Assessment of Biogas Potential of Pineapple Waste in Mono and Co- Digestion with Cow Dung at Ambient Temperature
Djonoumawou Mèmèvêgni Grâce Floriane Chidikofan1
*
 , Thierry Godjo2
, Thierry Godjo2
 , Josias Ayao Dossa1
, Josias Ayao Dossa1
 , David Gildas Farid Adamon2
, David Gildas Farid Adamon2
 , Melhyas Kplé3
, Melhyas Kplé3
 and Guevara Nonviho4
and Guevara Nonviho4

1
National School of Energy Engineering and Processes (ENSGEP),
National University of Sciences, Technologies,
Engineering and Mathematics (UNSTIM),
Abomey,
Benin
2
National Institute of Industrial Technology (INSTI),
National University of Sciences, Technologies,
Engineering and Mathematics, (UNSTIM),
Abomey,
Benin
3
National University of Agriculture (UNA),
Kétou,
Benin
4
National School of Technical Education (ENSET),
National University of Sciences, Technologies,
Engineering and Mathematics (UNSTIM),
Abomey,
Benin
Corresponding author Email: gchidi2008@gmail.com
Copy the following to cite this article:
Chidikofan D. M. J. F, Godjo T, Dossa J. A, Adamon D. G. F, Kplé M, Nonviho G. Assessment of Biogas Potential of Pineapple Waste in Mono and Co- Digestion with Cow Dung at Ambient Temperature. Curr World Environ 2025;20(1).
Copy the following to cite this URL:
Chidikofan D. M. J. F, Godjo T, Dossa J. A, Adamon D. G. F, Kplé M, Nonviho G. Assessment of Biogas Potential of Pineapple Waste in Mono and Co- Digestion with Cow Dung at Ambient Temperature. Curr World Environ 2025;20(1).
Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2024-10-14 |
|---|---|
| Accepted: | 2024-12-23 |
| Reviewed by: | 
 Shravan Kumar
Shravan Kumar
|
| Second Review by: |

 Varsha Srivastava
Varsha Srivastava
|
| Final Approval by: | Dr. Gangadhar Andaluri |
Introduction
The anaerobic digestion of biomass represents a promising approach to mitigate the effects of global warming and combat the greenhouse effect. It offers a sustainable, green, and substitute energy source for non-renewable energy sources, whose supplies are running low at an accelerating rate.1
A biological process called anaerobic digestion breaks down organic materials into simpler ones to create energy (biogas) and fertilizer (digestate) by a microorganism's actions. Biogas is a combination of gases consisting mainly of methane (40-75%), and carbon dioxide (25-50%), with smaller amounts of additional gases, including hydrogen sulfide (H2S), ammonia (NH3), oxygen (O2), hydrogen (H2), nitrogen (N2), and carbon monoxide (CO).2-3 Methane contained in biogas is an excellent source of heat and electrical power generation.4 The heating value of well-purified biogas is close to that of natural gas.5 Anaerobic digestion technology has the advantage of treating main organic waste,6 giving biogas a strong potential for sustainability.7 Organic waste can be classified as follows: vegetable waste, animal waste, food waste, fruit waste, and agricultural residues.8
Pineapple, Ananas comosus (L.) Merr., a tropical edible fruit belonging to the Bromeliaceae family, is regarded as a significant fruit because of its accessibility to a large number of people, excellent nutritious content and a delicious taste.9,10 In Benin, pineapple is among the most significant fruit varieties, representing the third most valuable export potential after cotton and cashew. In 2019, it contributed 0.42% of the national GDP and 1.95% of the agricultural GDP.11 They come in the varieties known as Smooth Cayenne and Sugarloaf. It is more prone to deterioration since it contains roughly 85% moisture, necessitating manufacturing or instantly consumption.12 According to estimates, approximately 264,834 tons of raw pineapple were marketed in 2020-2021.13 Only around 99,814 tons are processed mainly into juice by all the units counted.11 These quantities undergo processing at three distinct scales: artisanal, semi-industrial, and industrial. The common characteristic of units is to generate a finished product to consume. They coexist in a state of equilibrium, forming a continuum of activities with well-defined characteristics and responding to different market segments.14
Processing pineapple into juice is very energy-intensive. Most semi-industrial and industrial units use wood, butane gas, conventional electricity, and diesel. However, due to the inflation in energy costs over the last decade, most processing units are encountering significant challenges in procuring supplies.14 Pineapple manufacturing produces wastes (pulp, peel, core, stem, crown, and leaves) representing 60% (w/w) of the entire weight of the pineapple. These have many unique properties that warrant further investigation.12 This organic waste is unutilized and continually stored close to the units. Consequently, this accumulation of waste can contribute to (i) the pollution of soil, surface, and groundwater, (ii) the degradation of air quality due to the putrefaction of this waste giving off unpleasant odors and irritating vapors, and (iii) the emission of greenhouse gases into the atmosphere and global warming. Pineapple waste is the usual instance of biomass made of lignocellulosic material with a huge capacity for energy production but is not yet used as a durable, ecological, and clean source.15 Mamo T et al.16 reported that biogas outputs from pineapple peels range from 0.41- 0.67-meter cube per kilogram (m3/kg) volatile solids with a 41–65% methane concentration.
Biogas productivity of substrates is affected by physico-chemical conditions such as feedstock type, pH, temperature, carbon (C) and nitrogen (N) content, chemical oxygen demand (COD), etc. Anaerobic co-digestion might represent a promising approach for enhancing methane yields.1 In order to improve the anaerobic digestion process, co-digestion entails treating substrates jointly to give the microorganisms the necessary carbon and nutrients.17 Although microbial activity can be supported by separate substrates, mixing substrates frequently produces a synergistic effect in which the mixture's methane yield is greater than the sum of its parts. However, depending on the makeup and ratios of the substrates, antagonistic effects, including a decrease in methanogenic activity, can also happen.18 The potential of codigestion for methane optimisation is demonstrated by studies. Study,19 for instance, showed that a 1:1 ratio of food waste to fruit and vegetable waste stabilised the digestion process and enhanced methane generation to 0.49 m³ CH4/kg volatile solids. In a similar vein, study20 found that co-digesting jackfruit waste, pineapple peels, and banana peels with 25% cow dung increased biogas output by two to three times. By co-digesting tomato pulp with animal dung, study21 was able to generate the maximum methane yield, 404 ml CH4/gVS, underscoring the significance of preserving a higher proportion of animal dung for best outcomes. On the other hand, Sitorus and associates 22 found that the high acidity of plant waste may prevent methanogenic activity because of the quick acidification and volatile fatty acid buildup. Notwithstanding these challenges, co-digestion with animal waste stabilises the anaerobic digestion process by providing vital nutrients and buffering capacity.3 This was shown by Mukumba and partners,23 who discovered that a 75% methane composition was produced by an equal mixture of cow, goat, donkey, and horse excrement.
Any project of residue valorization through anaerobic digestion requires the knowledge of biogas generation potential to provide the technical elements needed for an economic feasibility study. However, this knowledge on pineapple residues remains limited. Several methods exist to determine the biogas or biomethane potential of a given residue or combination of residues. Estimates can be made based on the chemical or biochemical composition of the residue.24 These estimates do not take into account several factors: inhibition phenomena due to the presence of certain substances or overloading, the biodegradability of the material, the conditions of the reaction medium, and the proportion of the substrate consumed by the microbial flora for its growth.25 Biochemical Methane Production (BMP) test also can be used to evaluate objectively this potential.24,26
This article aims to optimize the production of biogas from pineapple residues. It investigates the biogas production potential of pineapple waste in mono and co-digestion with cow dung at ambient temperature and to determine which organic waste mixture is best suited to biogas production.
Materials and Methods
Research area
The investigation was conducted in the West African nation of Benin (114.763 km2), which is situated between 06°15 and 12°25' north latitude and 0°40' and 3°45' east longitude. Throughout the country, average annual temperatures vary between 26 and 28°C. The yearly temperature range is minimal in the southern region (5 to 10°C), although it is higher (11 to 13°C) in the north (from latitude 8°N northwards). The estimated population of Benin is 9,983,884 inhabitants.
Sampling, preparation, and characteristics of wastes
The substrates used in this research were pineapple cakes (pulp residues and peels), crowns, and cow dung.
Pineapple waste comes from Promo Fruits Bénin, situated in Allada (Benin) in southern Benin, 90 km from the capital. The processing capacity of this company is 21,900 tons per year.14 The cow dung, used as inoculum, comes from the Saclo’s slaughterhouse in Bohicon.
In the laboratory, the waste samples were sorted to remove undesirable matters then individually mixed in a blender to minimise their granulometry and enhance their surface area during the test. Then, the substrates were diluted with water to a total volume that was twice the volume of the substrates.27 The physico-chemical properties of the waste are shown in table 1.
Table 1: Physico-chemical properties of substrates
Parameters | Crown28 | Cake29 | Cow dung30 |
Moisture (%) h | 83.4 ± 1.8 | nd | nd |
Dry matter (MS) (%) h | 16.8 ± 1.8 | 26 | 21 |
Volatile solid (MV) (%) s | 94.9 ± 0.6 | nd | nd |
A (% ash) s | 5.1 ± 0.6 | 2.8 | nd |
Total soluble sugar s | 38.14 ± 4.2 | 4.8 | nd |
Cellulose (%) s | 11.63 ± 0.6 | 34.9 | nd |
Hemicellulose (%) s | 13.32 ± 24 | 28.2 | nd |
Lignin (%) s | 15.03 ± 0.6 | 2.2 | nd |
Reducing sugars s | 5.23 ± 0.1 | nd | nd |
C | 44.05 | nd | 31 |
H | 5.81 | nd | 85 |
N | 0.87 | nd | 1.46 |
O | 49.27 | nd | 17.46 |
C/N ratio | 50.63 | 43.3 | 21.4 |
pH | nd | 5.6 | 7.5 |
Biogas generation potential tests
To assess the biogas generation potential of the waste, samples were placed in 500 ml serum bottles, which served as reactors with a gas release valve attached to a screw cap. The tests were conducted in batch mode. The experimental design was defined using Minitab's statistical design software (Minitab.19) based on the Simplex-Centroid Designs method,31 which was selected due to its simplicity, time, and material availability. Table 2 presents the composition of the substrates in each reactor.
Table 2: Mixing design experiment matrices
Proportion (%) | |||
Formulation | C (crown) | T (Cake) | B (cow dung) |
R1 | 100 | 0 | 0 |
R2 | 0 | 100 | 0 |
R3 | 0 | 0 | 100 |
R4 | 50 | 50 | 0 |
R5 | 0 | 50 | 50 |
R6 | 50 | 0 | 50 |
R7 | 33.33 | 33.33 | 33.33 |
Batch reactor Displaced water vessel Water vessel
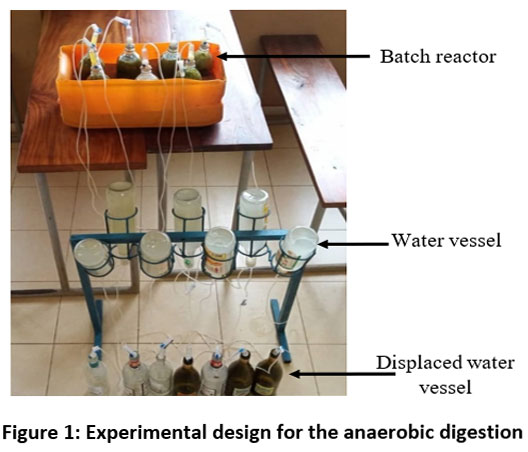 | Figure 1: Experimental design for the anaerobic digestion
|
The physico-chemical parameters analysed were pH and temperature. The pH of the mixtures was determined weekly using pH paper. Temperature was measured daily using a Benetech laser thermometer. The biogas produced was measured daily using a one-liter bottle calibrated to the nearest millimetre. The downward water displacement method at air pressure for each reactor is used.28 Lemon water was used to prevent the CO2 in the biogas from being trapped. The amount of water displaced was proportional to the volume of biogas produced. Measurements were taken every day at the same fixed time (01:00 pm). The tests lasted a maximum of 45 days, corresponding to the complete digestion of the last substrate formulation. Each test's cumulative biogas production was calculated by adding together the daily biogas production. Biogas production was monitored and measured until biogas the water level in the bottles stabilised on at least 3 successive days.
Data processing and analysis
All experimental data were recorded in Excel spreadsheets. First, the averages were calculated and graphs were created to analyze how the parameters changed over time. Then, biogas production data and mixing factors were used to develop a mathematical simulation model in Minitab 19 using the "Analyze a mixing plan" function. To verify the accuracy of the model, the experimental biogas production values were compared with those obtained from the model. The average difference between the predicted and actual values of the model is measured by the relative residual root mean square error (rRMSE) which is the standardised residual root-mean-square error. The model's ability to predict biogas output is indicated by the coefficient of determination (R2), which ranges from 0 to 1. The closer the model's R2 is near 1, the more accurate its predictions will be.
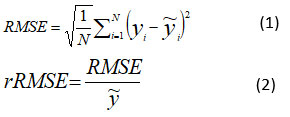
N: number of measurements performed

With
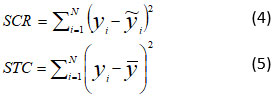
SCR: Sum of squared residuals
Results and discussion
Evolution of the average temperature
Figure 2 illustrates the average temperature changes in the digester throughout the experiment. The overall trends appear to be consistent. The average temperatures ranged from 23°C to 33°C, with a mean of 26.5°C ±0.33. These mean temperatures fall within the mesophilic temperature range. However, they are lower than the optimal temperatures suggested by the researchers 32,33 for cow dung's mesophilic digestion (29 and 35°C) and the researcher 34 for anaerobic co-digestion of pig slurry with bio-waste from pineapple peel in a continuously stirred reactor (37 ± 1 ºC). Maximum temperatures close to these optimal ranges for the substrates (T, B, T+B, B+C) were only recorded on around 9 days out of the 45-day trial. This is comprehensible given that the tests were conducted during the rainy season and by the addition of extra water to the waste. Adding water to cow dung in a 1:2 ratio35 found that this required a large amount of heat to keep the temperature high enough to allow bacterial activity. According to study36, the wastes in the present study are being evaluated for their suitability for biogas production under sub-optimal conditions. Nevertheless, according to a number of recent research, the mesophilic degradation temperature of agricultural waste should be between 21 to 40°C.37,38
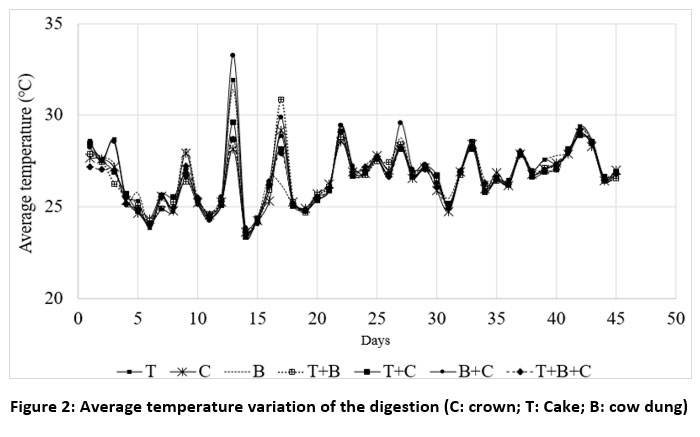 | Figure 2: Average temperature variation of the digestion (C: crown; T: Cake; B: cow dung)
|
Evolution of average pH of substrates
Figure 3 illustrates the evolution of average pH during the experimentation.
The initial pH of substrates composed of cow dung (B) and the mixture of cow dung and crown (B+C) are close to neutral at 7.1 and 6.7 respectively. The acidic nature of the other substrates, especially the cakes + crowns (T+C), emphasizes the positive impact of blending vegetable and animal matter in specific proportions.
The trends observed during the experiment are similar for all the trials. There was a fluctuation in pH over the initial 3 weeks, resulting in a relative drop. This decrease in pH is attributable to the production of volatile fatty acid (VFA) by acid-generating bacteria at the initial stages of the process.
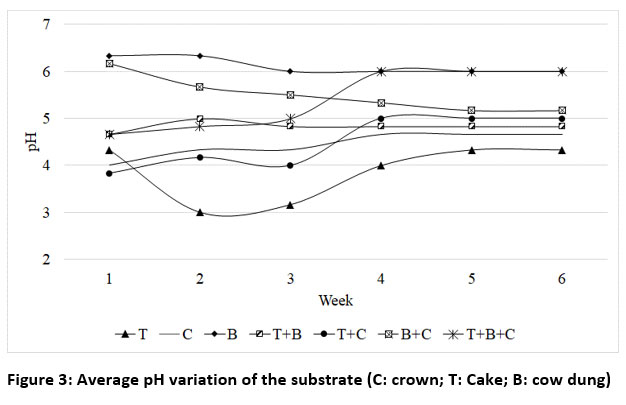 | Figure 3: Average pH variation of the substrate (C: crown; T: Cake; B: cow dung)
|
This trend in pH evolution is similar to that achieved by researchers.39,35 It was not until the 4th week that pH values became stable. This stability was not preceded by a substantial increase, as in the results obtained by researchers39,35 where pH increased to its normal operating value before stabilizing. According to these authors, the gradual increase in pH at the end of start-up appears when VFAs are consumed by methanogens and transferred to methane. Additionally, protein hydrolysis and amino acid production could also lead to an increase in pH. This was not observed in the present study.
Furthermore, the pH values of all substrates in the present study remained below the optimal pH range for methanogens (6.8-7.6). At pH values below 6.6, the growth of these bacteria is significantly reduced, resulting in suboptimal performance. This imbalance and the low pH can be corrected by incorporating NaOH or HCl in the pre-treatment or during the fermentation period, as previously described by researchers.35,40 Similarly, researcher41 proposes that in the instance of an anaerobic digestion process run at low initial pH values of 4.5-5.5, buffers (e.g., H2CO3/HCO3-/CO32-) and/or nutrients must be provided to make the alkalinity of the substrates higher to keep up neutral pH.
Evolution of daily biogas production
Figure 4 shows the average daily biogas production.
Biogas production started immediately from the 1st day of the experimental start-up date. This observation indicates that biogas production commenced at an early stage for all substrates, thus reducing the time required for startup.
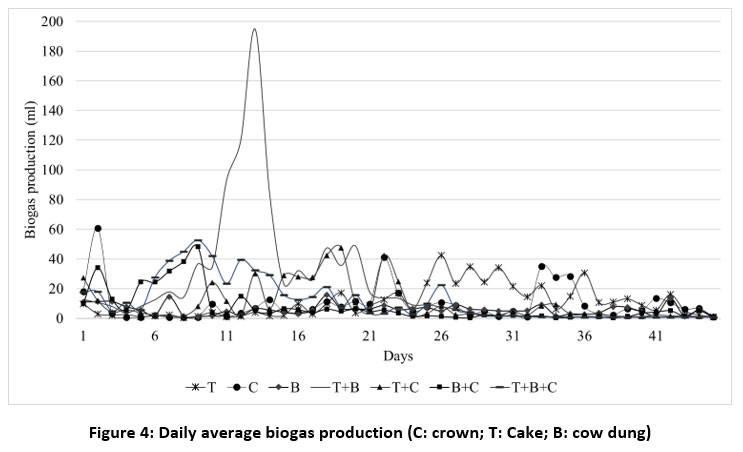 | Figure 4: Daily average biogas production (C: crown; T: Cake; B: cow dung)
|
This may be explained by the fact that microorganisms acclimated to the substrate during storage at the production site. These results are similar to those of researchers.33,42 After this start-up phase, daily biogas production fluctuated sharply. For substrate T, daily production ranged from 0.4 to 42.4 ml recorded on day 26th. For substrate C, production varied between 0.7 and 60.9 ml, with the highest levels recorded on days 2, 22, and 33. Substrate B produced very low daily yields ranging from 0.9 to 15.7ml (on the 18th day). Daily production of substrate T+B ranged from 1 to 194.7ml (obtained on the 13th day). For the T+C mix, high production levels were recorded on days 19 and 22, with variations ranging from 1.3 to 47.6ml. For mixture B+C, values ranged from 0.4 to 48.1 ml (on day 10). The peak production period is between days 1 and 13.
For the T+B+C mixture, production varies between 0.7 and 52.6 ml (on day 9). These daily fluctuations can be attributed to three factors. Firstly, the variation in temperature for the trials.43-45 Study46 showed that temperature spikes between 35°C and 30°C and between 30°C and 32°C were responsible for a decline in the output of biogas rates. However, no lasting damage to digestion performance was observed once temperatures had recovered. Secondly, they can be justified by the poor contact of the substrate with the microorganisms, which limited the conversion of the substrate into biogas47,48 and thirdly, the acidity of the reaction medium induced a low pH. Single digestion of cow dung (B), cake (T) and crowns (C) shows the lowest daily biogas production compared to co-digestion. These results are similar to those of the study.42 Biogas production stabilized and almost ceased on day 28 for substrate T+B, and by day 35 for substrate T+B+C. For all other substrates, production continued, but very weakly, until day 45. This observation indicates that the residence times for these two substrates are shorter than those for the others. The cessation or reduction in biogas production is linked to the lack of new nutrients. Anaerobic microorganisms process the substrate containing proteins and carbohydrates. As the quantity of these substances decreases, the biogas production process also slows down.49
Cumulative biogas production
Each substrate's overall biogas production volume in the reactors is illustrated in Figure 5. This figure presents the sum of the daily biogas production over the 45-day experimental period. The volume produced in three replicates for each treatment was averaged daily.
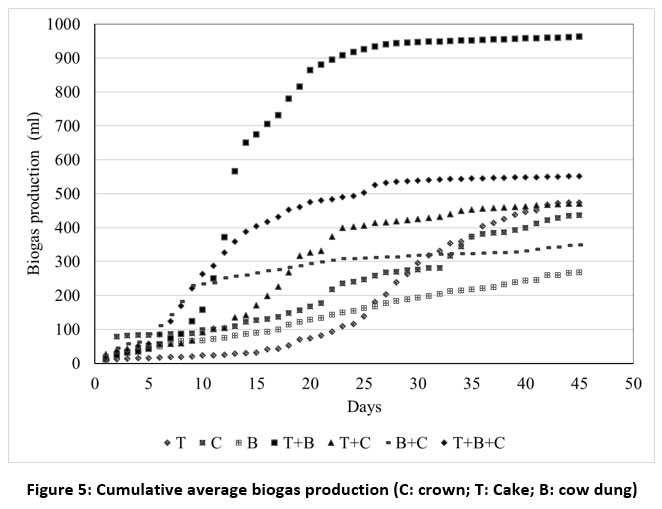 | Figure 5: Cumulative average biogas production (C: crown; T: Cake; B: cow dung)
|
The biogas production kinetics are ranked in the following order: (T+B) 883.033ml > (T+B+C) 599.063ml > (T) 467.11ml > (T+C) 425.675ml > (C) 384.241ml > (B+C) 311.975ml > (B) 259.71ml. The mixture of cakes with cow dung (T+B) yielded the highest biogas production. A comparison of this biogas production with that of the mixture of cake, cow dung, and crown (T+B+C) and the production of cake only (T) with that of cake and crown (T+C) indicates that the addition of the crown had an inhibitory effect on production. This is due to the slow degradation of this waste material, which is composed of a complex lignocellulosic structure, making it difficult for bacteria to digest the substrate. From table 1 related to physico-chemical properties of substrates, % lignin > % hemicellulose > % cellulose in the crown. Inversely, % lignin < % hemicellulose < % cellulose in the cake. Study50 highlighted the fact that the digestion of cellulose is superior to that of hemicellulose, which is also superior to that of lignin, while highlighting the fact that lignin is very difficult to digest. The efficiency of the process is limited during hydrolysis when microbial enzymes are unable to degrade the substrates.51
To optimize the digestion process of pineapple peels, researcher 52 carried out a pre-treatment using chemical agents such as sulphuric acid and alkaline hydrogen peroxide (H2O2) to solubilize the lignin in the matter. The use of a potassium hydroxide (KOH) solution has also been demonstrated to be effective.40 The low biogas production of substrates T, T+C, C, and B+C in comparison to the other substrates (T+B and T+B+C) can be attributed to a great C/N ratio. Conversely, in the instance of cow dung (B), it can be justified by a low C/N ratio in comparison to the recommended optimum can be observed. A very great C/N ratio results in a nitrogen concentration that is insufficient for microbial growth53 and an accumulation of unreacted carbon54.
Conversely, a low C/N ratio, i.e. a high nitrogen content, leads to a build-up of nitrogen from ammonia and subsequent inhibition of the digestion process.53,55 Consequently, microorganisms need a C/N ratio that is suited to their metabolic processes.56 For optimal biogas production, organic material must have a C/N ratio nearly 20-30. A C/N ratio of 30 has been found to result in biogas production that is 13.3-66.5% more than that with a C/N ratio of 25.40 A study by researcher57, showed that nitrogen-rich supplements, such as swine manure and urea, corrected the imbalances caused by a high C/N ratio, highlighting the fact that urea is the best solution as it not only increases the nitrogen content of the digestate, but also promotes lignin degradation by speeding up the hydrolysis process.
It is challenging to make direct comparisons between the present results and the various biogas production curves found in the literature due to the differing experimental approaches employed, which result in data expressed in different units.58 Table 3 compares the results obtained with cow dung with other studies at mesophilic temperatures.
Table 3: Comparison of the cow dung results with others studies at mesophilic temperature
Quantity of biogas (liters/kg) | Test duration (day) | |
Present study | 2.6 | 45 |
Study 59 | 15.6 | 25 |
Study 35 | 57.45 | 63 |
Study 60 | 0.38 | 41 |
The biogas production observed in this study with cow dung (2.6 liters/kg) is notably lower than that reported by study59 (15.6 liters/kg). In the case of this study, the batch reactor is made of glass, whereas in study59 study, the batch reactor is made of plastic. The reactor material can therefore also have an impact on the difference in biogas yield from cow dung through temperature variation. According to researcher61, biogas production efficiency is influenced by substrate composition.
In particular, animal manure may contain inhibitory agents, including recalcitrant or toxic materials like ammonia (or its excess), sulfide, both light metal ions and heavy metals like Na, K, Mg, Ca, and Al 62-64. These compounds originate from commercial feeds or animal feed additives that encourage rapid growth and guard against cattle illnesses. No analysis of possibly hazardous or inhibiting substances was done in this study. Their compositions depend on the composition of their feed.49, 65
Nevertheless, the results indicate that all the substrates tested can be utilized for biogas production.
Biogas production predictive model
The analysis of the mixing plan carried out in MINITAB has enabled us to propose a law that predicts biogas production as a function of the proportions of materials added. The linear model was selected to fit the experimental results and is presented by the following equation:
![]()
with T+B+C =1
The value of the R² coefficient associated with the model estimate is 87.17%, and that of the adjusted R²aj coefficient is 82.9%. This indicates good predictive quality of the biogas potential. This predictive quality is confirmed by Figure 6, which illustrates the biogas potentials obtained by the relationship and their mean values measured experimentally. The fitted values overlap well with the mean experimental values.
The curves show increasing cumulative production from the 1st to the 28th day for substrates T+B, T+B+C, T+C, B, and B+C. For the remainder of the digestion period, cumulative production tends to stabilize. For the total digestion time (45 days), biogas production kinetics are ranked in the following order: (T+B) 962.4ml > (T+B+C) 551.71ml > (T) 473.92ml > (T+C) 471.24ml > (C) 436.52ml > (B+C) 311.975ml > (B) 267.58ml.
Figure 6. Biogas potentials obtained by the predictive model and mean experimental values
The results of model validation by analysis of variance are presented in Table 3. Given that the value of p for T*B is less than 0.05%, we can deduce that production performance depends in particular on the proportions of cake and cow dung.
Table 4: Variance analysis
Source | DL | SomCar seq | SomCar ajust | CM ajust | F Value | p-Value |
Regression | 5 | 874916 | 874916 | 174983 | 20.39 | 0.000 |
Linear | 2 | 156932 | 72516 | 36258 | 4.23 | 0.035 |
Quadratic | 3 | 717985 | 717985 | 239328 | 27.89 | 0.000 |
T*B | 1 | 713287 | 703375 | 703375 | 81.97 | 0.000 |
T*C | 1 | 863 | 1037 | 1037 | 0.12 | 0.733 |
B*C | 1 | 3835 | 3835 | 3835 | 0.45 | 0.514 |
Residual error | 15 | 128720 | 128720 | 8581 | ||
Inadequate fit | 1 | 22042 | 22042 | 22042 | 2.89 | 0.111 |
Pure error | 14 | 106677 | 106677 | 7620 | ||
Total | 20 | 1003636 |
Conclusion
This study evaluated the biogas production potential of pineapple waste (cakes and crowns) in mono and co-digestion with cow dung using different mixing ratios. The study was conducted at ambient temperature, with a variation between 23 and 33°C. The results demonstrated that the cake-cow dung (T+B) and cake-dung-crown (T+B+C) mixtures exhibited the highest biogas production performance. It is noteworthy that the pH of the various mixtures was not optimal for the digestion tests, as they remained below neutral throughout. It would therefore be beneficial to investigate the incorporation of buffers and/or nutrients into the substrates to enhance biogas production performance while keeping the reaction temperature stable. Tests combustion of biogas from each substrate also deserve to be done.
Acknowledgement
We would like to thank Promo Fruit Benin for collecting pineapple waste.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Conflict of interest
The author(s) declares no conflict of interest.
Author Contributions
Djonoumawou Mèmèvêgni Grâce Floriane CHIDIKOFAN: Conceptualization, Analysis, Supervision, Writing – Review and Editing.
Thierry Gorlon GODJO: Supervision and reading.
Josias Emen Ayao DOSSA: Conceptualization, Methodology, Data Collection, Analysis, and Writing – Original Draft.
David Gildas Farid ADAMON, Melhyas Kplé and Guevara Nonviho: Manuscript reading and approval.
References
- Beniche I., Hungría J., El Bari H., Siles J. A., Chica A. F., et Martín M. A., « Effects of C/N ratio on anaerobic co-digestion of cabbage, cauliflower, and restaurant food waste », Biomass Conv. Bioref., 2021; 11(5): 2133-2145, doi: 10.1007/s13399-020-00733-x.
CrossRef - Okolie, J.A., Tabat, M.E., Ogbaga, C.C., Okoye, P.U., Davis, P., Gunes, B., Economic and environmental assessments of a novel integrated process for biomethane production and ammonia recovery from potale. Chem. Eng. J. 2022; 46, 137234. doi:10. 1016/j.cej.2022.137234.
CrossRef - Nwokolo N., Mukumba P., Obileke K., et Enebe M., « Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production », Processes. 2020; 8 (10): 1224, doi: 10.3390/pr8101224.
CrossRef - Karki, R., Chuenchart, W., Surendra, K.C., Shrestha, S., Raskin, L., Sung, S., Hashimoto, A., Kumar Khanal, S. Anaerobic co-digestion: current status and perspectives. Bioresour. Technol. 2021; 330, 125001. doi: 10.1016/J.BIORTECH.2021.125001
CrossRef - Rogala, Z., Stanclik, M., Luszkiewicz, D., & Malecha, Z. Perspectives for the Use of Biogas and Biomethane in the Context of the Green Energy Transformation on the Example of an EU Country. Energies. 2023; 16(4): 1911.
CrossRef - Mignogna D, Ceci P, Cafaro C, Corazzi G, Avino P. Production of Biogas and Biomethane as Renewable Energy Sources: A Review. Applied Sciences. 2023; 13(18):10219. https://doi.org/10.3390/app131810219
CrossRef - Tagne R. F. T., Dong X., Anagho S. G., Kaiser S., et Ulgiati S., « Technologies, challenges and perspectives of biogas production within an agricultural context. The case of China and Africa », Environ Dev Sustain. 2021; 23(10): 14799-14826. doi: 10.1007/s10668-021-01272-9.
CrossRef - Edem I. E. et Olugbade Q. O., « Biogas substrates performance ranking based on sustainability potential, biodegradability, and yield characterization: the case of an area in Southwest Nigeria », Nig. J. Tech. 2022; 41(3): 444-453. doi: 10.4314/njt.v41i3.4.
CrossRef - Samreen, Chilukuri S., Lingathoti E., et Beera V., « Physicochemical Characteristics of Pomegranate and Pineapple Juice », Indian J Ecol. 2020; 43, 60-63.
- Ali A., Chen Y., Liu H., Yu L., Baloch Z., Khalid S., Zhu J., and Chen L., « Starch-based antimicrobial films functionalized by pomegranate peel », International Journal of Biological Macromolecules. 2019; 129, 1120-1126. doi: 10.1016/j.ijbiomac.2018.09.068.
CrossRef - Kinha D. C., Payen S., Sohinto D., Govindin J. C., et Padonou F., « Analyse de la chaîne de valeur ananas au Bénin. », Bénin, agrinatura for European Commission, Value Chain Analysis for Development, 2019.
- Singh T. A., Sarangi P.K., et Singh N. J. R, « Tenderisation of Meat by Bromelain Enzyme Extracted from Pineapple Wastes », Int. J. Curr. Microbiol. App. Sci. 2018; 7(09): 3256-3264, doi: 10.20546/ijcmas.2018.709.404.
CrossRef - Adda C., Edah L., et Latifou A. B., « Evaluation la performance de la productivité, de la Transformation et de la commercialisation de l’ananas au Bénin », International Journal of Progressive Sciences and Technologies. 2021; 26(2): 310-321.
- Akpassonou R., Godjo T., Adamon F., Satoguina H., Guidi C., et Gbenou J., « Energy recovery of pineapple juice processing waste at Promo fruits Benin: Feasibility study and solution emergence », International Journal of Innovation and Applied Studies. 2023; 38 (4): 948-969.
- Tanamool V., Chantarangsee M., et Soemphol W., « Simultaneous vinegar fermentation from a pineapple by-product using the co-inoculation of yeast and thermotolerant acetic acid bacteria and their physiochemical properties », Biotech. 2020; 10(3): 115. doi: 10.1007/s13205-020-2119-4.
CrossRef - Zinare Mamo T., Dutta A., et Jabasingh S. A., « Start-up of a pilot scale anaerobic reactor for the biogas production from the pineapple processing industries of Belgium », Renewable Energy. 2019; 134, 241-246. doi: 10.1016/j.renene.2018.11.058.
CrossRef - Zhang C., Su H., Baeyens J., et Tan T., « Reviewing the anaerobic digestion of food waste for biogas production », Renewable and Sustainable Energy Reviews, 2014; 38, 383-392. doi: 10.1016/j.rser.2014.05.038.
CrossRef - Avena L. G., Almendrala M., Marron E. J. and Obille J. A. Biogas Production from the Co- and Tri-digestion of Pineapple Wastes with Food Wastes and Pig Manure, E3S Web Conf. 2024; 521, 01004. DOI: https://doi.org/10.1051/e3sconf/202452101004
CrossRef - Lin J., Zuo J., Gan L., Li P., Liu F., Wang K., Chen L., Gan H., « Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China », Journal of Environmental Sciences. 2011; 23(8): 1403-1408. doi: 10.1016/S1001-0742(10)60572-4.
CrossRef - Mibulo, T. , Nsubuga, D. , Kabenge, I. , Kiggundu, N. and Wydra, K. Comparative Study of Biogas Production from Jackfruit Waste, Banana Peels, and Pineapple Peels Co-Digested with Cow Dung. Journal of Sustainable Bioenergy Systems, 2023; 13, 1-15. doi: 10.4236/jsbs.2023.131001.
CrossRef - Parralejo A. I., Royano L., González J., et González J. F., « Small scale biogas production with animal excrement and agricultural residues », Industrial Crops and Products. 2019; 131, 307-314. doi: 10.1016/j.indcrop.2019.01.059.
CrossRef - Sukandar B. S., et Panjaitan S. D., « Biogas Recovery from Anaerobic Digestion Process of Mixed Fruit-Vegetable Wastes », Energy Procedia. 2013; 32, 176-182. doi: 10.1016/j.egypro.2013.05.023.
CrossRef - Mukumba P., Makaka G., Mamphweli N., et Xuza V., An assessment of the Biodegradability of a biogas digester fed with substrates at different mixing ratios. Waste-to-Energy (WTE). 2019; 107–126.
- Pham C. H., Triolo J. M., Cu T. T. T., Pedersen L., et Sommer S. G., « Validation and Recommendation of Methods to Measure Biogas Production Potential of Animal Manure », Asian Australas. J. Anim. Sci. 201; 26(6): 864-873. doi: 10.5713/ajas.2012.12623.
CrossRef - Thomsen I. K., Olesen J. E., Møller H. B., P. Sørensen, et B. T. Christensen, « Carbon dynamics and retention in soil after anaerobic digestion of dairy cattle feed and faeces », Soil Biology and Biochemistry. 2013; 58, 82-87. doi: 10.1016/j.soilbio.2012.11.006.
CrossRef - Holliger C., Alves M., Andrade D., Angelidaki I., Astals S., Baier U., Bougrier C., Buffière P., Carballa M., de Wilde V., Ebertseder F., Fernández B., Ficara E., Fotidis I., Frigon J-C., de Laclos H. F., Ghasimi D. S. M., Hack G., Hartel M., Heerenklage J., Horvath I. S., Konrad Koch P. J., Krautwald J., Lizasoain J., Liu J., Mosberger L., Nistor M., Oechsner H., Oliveira J. V., Pauss M. P. A., Pommier S., Porqueddu I., Raposo F., Ribeiro T., Pfund F. R., Strömberg S., Torrijos M., van Eekert M., van Lier J., Wedwitschka H. and Wierinck I., « Towards a standardization of biomethane potential tests », Water Science and Technology. 2016; 74(11): 2515-2522. doi: 10.2166/wst.2016.336.
CrossRef - Barbosa, F.J.L., Cabral, A.R., Capanema, M.A. # and Schirmer, W.N. Biogas Generation Potential of Anaerobic Co-Digestion of Municipal Solid Wastes and Livestock Manures. Journal of Solid Waste Technology and Management, 2018, 44(3): 248-258.
CrossRef - Kumar M., Jacob S., Upadrasta L., et Banerjee R., « Biomethanisation of pineapple wastes using potent anaerobic consortia substituting cow manure », Environ. Eng. Manag. J. 2017; 16(11): 2647-2655. doi: 10.30638/eemj.2017.275.
CrossRef - Perraud I., « Etude de la fermentation en milieu solide du tourteau d’ananas », ORSTOM, Fort-de-France, 1994. [En ligne]. Available on : https://www.documentation.ird.fr/hor/fdi:41441
- Sakouvogui A., Baldé Y. M., Barry M. F., Kanté C., et Keita M., « Évaluation du potentiel en biogaz de la bouse de vache, de la fiente de poule en mono et en codigestion à Mamou, République de Guinée », Afrique Science. 2018; 14(5): 147-157.
- Goupy J.et Creighton L., Introduction aux plans d’expériences, 3e édition. Paris: Dunod: L’Usine nouvelle, 2006.
- Ajay K. J., Jianzheng L., Loring N., et Liguo Z., « Research advances in dry anaerobic digestion process of solid organic wastes », Afr. J. Biotechnol. 2011; 10(65): 14242-14253, doi: 10.5897/AJB11.1277.
CrossRef - Kayode G. L. et Enahoro S. A., « Modelling the Kinetics of Biogas Production from Mesophilic Anaerobic Co-Digestion of Cow Dung with Plantain Peels », IJRED. 2015; 4(1): 55-63, doi: 10.14710/ijred.4.1.55-63.
CrossRef - Azevedo A., Gominho J., et Duarte E., « Performance of Anaerobic Co-digestion of Pig Slurry with Pineapple (Ananas comosus) Bio-waste Residues », Waste Biomass Valor. 2021; 12(1): 303-311, 2021, doi: 10.1007/s12649-020-00959-w.
CrossRef - Jha A. K., Li J., Zhang L., Ban Q., et Jin Y., « Comparison between Wet and Dry Anaerobic Digestions of Cow Dung under Mesophilic and Thermophilic Conditions », Advances in Water Resource and Protection. 2013; 1(2): 28-38.
- Yusuf M. O. L., Debora A., et Ogheneruona D. E., « Ambient temperature kinetic assessment of biogas production from co-digestion of horse and cow dung », Res. Agr. Eng. 2011; 57(3): 97-104.
CrossRef - Weide T., Baquero C. D., Schomaker M., Brügging E., et Wetter C., « Effects of enzyme addition on biogas and methane yields in the batch anaerobic digestion of agricultural waste (silage, straw, and animal manure) », Biomass and Bioenergy. 2020; 132, 105442, doi: 10.1016/j.biombioe.2019.105442.
CrossRef - Zhang L., Loh K. C., Zhang J., Mao L., Tong Y. W., Wang C. H., et Dai Y.., « Three-stage anaerobic co-digestion of food waste and waste activated sludge: Identifying bacterial and methanogenic archaeal communities and their correlations with performance parameters », Bioresource Technology. 2019; 285, 121333. doi: 10.1016/j.biortech.2019.121333.
CrossRef - Jitpupakdee, J., Pattharaprachayakul, N., Rungsardthong, V., Suvajittanont W. and Uttapap D. Enhancement of biogas production from industrial solid pineapple wastes by two-stage anaerobic digestion systems. J Mater Cycles Waste Manag. 2023; 25, 3734–3746. https://doi.org/10.1007/s10163-023-01790-w
CrossRef - Budiyono B., Matin H. H. A., Yasmin I. Y., et Priogo I. S., « Effect of Pretreatment and C/N Ratio in Anaerobic Digestion on Biogas Production from Coffee Grounds and Rice Husk Mixtures », Int. J. Renew. Energy Dev. 2023; 12(1): 209-215. doi: 10.14710/ijred.2023.49298.
CrossRef - Marone A., Izzo G., Mentuccia L., Massini G., Paganin P., Rosa S., Varrone C., Signorini, A., « Vegetable waste as substrate and source of suitable microflora for bio-hydrogen production », Renewable Energy. 2014; 68, 6-13. doi: 10.1016/j.renene.2014.01.013.
CrossRef - Taghinazhad J., Abdi R., et Adl M., « Kinetic and Enhancement of Biogas Production For The Purpose of Renewable Fuel Generation by Co-digestion of Cow Manure and Corn Straw in A Pilot Scale CSTR System », IJRED. 2017; 6(1): 37-44. doi: 10.14710/ijred.6.1.37-44.
CrossRef - Anderson K., Sallis P., et Uyanik S., Anaerobic treatment processes. In Handbook of Water and Wastewater Microbiology., Academic Press., 2003; 24. in Mara, D. and Horan, N., 24. London, 2003.
CrossRef - Görisch U. et Helm M., Éd., Biogasanlagen: Planung, Errichtung und Betrieb von landwirtschaftlichen und industriellen Biogasanlagen; 37 Tabellen. Stuttgart: Ulmer, 2006.
- Leven L., Eriksson A. R. B., et Schnürer A., « Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste », FEMS Microbiology Ecology. 2007; 59, 683-693.
CrossRef - Chae K. J., Jang A., Yim S. K., et Kim I. S., « The effects of digestion temperature and temperature shock on the biogas yields from the mesophilic anaerobic digestion of swine manure », Bioresource Technology. 2008; 99(1): 1-6. doi: 10.1016/j.biortech.2006.11.063.
CrossRef - Ganidi N., Tyrrel S., et Cartmell E., « Anaerobic digestion foaming causes – A review », Bioresource Technology. 2009; 100(23): 5546-5554. doi: 10.1016/j.biortech.2009.06.024.
CrossRef - Bollon J., « Etude des mécanismes physiques et de leur influence sur la cinétique de méthanisation en voie sèche: essais expérimentaux et modélisation », Institut National des Sciences Appliquées, Lyon, France, 2012.
- Zagorskis A., Baltrenas P., Misevicius A., et Baltrenait E., « Biogas production by anaerobic treatment of waste mixture consisting of cattle manure and vegetable remains », Environmental Engineering and Management Journal. 2012; 11, 849-856.
CrossRef - Li, W., Khalid, H., Zhu, Z., Zhang, R., Liu, G., Chen, C., and Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Applied Energy, 2018; 226, 1219–1228. doi:10.1016/j.apenergy.2018.05.05.
CrossRef - Max Rowan, Great C. Umenweke, Emmanuel I. Epelle, Inioluwa Christianah Afolabi, Patrick U. Okoye, Burcu Gunes, Jude A. Okolie, Anaerobic co-digestion of food waste and agricultural residues: An overview of feedstock properties and the impact of biochar addition, Digital Chemical Engineering. 2022; 4, 100046, https://doi.org/10.1016/j.dche.2022.100046.
CrossRef - Dahunsi S. O., « Liquefaction of pineapple peel: Pretreatment and process optimization », Energy. 2019; 185, 1017-1031, doi: 10.1016/j.energy.2019.07.123.
CrossRef - Banks C. J. et Heaven S., « Optimisation of biogas yields from anaerobic digestion by feedstock type », in The Biogas Handbook, Elsevier, 2013; 131-165. doi: 10.1533/9780857097415.1.131.
CrossRef - Chandra R., Takeuchi H., et Hasegawa T., « Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production », Renewable and Sustainable Energy Reviews. 2012; 16(3): 1462-1476. doi: 10.1016/j.rser.2011.11.035.
CrossRef - Dioha I. J., Ikeme C. H., Nafi’u T., Soba N. I., et Yusuf M. B. S., « Effect of Carbon to Nitrogen Ratio on Biogas Production », International Research Journal of Natural Science. 2013; 2, 30-39.
- Hassan A. N. et Nelson B. K., « Invited review: Anaerobic fermentation of dairy food wastewater », Journal of Dairy Science. 2012; 95(11): 6188-6203. doi: 10.3168/jds.2012-5732.
CrossRef - Zhang, M., Wang, Z., Zhang, X., Qian, X., & Shen, G. Biogas and quality fertilizer production from dry anaerobic digestion of rice straw with nitrogen addition. Bioresource Technology Reports, 2020; 100509. doi:10.1016/j.biteb.2020.100509.
CrossRef - Angelidaki I., Alves M., Bolzonella D., Borzacconi L., Campos J. L., Guwy A. J., Kalyuzhnyi S., Jenicek P. and van Lier J. B., « Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays », Water Science and Technology. 2009; 59(5): 927-934, doi: 10.2166/wst.2009.040.
CrossRef - Sakouvogui A., Kamano M., Bangoura M., et Keita M., « Production du biogaz à partir du lisier de porc et de la bouse de vache en mono et en codigestion à l’Université de N’zérékoré, République de Guinée », Rev. Ivoir. Sci. Technol. 2021; 38, 281-295.
- Pandey P. K., and Soupir M. L. Impacts des températures sur la production de biogaz dans la digestion anaérobie du fumier laitier. International Journal of Engineering and Technology, 2012; 4(5) :629-632. Doi:10.7763/IJET.2012.V4.448
CrossRef - Maile I. et Muzenda E., « Production of Biogas from Various Substrates under Anaerobic Conditions », in International conference on Innovative Engineering Technologies (ICIET’2014) Dec. 28-29, 2014 Bangkok (Thailand), International Institute of Engineers, déc. 2014. doi: 10.15242/IIE.E1214065.
CrossRef - Chen J. L., Ortiz R., Steele T. W. J., et Stuckey D. C., « Toxicants inhibiting anaerobic digestion: A review », Biotechnology Advances. 2014; 32(8): 1523-1534. doi: 10.1016/j.biotechadv.2014.10.005.
CrossRef - Yenigün O. et Demirel B., « Ammonia inhibition in anaerobic digestion: A review », Process Biochemistry. 2013; 48(5): 901-911. doi: 10.1016/j.procbio.2013.04.012.
CrossRef - Rajagopal R., Massé D. I., et Singh G., « A critical review on inhibition of anaerobic digestion process by excess ammonia », Bioresource Technology. 2013; 143, 632-641, doi: 10.1016/j.biortech.2013.06.030.
CrossRef - Mata-Alvarez J., Dosta J., Romero-Güiza M. S., Fonoll X., Peces M., et Astals S., « A critical review on anaerobic co-digestion achievements between 2010 and 2013 », Renewable and Sustainable Energy Reviews. 2014; 36, 412-427. doi: 10.1016/j.rser.2014.04.039.
CrossRef







