Water quality criteria and Arpa river water of Bilaspur city (India)
Sudeshan Verma1 * and S.A. Khan2
1
Chemistry C.M.D. P/G College,
Bilaspur,
India
2
Principal Government Girls College,
Korba,
India
DOI: http://dx.doi.org/10.12944/CWE.2.1.12
The present paper aims to study quality of water in the Arpa river of Bilaspur District (C.G.). Standard testing methods have been adopted for the measurement of water quality of Arpa river. Adoption of pollution abatement measures plays a great role in improving water quality.1
Copy the following to cite this article:
Verma S, Khan S.A. Water quality criteria and Arpa river water of Bilaspur city (India). Curr World Environ 2007;2(1):61-66 DOI:http://dx.doi.org/10.12944/CWE.2.1.12
Copy the following to cite this URL:
Verma S, Khan S.A. Water quality criteria and Arpa river water of Bilaspur city (India). Curr World Environ 2007;2(1):61-66. Available from: http://www.cwejournal.org/?p=633
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2007-02-18 |
|---|---|
| Accepted: | 2007-04-03 |
Introduction
Water quality is a terms used to define the physical, chemical biological or radiological characteristics by which a particular type of water may be evaluated to establish its acceptability for various beneficial uses.2-4
Materials and Methods
All the chemical used were of AR grade. Double distilled water was used for preparation of reagents. Six strategic locations were chosen for the sampling water. Those are Koni Petrol Pump (Station-I), Indira Setu (Station-II), Old Bridge (Station-III), submersible bridge (Station-IV), Kanoi paper Mill (Station-V) and Near Gatora Bridge (Station-VI). Water samples were collected in one-litre polythene bottles previously soaked with 8N.HNO3 and cleaned with detergent. The samples were acidified with 6N.HNO3 (8 ml/L) soon after sampling.
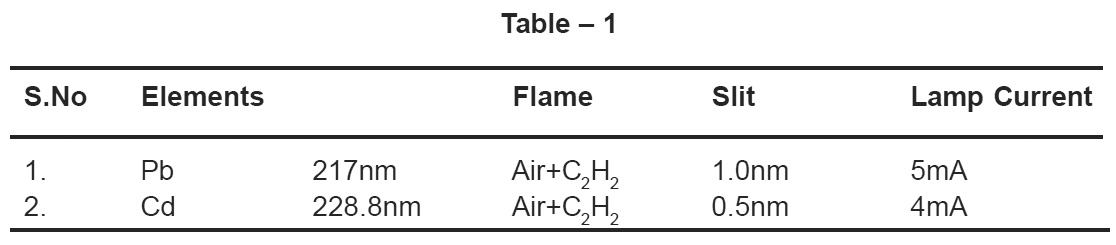 |
Table – 1 Click here to view table |
Analytical Methods
pH of the sample was measured by pH meter (Orion pH meter model 940). Cd and Pb were determined (after complexation and extraction) by AAS (Varion AA model 220). Conductivity of samples have been measured by Digital conductivity bridge (Systronics-304). The operation conditions for AAS have been described below in the Table 1. Dissolved oxygen (DO) and Biological Oxygen Demand (BOD) were determined by prescribed laboratory titrimetric methods.
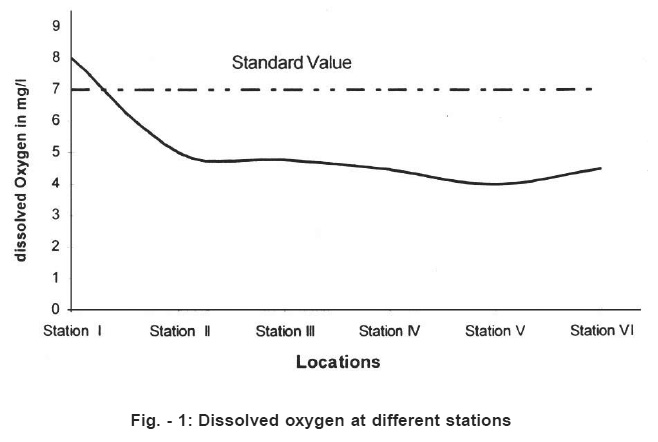 |
Figure - 1: Dissolved oxygen at different stations Click here to view figure |
Dissolved Oxygen (Wrinklers methds)
300ml sample was taken in BOD bottle, then 2ml. MnSO4 and 1 ml. Alkaline lodide Azide solution was mixed with it 2ml. H2SO4 acid was added and titrated with 0.025 N Hypo, using starch as indicator. The dissolved oxygen was then calculated using standard formula.5
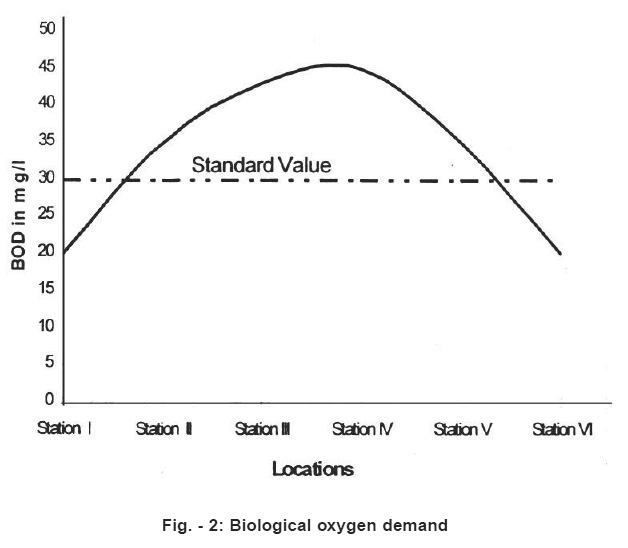 |
Figure - 2: Biological oxygen demand Click here to view figure |
[1ml of 025 N. Hypo solution = 1mg/l of dissolved oxygen]
Biological Oxygen Demand
Water sample were kept in the dark for five days. The dissolved oxygen was determined using the Winkler’s titration method and calculated BOD using standard formula5
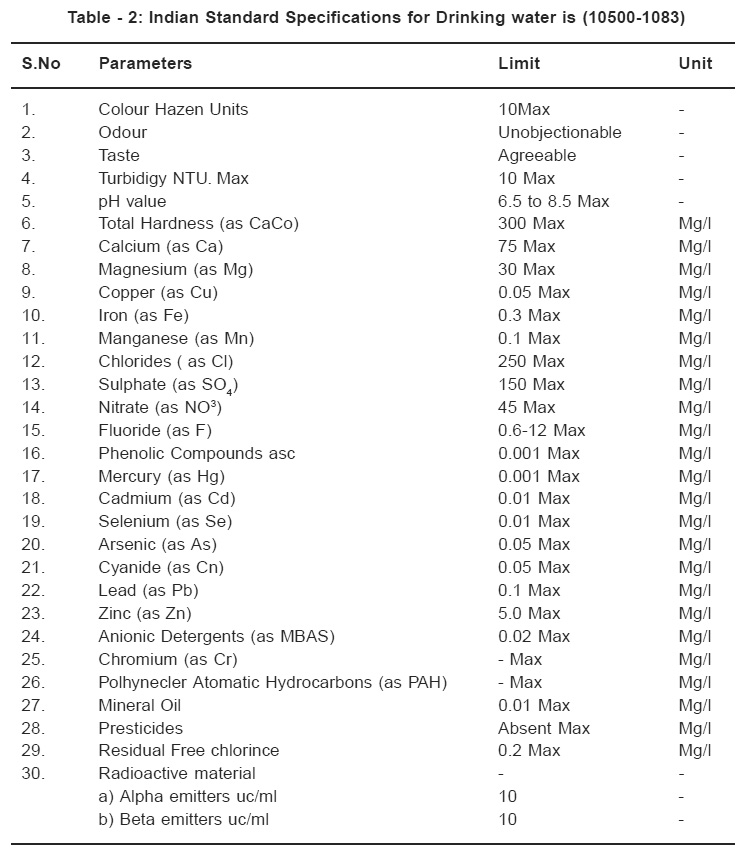 |
Table - 2: Indian Standard Specifications for Drinking water is (10500-1083) Click here to view table |
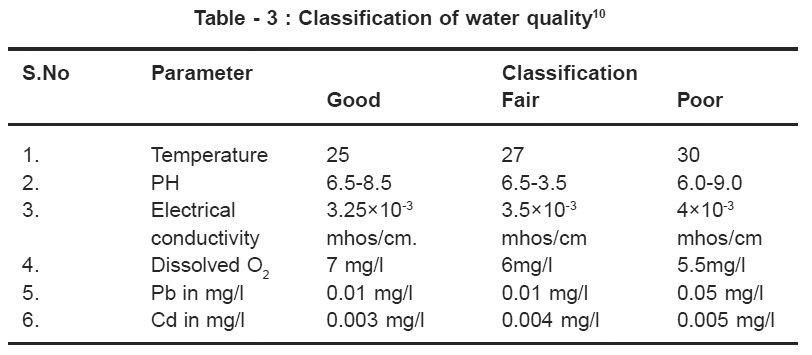 |
Table - 3 : Classification of water quality10 Click here to view table |
Chemical Oxygen Demand
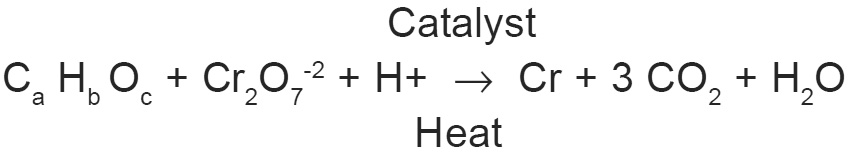
Organic substance in the samples were oxidized by K2Cr2O7 in 50% H2SO4 at a reflux temperature. Ag2SO4 was used as a catalyst. The excess of K2Cr2O7 was titrated with standard FeII (NH4) (SO4)2 (hypo) using ferroin as an indicator.
Principle reaction is :
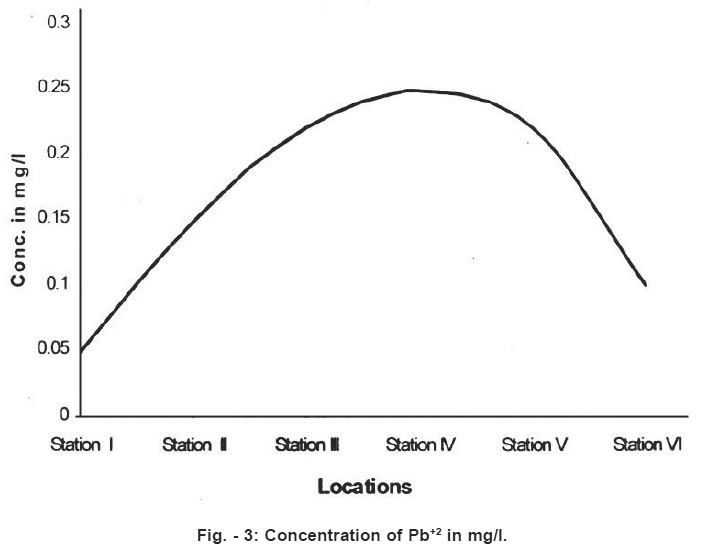 |
Figure - 3: Concentration of Pb+2 in mg/l. Click here to view figure |
Results and Discusiion
These figure inform about dramatic change in water quqlity parameters. Hence the Arpa river water is rendered unsuitable for drinking in the city area. The Arpa river is shallow but life line of Bilaspur city. Increasing population and rapid industrialization in the last few decades have icreased pollution in the potable rive water. Dissolved oxygen decreases from point no. 1 to 4. Paper mill at point 5, throws chemical containing alkali and plant degradation products. In the present study temperature ranged from 25°C to 29.5°C. The pH value ranged from 6.9 (Location) 8.69 (location 5) of the Arpa river. Location 5 and 6 show higher values than the prescribed limit.6
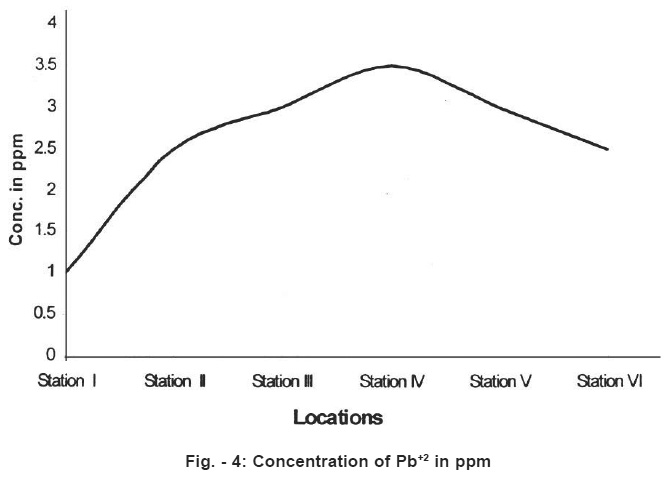 |
Figure - 4: Concentration of Pb+2 in ppm Click here to view figure |
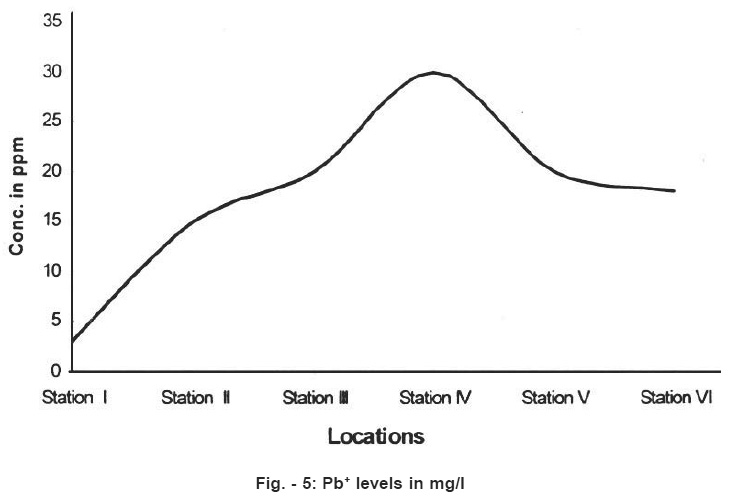 |
Figure - 5: Pb+ levels in mg/I Click here to view figure |
Total dissolved solids (TDS) ranged form 230 mg/l (station I) to 1500 mg/l (station 4,5,6). According to and Indian standards as shown in the Table No. 3 TDS should be less than 500mg/l for drinking water (Table No.3). The dissolved oxygen between 4 to 8 mg/l .The standard limit is 7mg/l for drinking water.8 The BOD ranged from 20mg/l to 45 mg/l. The standard limit is 30mg/l.9 Chemical oxygen demand (COD) was determined by titrimetric method. COD value informs about organic matter present in the drinking water.
Depletion of dissolved oxygen in water supplies can encourage microbial of NO3-1 to NO2-1 and SO4-2 to S-2 giving rise to odour problem. The Electrical conductivity value ranged between 0.50 to 6.15 × 10-3 mhos/cm to 6.15 × 10-3 mhos/ cm in the present work. Higher values are 3.30, 4,5.2×10-3 mhos/cm at locations 2,3,4,5 and 6 and water is unfit for drinking.
Standard values Pb and Cd concentrations for potable water are 0.01 mg/l and 0.003mg/l. In the present work higher values have been found in the stations 2,3,4,5 and 6. Classification of water quality has been described in the table No.3 below.
Conclusion
Rapid urbanization and increased anthropogenic activities have deteriorated the water quality parameter of Arpa river water. Therefore the pretreatment is essential before supplying for drinking purpose. (This also applies to ground water). Following methods have been suggested as remedial measures.
Remedial Measures
-
Flyash, a waste product from coal combustion can be employed as a low cost adsobenl for the11 treatment of Arpa river water et al have reported that fly ash removes metals, lowers oxygen demand. Some authors have also suggested that it removes colouring material from waste water.12
-
We propose treatment of Arpa river water by photo catalytic degradation of BOD, COD, iron and E-Coli bacteria. The solar detoxification is the process in which catalyst, for example TiO2 is exposed to the sun. The catalyst absorbs high-energy photons from UV. Portion of the solar spectrum and reactive free radicals (OH) are formed. These free radicals are powerful oxidizer and disinfectant. In general a concentration of 0.1% TiO2 is quite, effective in killing bacteria.13 This is quite in agreement with works of Kaushik N.D. et al.14
-
Vermiculite, a type of mica adsobs fluoride ion.15 Hence this can be used for treatment of water to remove fluoride ions. These abatement measures play a great role in improving water quality of Arpa river water.
References
-
F.Coulson and Mark.E., Proceedings of an International Forum. America. Sanfranciso. (1977) pp 51-56.
-
Hooda. S. and Kaur S., Laboratory manual for environmental chemistry. S. Chand and Co. ed pp (1999) 18-52.
-
CPCB. Pollution Controls Acts, Rules and Notifications Issued there under Sept (2001).
-
Hasan M.Z. and Pande S.P., Estimation of Cd and Pb. Ind. J. Envi. Hlth. (1978) 20, 234-34.
-
Allegria A., Barbera. R., Errecalde. F.et al. Environmental Cd, Pb and Ni and its possible relation between soil and vegetable content. Fresenius J. Anal Chem. Springer Verlag. (1991).
-
Kabata-Pendias A., Pendias H., Trace elements in plants and soils. CRC Press. Inc. Boca Raton. Florida. (1984).
-
Yoshikawa H. et al. Indian Health Ed. 1974, (1974) 12, 127-140.
-
Foulkes, E.C. Absorption of Cd in Handbook of Experimental pharmacology. Pp. 75-100 Berlin. New York (1986).
-
Environmental Science Series, Environmental Pollution of Cd. Bio. Physiological and Health effects. By Nath. R.P. Publihed by metha House, New Delhi (1990).
-
Jones. M. David. O, Johnstn J.T. et al., Chemistry & Society, Saunders College Publication,New York (1987) pp. 405.







