Anaylysis of drinking bore well's water of Bhopal (M.P.)
H.C. Kataria1 * and Shahila Bux1
1
Department of Chemistry,
Government Geetanjali Girls P.G. College,
Bhopal,
462 038
Madhya Pradesh
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.20
To assess and evaluate the water quality of Bhopal City, biochemical analysis of drinking water i.e. bore-well’s has been studied seasonally during 2006-07. The study area for this purpose has been chosen is T.T. Nagar and Bhadbhada road of Bhopal. Two readings of analysis have been observed in one season for preliminary analysis of water quality index. The parameters for study are temperature, pH, EC, Free CO2, Chloride, total alkalinity, total hardness, Ca-H, Mg-H, D.O., B.O.D., C.O.D., NO3-, SO42-, analyzed at different sampling stations.
Copy the following to cite this article:
Kataria H.C, Bux S. Analysis of drinking bore well's water of Bhopal (M.P.). Curr World Environ 2008;3(2):317-320 DOI:http://dx.doi.org/10.12944/CWE.3.2.20
Copy the following to cite this URL:
Kataria H.C, Bux S. Analysis of drinking bore well's water of Bhopal (M.P.). Curr World Environ 2008;3(2):317-320. Available from:http://www.cwejournal.org/?p=859
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-07-10 |
|---|---|
| Accepted: | 2008-09-03 |
Introduction
Water is one of the essential natural resources available to human beings. As fresh water resources in India is declining day-by-day and has an acute shortage of potable, drinking water of good quality. The socio-Economic growth of a region is severally constraining with lacking the fair chances of availability of safe drinking water.
The natural quality of bore-wells and hand-pump’s water tends to be degraded by human activities, over pumping and by some Geological changes in groundwater system municipal and sewage water may enter into aquifer or these water resources by percolation. Hence it became the need to assess and analyse the drinking water resources regularly in public interest.
WHO recent report is alarming due to poor sanitation, the fatality figures are dispersed over wide range of Sub-Causes; Water borne diseases like cholera, typhoid, dysentery, vector-borne diseases e.g. malaria, dengue and chikungunia, encephalitis and other viral infections.
Bhopal is the capital of Madhya Pradesh is situated on 23o16' N latitude and 77o26' E longitude on hard pink red sandstone of vindhyan region sampling of 9 bore-wells are collected seasonally to keep close watch on water quality i.e. BW1-BW9. BW2 of TT Nagar and Bhadhbhada road area i.e. densely and thinly populated area. The average rainfall recorded is about 1156 mm/year in Bhopal. Sampling of nine (09) bore-wells (drinking water) sources were selected from the study area. Water samples were collected seasonally in 2 L clean jerry canes in 2006-07.
As water is the most important commodity of environment, so the water quality becomes a major global concern due to increase of human developmental activities i.e. industrial, agricultural, transportation construction due to urbanization.
Material and Methods
The present investigation has been undertaken to continuous monitoring of the study area and to analyse and assess the Physico-Chemical parameters seasonally i.e. monsoon, summer and winter.
The methods for present study are used as prescribed by APHA & NEERI and Trivedi et al. Physico-chemical Parameters has analysed volumetrically, titrimetrically as well as spectrophotometrically. To detect the trace elements Fe, Cu, Mn, Zn, Ni and Cr the samples are aspirated into air-acetylene flame and the concentration is observed by AAS (Atomic Absorption Spectrophotometer) Water samples are digested with conc. HNO3 2 or 3 times and residue of digested dissolved in distilled water, Simultaneously a blank is digested and digested Sample is run on AAS for trace- element’s analysis at different wavelength (a), slit width and sensitivity check. The instrument is calibrated for a particular element.
For Bacteriological analysis sample is collected in presterilized glass bottles and brought to laboratory in ice-box using multiple tests is done on mac’conkey broth medium using multiple tubes techniques at 37°C for 48h. MPN of coliform is observed in terms of Index/100ml by using standard tables.
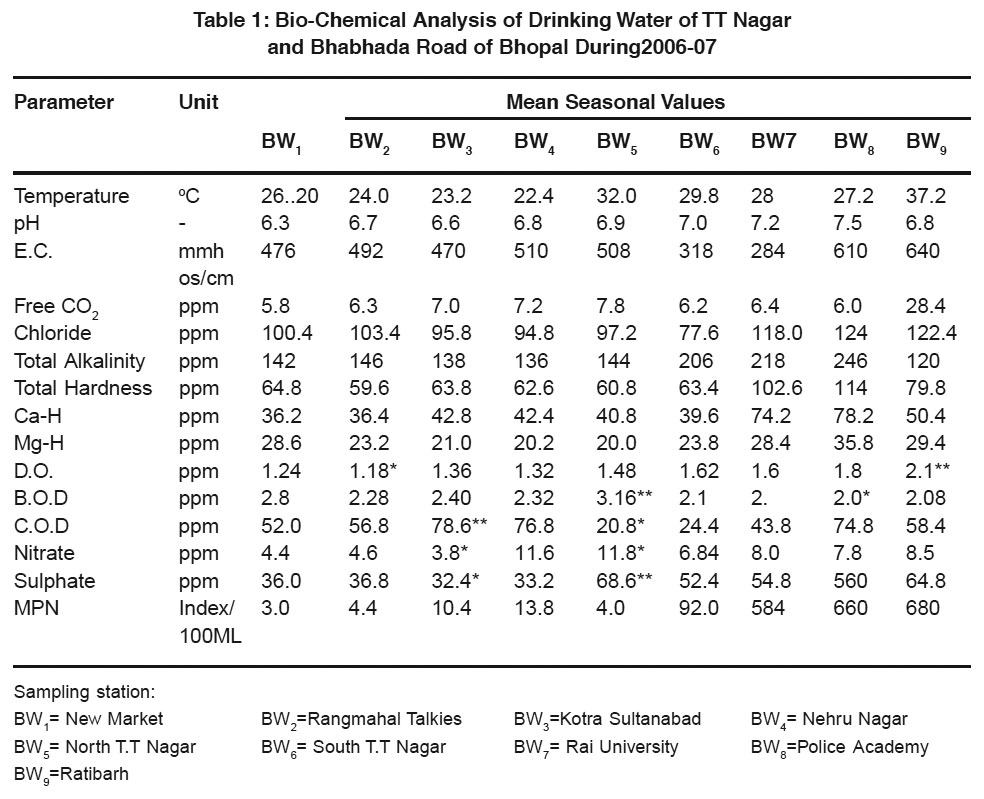 |
Table 1: Bio-Chemical Analysis of Drinking Water of TT Nagar and Bhabhada Road of Bhopal During 2006-07 Click here to view table |
Results and Discussion
The results of Physico-chemical and bacteriological analysis are summarized in Table 1 & of Trace elements analysis in Table 2.
In the present study, temperature ranges from 22.4-37.2oC. Higher value of temperature accelerates the chemical reaction in water. pH indicates the intensity of acidity and alkalinity and measures H+ ions in water. In this study minimum value of pH 6.3 at BW1 and maximum value of 7.5 is recorded at BW8 in summer. E.C. measures the dissolve ions to ranges from 284-640 mmhos/cm, at BW7 and BW9. Ground water is rich in CO2, as water comes from percolation through various states and absorbs a large amount of CO2. It ranges from 5.8-28.4 ppm at BW1 and BW9. In this study, Chloride, total alkalinity, T-H, Ca-H and Mg-H ranged from 77.6-124.0, 120.4-24.6, 59.6-114.0, 36.2-78.2 and 20-35.8 ppm respectively at different sampling stations. Higher values of alkalinity are due to leaching of soil during natural filtration of water from sewage. Hardness in sample is the result of geological formation of the water sources. The findings are similar with those of kataria et al., (1994, 95, 2005, and 2006)
Dissolved oxygen is the primary cause of corrosion of supply pipes. Higher B.O.D values are attributed to the stagnation of water body leading to the absence of self-purification cycle. C.O. and B.O.D here ranges form 1.18-2.16 and 2.0-3.16 ppm. Increase of C.O.D values is due to the pollution of input zones. In this study C.O.D has found in the range of 20.8-78.6 ppm at BW5 and BW3, Nitrate Concentration in groundwater is found due to leaching of nitrate with percolation of water ranges from 3.8-11.8 ppm. Sulphate is an important constituent of hardness with Ca and Mg. Excess amount of Sulphate in water has cathartic affect for human health. SO4- in this study ranges form 32.4-68.6 ppm at BW3 and BW5 respectively.
Handa (1994), Khurhshid et al., (1997), Kataria (2004), 2006 and 2006 has briefly described the status of groundwater contamination in India, Faridabad (Haryana) and Groundwater of Bhopal in Kolar, Kerwa and other reservoirs of Bhopal and Raisen districts. Concentration of trace elements in this study has observed in the range Cu (0.008-0.032), Fe (0.084-1.46), Mn (0.007-0.428), Ni(0.003-0.092, Pb (0.18-0.58) and Cd (0.002-0.070)ppm in respectively in ground water sources. Copper in ubiquitous in environment and hence frequently present in water, large doses of Cu irritate the stomach (ROSS, 1955) & Ernst (1991).
Excessive concentration of Iron may promote bacterial activities in water supply pipe and service mains, causing objectionable odours and red-rod disease. Manganese recorded a maximum level of 0.428 mg/L or ppm, it does not occur naturally as a metal but it is found in various salts and minerals frequently in association with iron. 0.3 mg/l is maximum permissible limit of Fe for drinking water (BIS, 1991),. Lead in drinking water occurs primarily due to corrosion of lead pipes and solders, especially in areas of soft water. Cadmium is a non-essential unbeneficial element known to have a high toxic potential. The ISI has prescribed 0.01 mg/l of Cd as desirable limit for drinking water. Hence drinking water of the study is is well suitable for drinking purpose. The removal of heave metals is possible by macrophytes e.g. Jassiacs repens; Hydrilla verticillate and some other plants could not enter into the human food chain and remove from water resources.
References
- APHA, AWWA and WPCF, Standard methods for the examination of water and waste water, 13th edn., New York (1971).
- BIS: Specification for drinking water IS: 10500; 1991. Bureau of Indian standards, New Delhi (1991).
- Handa, BK, Groundwater contamination in India, Proc. Regional workshop on environmental aspects of groundwater development, Kurukshetra (1994) 1-33.
- Brown, E., S.K. Ougland , N.W and Fishman, M.J. Methods for collection and analysis of water samples for dissolved minerals and gasses, U.S. Dept; Interior Book 5, 160 (1974); ISI (Indian Standard Institute ) Indian standard specification for drinking water, IS:10500 (1983).
- Kataria, H.C and R.K. Sharma, Physico-Chemical analysis of River Pasa (Hoshangabad) M.P J. Hydrobiol, (1994) 10: 7.
- Kataria H.C. et al. Physico-Chemical analysis of water of Kubza river of Hoshangabad. Orient. J. Chem. (1995) 11(2): 157-159
- Kataria, H.C, Preliminary study of Drinking water of Pipariya Township, Poll Res. (2000) 19(4): 645-649.
- Kataria, H.C, Analytical study of trace elements in groundwater of Bhopal city, Indian J. Env. Prot. (1JEP), (2004) 24(12): 894-896
- Kataria, H.C, Analytical study of trace elements in ground water of Bhopal city, BBR Asia, (2006) 3(1a): 161-162.
- Kataria, H.C, Detection of Trace elements in Kolar reservoir water, Bhopal, 1JEP, (2006) 26 (10): 923-925.
- NEERI, Manual on water and waste water analysis. National environmental Engineering Research Institute, Nagpur, 340 (1986).
- Ernst, M metals and their compounds in the environment VCH Weinheim, (1991) 894-1332.
- ROSS, SI (1975), Lancet 11, 87 e.f Holaein, W.S. (1970).
- WHO, Guidelines for drinking water quality 1 (1984).







