obtained.

Moisture content
Moisture content was measured by the gravimetric method18. A half-gram sample powder was taken and maintained at 110°C for 3 h. After chilling, the weight was estimated, and the percentage of moisture content was determined using the given formula15:

Degree of deacetylation (DD)
The DD was determined by the titration method of acid-base19. Sample (0.1 g) was dissolved in HCl solution (0.1 mol/l) and methyl orange (5 drops) was added. Finally, the dissolved chitosan solution was titrated with NaOH solution (0.1 mol/l).

Were,
HCl concentration (C1)
The volume of HCl solution (V1)
The concentration of standard NaOH (C2)
The Volume of NaOH solution (V2)
Dosage of a sample (M)
NH2 group equivalent weight (0.016)
NH2 compound proportion (0.0994)
Water Binding Capacity (WBC)
The 0.5 g of sample was dissolved with 10 ml of water and mixed on a vortex for 5 min and maintain at 29°C for 30 min. After, the bound particles were read at 3000 rpm for 30 min3.

Solubility
The sample was dissolved in acetic acid (1 %) for 30 min and cooled at 30°C. Then, the mixed particles were read at 10000 rpm for 10 min. The undissolved particles were separated and centrifuged at 10000 rpm and dried at 60°C for one day. Then, the residues were measured and calculated20.

Fat binding capacity (FBC)
Chitosan (0.5 g) was put in a tube with 10 ml oil and mixed on a vortex for 5 min. The residues were read at 3000 rpm for 25 min. After centrifuged, the tube was measured and calculated3.

Effluent sampling
The textile effluent was collected from a cotton spinning mill at Tirupur, Tamil Nadu, South India (Lat. 11.11°N, Long. 77.34°E). The effluent was collected in a sterile plastic can from the discharge tank of the textile mill. The pH and temperature of the sample were determined on the site by using a pH meter and a thermometer. The effluent was stored in a refrigerator and used for further analysis21.
Decolourization experiment
Sample (0.5, 1.0, 2.0 g) was added to varying concentrations (10, 20, 30, 40, 50, 60, 70, 80, 90, 100%) of the textile effluent. The initial absorbance was noted, and the absorbance was measured periodically at a time interval of 24 h at 500 nm for 30 days.

Fourier transform infrared spectroscopy (FTIR)
The spectra of FTIR were analyzed utilizing the software of Perkin Elmer Spectrum. A few milligrams of chitosan were placed in the ATR head from 4000 to 450 cm-1. For each spectrum, 16 scans were collected and averaged.
 | Figure 1: Extraction of chitosan
|
 | Figure 2: Chitosan Membrane.
|
Table 1: Physico-chemical and functional properties of P. monodon shell, chitin, and chitosan.
S. No |
Physicochemical / functional parameters (%) |
Raw shell
|
Chitin
|
Chitosan
|
1. | Protein content | 38.8 ± 0.6 | 3.82 ± 0.60 | 2.94 ± 0.05 |
2. | Lipid content | 5.1 ± 0.08 | 0.9 ± 0.25 | 1.3 ± 0.08 |
3. | Carbohydrate content | 75.42 ± 0.18 | 68.45 ± 0.45 | 73.42 ± 0.04 |
4. | Ash content | 17.2 ± 0.16 | 2.9 ± 0.08 | 1.5 ± 0.081 |
5. | Moisture content | 21.26 ± 0.52 | 9.6 ± 1.63 | 8.2 ± 0.16 |
6. | Deacetylation | - | 67.60 ± 0.26 | 79.03 ± 0.13 |
7. | Water binding capacity | 170 ± 8.16 | 640 ± 16.32 | 580 ± 16.32 |
8. | Fat binding capacity | 140 ± 16.32 | 420 ± 16.32 | 420 ± 8.16 |
9. | Solubility | 33 ± 2.44 | 97.3 ± 0.28 | 97.6 ± 0.53 |
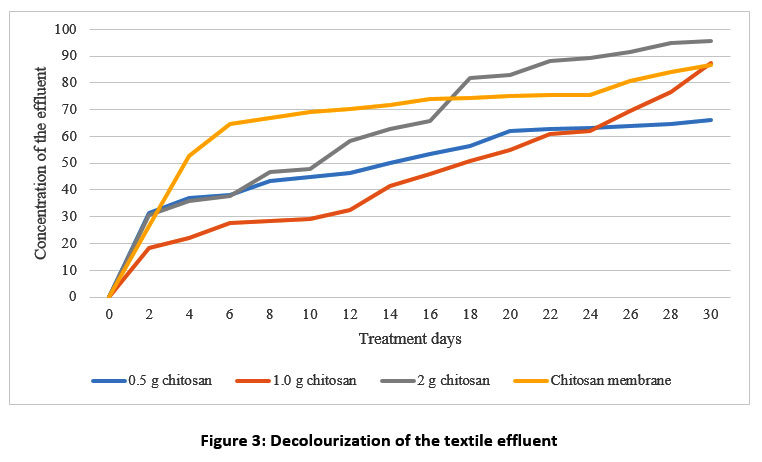 | Figure 3: Decolourization of the textile effluent.
|
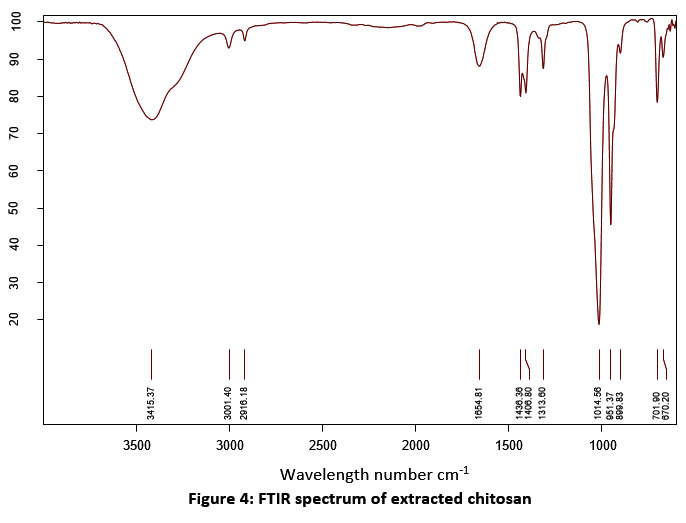 | Figure 4: FTIR spectrum of extracted chitosan.
|
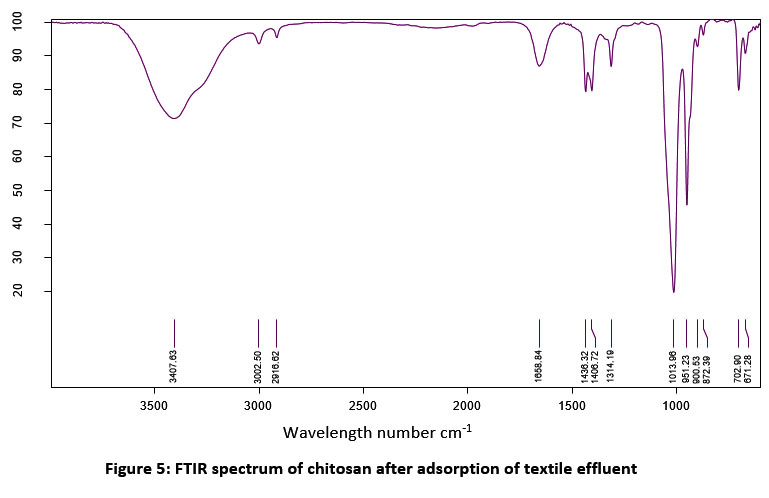 | Figure 5: FTIR spectrum of chitosan after adsorption of textile effluent.
|
Table 2: FTIR analysis (functional groups) of extracted chitosan.
Wavelength | Functional groups | Structure |
670.20 | Alkyl halides | R-Br |
701.90 | Amines | RNH2, R2NH |
899.83 | Amines | RNH2, R2NH |
951.37 | Misc. | P-H phosphine |
1014.56 | Misc. | P-H phosphine |
1313.60 | Carboxylic acids | RCO-OH |
1406.80 | Misc. | S=O sulfate |
1436.36 | Misc. | S=O sulfate |
1654.81 | Alkenes | 8-ring |
2916.18 | Alkanes | RCH2CH3 |
3001.40 | Aromatics | Ar-H |
3415.37 | Phenols | ArO-H bonded |
Table 3: FTIR analysis of chitosan after adsorption of textile effluent
Wave length | Functional groups | Structure |
671.28 | Alkyl halides | R-Br |
702.90 | Misc. | S-OR esters |
872.39 | Misc. | S-OR esters |
900.53 | Misc. | P-OR esters |
951.23 | Misc. | = NOH oxime |
1013.96 | Misc. | P-H phosphine |
1314.19 | Esters | RCOOR? |
1406.72 | Misc. | S=O sulfate |
1436.32 | Misc. | S=O sulfate |
1658.84 | Alkenes | 8-ring |
2916.62 | Alkanes | RCH2CH3 |
3002.50 | Aromatics | Ar-H |
3407.63 | Phenols | ArO-H bonded |
Results and Discussion
In the current study, the protein contents of raw shell, chitin, and chitosan were 38.8 ± 0.6, 3.82 ± 0.60, and 2.94 ± 0.05 respectively. In a similar report the protein content of shell wastes and chitin from shrimp shells were 32.13 ± 0.08 and 0.80 ± 0.15 respectively22. Generally, the raw shrimp shell has high protein content (38.81%), and after deproteinization, the chitin had a trace amount (3.82%) of protein. Deproteinization could not remove 100 % of proteins from the shrimp shells23.
The lipid content of raw shell, chitin, and chitosan was recorded as 5.1 ± 0.08, 0.9 ± 0.25, and 1.3 ± 0.08 respectively. Similarly, the lipid contents of the chitin, raw particles of chitosan, and extracted chitosan from shrimp shells’ exoskeleton and recorded them as 1.50, 2.00, and 2.60%24. The crude lipid content was 10%, which revealed the relationship with the crude protein contents of the shrimp shells.
The carbohydrate contents of raw shell, chitin, and chitosan were 75.42 ± 0.18, 68.45 ± 0.45, and 73.42 ± 0.04 respectively. In a similar work, the carbohydrate content of the extracted chitin, raw particles of chitosan, and extracted chitosan from the shell as 76.20, 79.18, and 77.55 %24.
In the current study, the ash content of the raw shell, chitin, and chitosan was noted as 17.2 ± 0.16, 2.9 ± 0.08, and 1.5 ± 0.081% respectively. Based on the presence of ash content, pure chitosan was measured. Ash is a highly-frequency parameter that affects characteristics such as viscosity and solubility25. The final process of demineralized chitosan resulted having 31-36% ash26. The chitosan having <1% ash is a good grade of chitosan2, 27.
The study described that the moisture contents of raw shell, chitin, and extracted chitosan were 21.26±0.52, 9.6±1.63, and 8.2±0.16% respectively. In a related study the moisture content of shrimp chitosan was 9.34%28. The commercial chitosan has a 10% moisture content29. Chitosan is hygroscopic, which is affected by low moisture absorption during storage20.
The DD of the extracted chitin, chitosan was 67.60 ± 0.26 and 79.03 ± 0.13%. In a similar work, the degree of deacetylation as 80% which is considered high-quality chitosan30. In chitin, the amount of DD depends on the raw material, deproteinization, and demineralization31.
Water binding capacity (WBC) of the raw shell, chitin, and chitosan were 170 ± 8.16, 640 ± 16.32, and 580 ± 16.32% respectively. Likewise, the WBC of chitosan ranged from 581 to 1150%32 The commercial, shrimp, and crab chitosan wherein the WBCranged from 458 to 805%33.
The FBC of raw shell, chitin, and extracted chitosan were 140 ± 16.32, 420 ± 16.32, and 420 ± 8.16% respectively. In a similar report, FBC in the range from 314 to 535%34. The level of FBC depends on deproteinization and deacetylation35.
The solubility of raw shell, chitin, and chitosan was observed as 33 ± 2.44, 97.3 ± 0.28, and 97.6 ± 0.53 respectively. In a related work, the shells treated with 50 and 60% NaOH solution gave high solubility ranged from 96.01- 97.2%. The chitosan solubility is influenced by temperature, period of deacetylation, concentration of alkaline and yield of chitin, various methods applied to chitin separation, and size36. Chitosan solubility increases proportionally with deacetylation degree.
In this study, figure 3 shows that the rate of decolourization increased from the 2nd to 18th days of observation (from 81 to 13%). In a similar study, the textile dye was decolourized by prawn shell waste from 93 to 70%37. This result indicates that the percentage of decolourization increased when the chitosan dosage was increased. In the chitosan membrane, the optimum decolourization of the effluent occurs at 86.82 to 41.54%. The adsorption sites are less available at high concentrations of textile effluent. So, the percentage of decolourization is highly dependent on the textile effluent cocentration38.
The absorption efficiency of extracted chitosan was determined by spectrometric assay. Figure 2 & 3 shows the comparison of the FTIR spectrum of extracted chitosan (before treatment) with textile effluent absorbed (after treatment) chitosan. The presence of similar groups were alkyl halides, P-H phosphine, alkenes, alkanes, aromatics, and phenols in the position of a peak at 600 cm -1 to 3500 cm -1. New peaks were recorded at 872.39 cm -1 which indicated the S-OR esters. The carboxylic acids group at the wavelength of 1313.60 cm -1 was modified into the esters group at the wavelength of 1314.19 cm -1. The P-H phosphine group was converted into = NOH oxime. In this study, the presence of an amine group at 899.83 cm-1 was noticed. In a similar study, chitosan oligomers polysaccharide structure at 899 cm-1 39. The functional groups of alkenes were found at 1654.81 cm-1. Similarly, the bands of bending vibration of NH2 groups were found at 1656.55 and 1658.48 cm-1 40. The presence of a full band stretch in the prepared chitosan compared to the standard band stretch indicates that adsorption is occurring on the chitosan oligomers.
Conclusion
Chitosan has been prepared from Penaeus monodon the steps including demineralization, deproteinization, and deacetylation process. The results of FTIR concluded that the shrimp shell chitosan can be used as a good bio-remediating agent for removing colour from textile effluent. Above all, the method employed in the study to prepare chitosan was economical and it could be a way to use the shrimp exoskeleton waste which would otherwise go unutilized as a mere waste polluting the environment.
Acknowledgment
The authors are thankful for the support from Manonmanium Sundaranar University, Tirunelveli, and the Scott Christian College (Autonomous), Nagercoil for providing permission and necessary facilities to carry out this research work.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
The authors received no financial support for the research, authorship and publication of this article.
References
- Rao, M.S., Yu, P., Stevens, W.F., Chandrkrachang, S., Kungsuwan, A., and Hall, G.M. The Proceedings of the Second Asia Pacific Chitin and Chitosan Symposium. Bangkok, Thailand. 1996.
- Mohammed, M.H., Williams, P.A. and Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocolloids. 2013; 31(2): 166-171.
CrossRef - Knorr, D. Functional properties of chitin and chitosan. J. Food Sci. 1982; 47: 593-595.
CrossRef - No, H.K. and Meyers, S.P. Preparation and characterization of chitin and chitosan-a review. J. Aquat. Food Prod. Technol. 1992; 4: 27-52.
CrossRef - Kalut, S. A. Enhancement of Degree of Deacetylation of Chitin in Chitosan Production. Faculty of Chemical Engineering and Natural Resources Universiti Malaysia Pahang. 2008; 5-31.
- Klug, M., Sanches, M. N. M., Laranjeira, M. C. M., Favere, V. T. and Rodrigues, C. A. Adsorption isotherms of Cu (II), Ni (II), Pb (II) and Zn (II) by N-(3, 4-dihydroxybenzyl) chitosan using nonlinear regression method. Quimica Nova. 1998; 21(4): 410–413.
CrossRef - Kubota, N., Tastumoto, N., Sano, T. and Toya, K. A simple preparation of half N-acetylated chitosan highly soluble in water and aqueous organic solvents. Carbohydrate Research. 2000; 324: 268–274.
CrossRef - Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Separation Purification Technology. 2004; 38: 43-74.
CrossRef - Dash, M., Chiellini, F., Ottenbrite, R.M. and Chiellini, E. Chitosan - A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011; 36: 981–1014.
CrossRef - Aranaz, I., Mengibar, M., Harris, R., Panos, I., Miralles, B., Acosta, N., Galed, G. and Heras, A. Functional Characterization of Chitin and Chitosan. Current Chemical Biology. 2009; 3(2): 203-230.
CrossRef - Zeenat, M.A., Abdul Jabbar, L., Abdul Khalique, A. and Mohammad, Y.K. Extraction and characterization of chitosan from Indian prawn (Fenneropenaeus indicus) and its applications on wastewater treatment of local ghee industry. IOSR Journal of Engineering, 2013; 3 (10): 28-37.
CrossRef - Mary, C.A. and Leena, R. A comparative study on color removal from textile industry effluent using shrimp and crab shell chitosan. Nature Environment and Pollution Technology. 2022; 21 (2): 675-681.
CrossRef - Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951; 193 (1): 256-275.
CrossRef - Foltch, J., Lees, M. and Sloane, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957; 226 (1): 497-509.
CrossRef - Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. Colorimetric method for determination of sugars and related substance. Anal. Chem. 1956; 28 (3): 350-356.
CrossRef - AOAC. Official methods of analysis, 15th edition, Association of Official Analytical Chemists, Washington, DC. 1990.
CrossRef - Blacke, G. and Hartge, K. Methods of soil analysis. Part I, American Society of Agronomy. Madison, Wisconsin, USA. 1965.
- Domard, A. and Rinaudo, M. Preparation and characterization of fully deacetylated chitosan. International Journal of Biological Macromolecules. 1983;5(1): 49-52.
- Fernandez-Kim, S.O. Physicochemical and functional properties of crawfish chitosan as affected by different processing protocols. Dissertation, Louisiana State University: Baton Rouge, La, USA. 2004.
CrossRef - APHA. Standards methods for the examination of water and waste water. American Public Health Association. 1998; 19 (1):10-150.
- Parthiban, F., Balasundari, S., Gopalakannan, A., Rathnakumar, K. and Felix, S. Comparison of the quality of chitin and chitosan from shrimp, crab, squilla waste. Current World Environment. 2017; 12(3): 672-679.
- Rutherford, F.A. and Austin, P.R. Marine chitin properties and solvents. International Conference on Chitin /Chitosan Cambridge, MA. 1978; 182-192.
CrossRef - Olafadehan, O.A., Amoo, K.O., Ajayi, T.O. and Bello, V.E. Extraction and characterization of chitin and chitosan from Callinectes amnicola and Penaeus notialis shell wastes. Journal of Chemical Engineering and Materials Science. 2021; 12(1): 1-30.
- Mohanasrinivasan, V., Mudit, M., Jeny, P., Suneet, S., Selvarajan, E., Suganthi, V. and Subathra, D. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste, 3 Biotech. 2014; 4(2): 167-175.
CrossRef - Bough, W.A., Salter, W.L., Wu, A.C.M., and Perkins, B.E. Influence of manufacturing variables on the characteristics and effectiveness of chitosan products. Biotechnology and Bioengineering, 1978; 20: 1931.
CrossRef - Divya K, Sharrel R. & Jisha M S. A Simple and Effective Method for Extraction of High Purity Chitosan from Shrimp Shell Waste. Proc. of the Intl. Conf. on Advances in Applied Science and Environmental Engineering – ASEE. 2014. doi: 10.15224/ 978-1-63248-004-0-93.
CrossRef - Puvvada, Y.S., Vankayalapati, S. and Sukhavasi, S. Extraction of chitin from chitosan Exoskeleton of shrimp for application in the pharmaceutical industry. International Current Pharmaceutical Journal. 2012; 1(9): 258-263.
- Li, Q., E.T. Dunn, E.W. Grandmaison and Goosen, M. F. A. Applications and properties of chitosan. J. Bioactive Compatible Polymer. 1992; 7: 370-397.
CrossRef - No, H.K. and Meyers, S.P. (1992). Utilization of Crawfish Processing Wastes as Carotenoids, Chitin, and Chitosan Sources. Journal Korean Soc. Food Nutrition, 21(3): 319-326.
CrossRef - Younes, I., Hajjia, S., Frachetb, V., Rinaudoc, M., Jelloulia, K. and Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment, antitumor, antioxidant and antimicrobial activities of chitosan. International Journal of Biological Macromolecules. 2014; 69: 489–498.
- Rout, S.K. Physicochemical, functional and spectroscopic analysis of crawfish chitin and Chitosan as affected by process modification. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA. 2001.
CrossRef - Cho, Y.I., No, H. K. and Meyers, S.P. Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J. Agric. Food Chem. 1998;46: 3839-3843.
- No, H.K. and Hur, E.Y. Control of foam formation by antifoam during demineralization of crustacean shell in preparation of chitin. Journal of Agricultural and Food Chemistry. 1998; 46(9): 3844-3846.
CrossRef - Rout, S.K. Physicochemical, functional and spectroscopic analysis of crawfish chitin and Chitosan as affected by process modification. Ph.D. Thesis, 2001 Louisiana State University, Baton Rouge, LA, USA.
CrossRef - Hossain, M. S. and Iqbal, A. Production and characterization of chitosan from shrimp waste. J. Bangladesh Agril. Univ. 2014; 12(1): 153–160.
- Ismail, A.M., Loganathan, M. and Theodor, P. Effect of bioadsorbents in removal of colour and toxicity of textile and leather dyes. Journal of Ecobiotechnology. 2012; 4(1): 1-10.
CrossRef - Natarajan, S.T., Jayaraj, R., Thanaraj, P and Prasath, P. The removal of heavy metal chromium (VI) from aqueous solution by using marine algae Graciliria edulis. J Chem Pharm Res. 2011; 3: 595-604.
- Kurita, K., Tomita, K., Ishi, S., Nishimura, S.I. and Shimoda, K. ?-Chitin as a convenient starting material for acetolysis for efficient preparation of N-cetylchitooligosaccharides. J poly Sci A Poly Chem. 2006; 31: 2393-2395.
- Ghannam, H.E., Talab, A.S., Dolganova, N.V., Hussein, A.M.S. and Abdelmaguid, N.M. Characterization of chitosan extracted from different crustacean shell wastes. Journal of Applied Sciences. 2016;16 (10): 454-461.
CrossRef - Khanday, W.A, Asif., M and Hameed, B.H. Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio–adsorbent for the removal of methylene blue and acid blue 29 dyes. Int J Biol Macromol. 2017; 95:895–902.
CrossRef






