Assessment of Contamination Potential in Okhla Landfill, New Delhi by Using Leachate Pollution Index
1
School of Interdisciplinary and Transdisciplinary Studies,
IGNOU,
New Delhi,
India
2
Instrumentation Laboratory,
Central Pollution Control Board,
New Delhi,
India
Corresponding author Email: sonamangmo111@rediffmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.1.11
Copy the following to cite this article:
Angmo S, Kharayat Y, Shah S. Assessment of Contamination Potential in Okhla Landfill, New Delhi by Using Leachate Pollution Index Curr World Environ 2023;18(1). DOI:http://dx.doi.org/10.12944/CWE.18.1.11
Copy the following to cite this URL:
Angmo S, Kharayat Y, Shah S. Assessment of Contamination Potential in Okhla Landfill, New Delhi by Using Leachate Pollution Index Curr World Environ 2023;18(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-09-28 |
|---|---|
| Accepted: | 2023-02-09 |
| Reviewed by: | 
 Umama Begum Ruba
Umama Begum Ruba
|
| Second Review by: |

 Prashant
Prashant
|
| Final Approval by: | Dr. C. P. Kumar |
Introduction
Landfilling is considered the least preferred step in the integrated waste management hierarchy. In many developing countries, solid waste has directly dumped in landfill. Municipal solid waste landfills are the main sources of various broad ranges of environmental pollutants 1. In developing countries uncontrolled dumping of waste in active landfills because of it a toxic liquid called leachate generates as a product of the degradation of solid waste. Because of the lack of baseliners and collection facilities, leachate finally finds its way into the terrestrial and aquatic environment2. Landfill leachates and emission landfill gas because of various physical and biochemical reactions taking place in landfills 3,4,5,6, 7.
Fast generation of solid waste on daily basis because of urbanization, industrialization, lifestyle changes, and population exploration lead to the degradation of soil, water, and air which are main environmental issues, and unscientific management of solid waste has a serious threat from leakages of toxic liquid called leachate which effect on the environment and human health8,9. Contaminations of groundwater and an increase in global warming are contributed by landfills various health issues faced by people living near the landfill, particularly during monsoon and post-monsoon seasons 10.
As per Arbastan and Gitipour,11 single percent of municipal solid waste are domestic hazardous waste which is the origin of such type of waste as Compact fluorescent lamps (CFL), broken thermometer, paint, used syringes, and discarded medicines which is the mains source of heavy metals10. Landfill leachate is formed beneath the solid waste after some time due to biochemical and microbial degradation of organic matter contained in solid waste. 12Various pollutants occurred in leachate (TOC, BOD, COD, TSS, TKN, AN, TCB, pH) and Heavy metals (Arsenic, Copper, Chromium, Iron, Zinc, Nickel, Cadmium, Lead, Mercury, Cobalt, Vanadium).
The concentration of leachate varies with seasons, so seasonal analysis and assessment of contamination potential by calculating LPI are very necessary for the effective management and treatment of leachate 13, 14.
LPI is an environmental indicator for calculating the hazardous capacity of landfill leachates of municipal solid wastes, provides an appropriate means of investigating complex data, and promotes its reports to the people, area specialists, and policymakers developed and reported by Kumar and Alappat 15. The value of LPI denotes the level of leachate toxicity possibilities of a specific landfill. It is a solo number ranging between 5-100, based on the Delphi technique 16. This value reveals the total contamination capacity of a landfill built on some parameters of leachates at a given time 17. LPI is a growing environmental indicator index, where the high values validate the poor situations of the landfills, and the low value represents less contamination potential 18. Minimized generation of the landfills leachate can be possible by adopting sustainable waste management such as a framework for waste reductions, an assortment of segregated types of waste, well refining equipment, and the treatment method reduces landfilling19.
Several researchers have calculated an overall LPI of a particular landfill or a group of the landfill, but very few have focused on the calculation of sub-indices of LPI (LPIorg, LPIin, and LPIhm). To date, limited publications were available that discussed seasonal variations of sub-LPI and heavy metals in landfills.
Aim of the study: - The aim of the study was to analyze the seasonal variation of heavy metals, the quality of leachate, and its contamination potential, and it was observed that results are indicative of the kind of treatment method suitable for the Okhla landfill leachate treatment and this study also supports decentralized waste segregation unit, proper composting of biodegradable waste and extension of present waste to energy plants and reduce landfilling.
Material and Methods
Study area
Okhla landfill is located at a latitude and longitude of 28°30'40.05"N, 77°17'4.47"E just adjacent to ESIC hospital Tughlagabad, in the district of Southeast Delhi shown in Fig. 1. Okhla landfill is active, uncontrolled, and a non-engineered landfill, which is commissioned in 1994 and starts dumping of waste from 1996 and exhausted its capacity in 2010 and decommissioned in 2018 but, still wastes are dump in the landfill without giving any attention of segregation. Around 2000 tons of waste are dumped daily, including municipal solid waste construction and demolition waste, and also from agriculture production (APMC). At the landfill, waste comes from four-zone of South Delhi (Central, South, West, and Najafgarh). Fifty-one percent (51%) of the total average waste from these zones is directly into Okhla landfill, 46% is used waste to an energy plant for electricity generation, and 3% is transported to a composting plant for the production of compost 20
 | Figure 1: Okhla Landfill, New Delhi.
|
Sampling has done in three seasons of the year 2019-2020. Pre-monsoon sampling was in the month of May, monsoon sampling was in the month of July, and post-monsoon sampling in was the month of November and 30 numbers of samples were collected during each season and total 90 samples were collected for this study. The grab leachate samples were collected in such a manner that each sample truly represents the leachate. High-Density polyethylene and glass containers were used for sample collection as prescribed for the sample collection of samples for physiochemical and biological parameter analysis. Samples were preserved as prescribed in APHA for physico-chemical parameters and heavy metals. The collected samples were labeled and preserved in the ice box and transported to the laboratories, kept in the refrigerator at 40C till the analysis started.
Physicochemical analysis
Samples of collected landfill leachates for physiochemical analysis (including heavy metal) were analyzed using standard method 21. Electrical conductivity and pH were analyzed by using a Multi-parameter instrument. TSS and TDS were analyzed by Gravimetric methods, COD was determined by the Open reflux method, BOD was determined by Winkler’s method, NH4-N was analyzed by the Distillation method, TKN by Kjeldahl method, Cl- by Argentometric method and phosphate by Stannous chloride method. The heavy metals (Cu, Cr, Co, Cd, Ni, Zn, Ni, Fe, Mn, Hg, V), and Se, Sb were analyzed by using Induced coupled Plasma-Optical Emission Spectrophotometer (ICP-OES), Perkin Elmer make)whereas Total coliform was analyzed according to the Multiple Tube fermentation Technique (MTF).
Leachate Pollution Index: Various steps to determine the leachate toxicity potential of landfills are discussed below.
Variable Selection
Eighteen (18) parameters of leachate pollution were chosen for incorporation in the Leachate Pollution Index. These are pH, Total dissolved solids, Biological oxygen demand, Chemical oxygen demand, Total coliform bacteria, Total Kjeldahl nitrogen, Ammonical nitrogen, Chlorides, Arsenic, Zinc, Copper, Nickel, Iron, Chromium, Mercury, Lead, Phenol and Cyanide.
Variable Weights
Based on the significance level of each pollutant, the weights of 18 pollutants parameters were determined. The values of individual pollutant variables to the total leachate contamination were designated by the weight factor. In case, the weight factor for total iron is 0.045, which is the least important as compared to the weight factor of Cr, which is 0.064 and is the most valuable variable than others included in LPI 15.
Sub-Index Curve: Averaged sub-index curve shows the relation between concentration or strength and leachate pollution. For individual parameters, the averaged sub-index curve was produced to show the relation. All the selected pollutants variables sub-index curves are reported by Kumar and Alappat 15.
Variable Aggregation
To summarize the nature of all the pollutants the weighted sum linear summations function and various potential aggregations were assessed by Kumar and Alappat15. The computation of LPI when all the eighteen pollutants of leachate are available by the given equation.

wi is ith pollutants weight
pi is ith pollutants sub-index value
n = No. of pollutants selects in LPI calculations
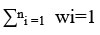
However, LPI can also be determined when <18 leachate pollutants are available. In such cases, LPI is calculated by the given equation.
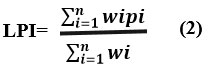
Where n depicts No. of parameters of pollutants, when 18Ewi <1.
Sub-Indices of LP
LPI was subdivided into three sub-indices such as (LPIorg, LPIin, and LPIhm) to make LPI more valuable and helpful for field professionals and the scientific community. These sub-indices indicate the dominance of pollutants based on their concentrations in the collected samples of landfills leachate. To find the occurrences of a particular contaminant in leachate, there is a need for the physicochemical, and biological studies of the leachate sample of a particular landfill.15 The physical characteristics of landfill leachate comprise temperature, odors, color, and solids extra. Similarly, the chemical characteristic can be classified into inorganic and organic components. Various infectious viruses and bacteria are found as biological components.Out of all the pollutants only eighteen (18) were selected. These are further grouped into three sub-LPI which are given below.
LPI organic (COD, BOD, TCB, Phenol)
LPI inorganic (NH4-N, TKN, Cl, TDS, pH)
LPI heavy metal (Cr, As, Co, Cu, Ni, Zn, Fe, Hg, CN-)
Calculation of Sub-LPI and Overall LPI
Sixteen leachate pollutants such as (COD, BOD, TCB NH4-N, TKN, Cl, TDS, pH, As, Cu, Cr, Co, Zn, Ni, Fe, Hg) were analyzed and selected for LPI and further divided into three sub-LPIs
LPI organic (COD, BOD, TCB) (b) LPI inorganic (NH4-N, TKN, Cl, TDS, pH) (c) LPI heavy metal (Cr, As, Co, Cu, Ni, Zn, Fe, Hg)
The steps involved in the calculation are given below:
Each parameter of the sub-index scores was determined from the Fig. mentioned in Kumar and Alappat17. Concerning the concentration of analyzed pollutants from collected leachates samples
Sum up the sub-index values for three sub-LPIs of LPI.
The sub-indices in regards to (LPIorg, LPIin, and LPIhm) computed by applying the weight factors table given in Kumar and Alappat17 based on the aggregation function from Equation (2),

Combinations of the (LPIor, LPIin, and LPIhm) sub-indices to obtain overall LPI
The overall LPI can be computed
LPI = 0.232LPIor + 0.257 LPI in+ 0.511 LPIhm (3)
Result And Discussions
Seasonal Variation of Leachate Quality
In the present study, 25 leachate parameters including heavy metals were analyzed which are shown in Table 1. The leachate parameters were compared with the Leachate Disposal Standard (2016)22. It was observed that all the organic and inorganic parameters except pH and most of the heavy metals have shown concentrations beyond the standard disposal limit. During the study, the pH value of leachate was observed between 6.6-7.9 .The alkalinity is the indicator for the maturity of landfill 23. Okhla landfill was commissioned in the year of 1996 and it is more than 20 years old and comes under the category of mature landfill.
Table 1: Seasonal characterization of landfill leachate and comparison with Standard (SWM, 2016)
| Leachate | Premonsoon pollutant concentration | Monsoon | Post monsoon | Unit | Leachate Disposal Standard (2016)* |
S. No | Pollutant | Pollutant concentration | Pollutant concentration | |||
Variable | ||||||
1 | pH | 6.63±0.01 | 7.34±0.427 | 7.95±0.144 | - | 5.5-9 |
2 | EC | 5076±3.46 | 394±2.3 | 4075±5 | µS/cm | No standard |
3 | COD | 59136±7.02 | 42856.3±30.8 | 47485.3±7.80 | mg/L | 250 |
4 | BOD | 36311±5.50 | 18960.7±4.04 | 25202.3±5.29 | mg/L | 30 |
5 | BOD/COD | 0.6±0.01 | 0.4±0 | 0.5±0.01 |
| - |
6 | DO | 6.66±0.58 | 6.65±0.02 | 6.8±0.17 | mg/L | No standard |
7 | TDS | 62821±10.50 | 15933.3±18.34 | 20220.3±52.85 | mg/L | 2100 |
8 | Cl | 12000±250.6 | 1040±15 | 14600±15.2 | mg/L | - |
9 | TKN | 812±10.06 | 330±12.58 | 5020±2.64 | mg/L | - |
10 | NH3-N | 1525±27.53 | 776±6.24 | 1043.33±7.87 | mg/L | 50 |
11 | TCB | 433.3±34.7 | 9480±40.41 | 5084.2±7.7 | MPN/100ml | No standard |
12 | As | 0.093±0.158 | 0 | 0.449±0.210 | mg/L | 0.2 |
13 | Cd | 0.018±0.066 | 0 | 0±0 | mg/L | 2 |
14 | Co | 0.220±0.137 | 0 | 0.134±0.064 | mg/L | No standard |
15 | Cr | 0.414±0.515 | 0.0432±0 | 0.983±0.718 | mg/L | 2 |
16 | Cu | 0.399±0.270 | 0.474±0.37 | 0.496±0.343 | mg/L | 3 |
17 | Ni | 1.172±0.66 | 0.195±0.11 | 0.392±0.167 | mg/L | 3 |
18 | Pb | 1.442±3.99 | 0±0 | 0.395±1.177 | mg/L | 0.1 |
19 | Sb | 0.009±0.027 | 0.051±0.05 | 0.008±0.015 | mg/L | No standard |
20 | Se | 0.0267±0.074 | 0.020±0.04 | 0.011±0.029 | mg/L | No standard |
21 | V | 0.940±0.698 | 1.317±0.59 | 1.583±0.796 | mg/L | No standard |
22 | Zn | 1.239±1.383 | 0.766±0.86 | 0.739±0.765 | mg/L | 5 |
23 | Fe | 306.683±194.55 | 62.357±63.1 | 15.437±11.344 | mg/L | No standard |
24 | Mn | 27.910±15.73 | 6.036±7.64 | 0.514±0.367 | mg/L | No standard |
25 | Hg | <0.001 | <0.001 | <0.001 | mg/L | 0.01 |
*Solid Waste Management Rule, 2016. (SWM, Rule 2016)
Higher values of COD and BOD was found during pre and post-monsoon may be due to high evaporation in pre-monsoon and dryness in post-monsoon season and low concentration of organic pollutants throughout monsoon season as rainfall plays an important role as dilution 24.
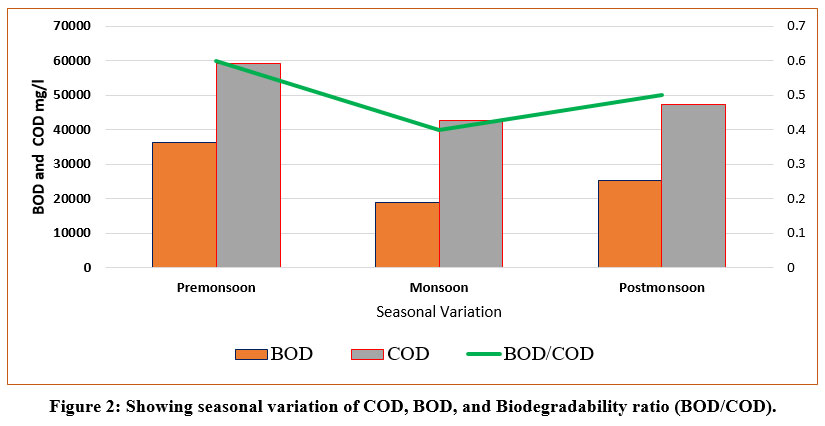 | Figure 2: Showing seasonal variation of COD, BOD, and Biodegradability ratio (BOD/COD).
|
The BOD/COD ratio of the Okhla landfill was observed 0.5, 0.4 and 0.6 during post-monsoon, monsoon and post-monsoon, respectively. If BOD/COD ratio found less than 0.5, indicates an intermediate biodegradability and ratio more than 0.5, was mainly for young landfill, as shown in Fig.2.During post-monsoon and monsoon, landfill showed/revealed intermediate biodegradability, but during pre-monsoon, landfill show high degradation. Okhla landfill finished its commission in the year of 2018 and now it is a mature landfill, but still dumping of fresh waste and waste deposition continues without any restriction against its capacity, which could be the reason that the biodegradability ratio was high as a young landfill. In pre and post-monsoon seasons, biodegradation was 0.6 and 0.5, but during monsoon, it was 0.4 because of dilution by the rainy season. BOD/COD ratio is a measure of the biodegradability and maturity of a landfill and is dependent on the age of the landfill and decreases with decomposition and biodegradation with time and its ratio was found between 0.4 to 0.6 values. The Biodegradability ratio became greater than 0.5 value for new landfills (<5 years) and this value is going on a decline with time as the biodegradability ratio for mature landfills (>10 years) became less than 0.1 value 25
The electrical conductivity value of leachate in the period of pre and post-monsoon seasons were too high, which indicate the presence of highly dissolved inorganic like anion (chloride, nitrate, phosphate) and cation such as Na, Mg, Ca, and Fe.26,27
The concentration of Chloride was observed to be higher in pre and post-monsoon seasons 12000 mg/L, and 14600 mg/L and slightly less in the monsoon season 1040mg/L and the concentration value was observed beyond the standard set by MoEF&CC as mentioned in (SWM Rule 2016) 22. Leu etal.28 reported that chloride is highly movable and biochemically it can’t be sorbed or transformed easily therefore, leachate content in landfill leachate is always high. TDS was observed beyond the standard in all the seasons slightly less in monsoon season and TDS is the parameter used as criteria for the discharge of leachate in almost all the countries 29. High TDS was observed, maybe because of leaching ions from waste and concentration decrease in monsoon as a result of dilution.
Ammonical nitrogen was observed from the collected samples of leachate in pre-monsoon 1525 mg/L, monsoon 776 mg/L, and post-monsoon 1043.33 mg/L. Ammonical nitrogen (NH4-N) presence in the landfill is assigned to the degeneration of amino acids and other waste constituents of nitrogenous (primarily food and animal waste)30. Ammonical nitrogen NH4-N has been recognized as a priority parameter responsible for the contamination of landfill and could have a detrimental effect even as an air pollutant upon its evaporation from the leachate when it is beyond 0.50 mg/L31. Okhla landfill has a greater concentration of nitrogen compounds, compared with the Ghazipur and Bhalswa landfill sites32. Seasonal variation of leachate characteristics from landfill in Ludhiana city of Punjab was studied by Bhalla et al.33 and they found that leachates enclosed peak concentrations of organic and inorganic pollutants but heavy metals in traces amount. Likewise in the present study area, organic and inorganic pollutants were found in high concentration but heavy metals were in low concentration.
Heavy Metal Analysis
The analysis of heavy metals such as As, Pb, Cd, Zn, Co, Cr, Fe, Cu, Ni, Mn, Hg, and V was performed in the leachate samples, which were collected at the time pre-monsoon, monsoon, and post-monsoon seasons during the year (2019-2020). It has been observed visually that the present study site i.e Okhla Landfill receives waste from an area that comes under the authority of the South Delhi Municipal Corporation and also receives some rejected waste from waste to energy plants and composting plants. This landfill receives waste such as solid waste, demolition waste from construction sites, and other such as domestic hazardous waste may present because during waste disposal authorities didn’t give attention to segregation. Many researchers studied landfill leachate and reported that high concentrations of heavy metals were analyzed from collected leachate samples such as Fe, Ni, Zn, Cr, Cd, Cu34, 35, 36, 32, 37, 38
During pre-monsoon season, heavy metals such as (As, Co, Cr, Cd, Cu, Ni, Zn, Pb, Fe, and Mn) concentrations were observed (0.09mg/L, 0.01mg/L, 0.22mg/L, 0.41mg/L, 0.39mg/L, 1.17mg/L, 1.44mg/L, 1.23mg/L, 306.68mg/L and 27.9mg/L,) respectively. Likewise in monsoon season, concentrations of Cr, Cu, Ni, Zn, Fe, and Mn were observed (0.04 mg/L, 0.47mg/L, 0.19 mg/L, 0.76mg/L, 2.35mg/L, 6.03mg/L,) respectively and concentration of (As, Cd, Co, and Pd) were found below detection limit i.e. (mentioned the value). And during the post-monsoon season, the concentration of (As, Co, Cr, Cu, Ni, Pb, Zn, Fe, and Mn) was observed as 0.44mg/L, 0.13mg/L, 0.98mg/L, 0.49mg/L, 0.39mg/L, 0.39mg/L, 0.73mg/L, 15.43 mg/L and 0.51mg/L, respectively and Cd was found below the detection limit. It was observed in the study that Pb concentration in pre and post-monsoon was found beyond the standard limit, as set in SWM (Solid Waste Management) Rule 2016 22. Occurrences of high concentrations of Lead (Pb) indicated that the toxicity cause can be the dumping of lead acid batteries and it has a possibility of cancer risk 39.
Likewise, Arsenic (As) concentration in collected samples in post-monsoon was observed to exceed the set limits for discharge of leachate on inland, subsurface, or public sewer, and in Monsoon Arsenic (As) was not detected, and in pre-monsoon, the concentration of Arsenic (As) was under permissible limit. According to Al-Abed and Jegadeesan40 a main source of Arsenic (As) from landfill leachate was the disposal of Cromated copper arsenate-treated timber from construction and demolition (C&D) also from arsenic-bearing solid residual from the water treatment plant and it is also a source from sediments and natural iron occurring soil. WHO41 reported that Arsenic polluted water sources can cause harm to the vital organs as well as the blood-pumping system, sinusitis, peripheral vascular disease, skin lesions, and peripheral nervous system may also result. Chaudhary, Nain, and Kumar42 also evaluated that in the year 2015, Okhla dumpsite exhibits elevated Pb concentration and, thus surpassed the standard threshold value of 0.1 mg/L.
During pre-monsoon, monsoon, and post-monsoon Iron (Fe) in collected leachate samples was found in the concentration of 306.68mg/L, 62.35mg/L, and 15.43mg/L but there is no standard limit mentioned for disposal of leachate having a high concentration of iron (Fe) in the public sewer, inland water or subsurface water. The occurrences of Iron in the leachate are linked to the existence of ferrous metal fragments in the drain water, left off after scavengers had disorganized through the in-place waste. The exhausted dissolved oxygen and the attributed brown color of the leachates so obtained is due to corrosion of the (Fe2+) to the Fe(OH)3 colloid and other ferric forms is partly responsible in this research 30,41. Iron (Fe) concentrations in landfill leachates the case of the Okhla landfill, have reduced from 2003 to 2012 and further raise from 2014 to 2017 34.
However, Manganese (Mn) concentration in collected leachate samples in pre-monsoon, monsoon, and post-moon was 27.9mg/L, 6.03, and 0.51mg/L again there is no standard limit set for the concentration of manganese for disposal of leachate having Manganese in Public sewer, Inland water, and Surface water.
During all the three season, Mercury (Hg) from leachate samples were analyzed and observed under the permissible limit. High concentrations of heavy metals like Fe, Cu, and Cr indicate toxicity because of their long-lasting persistence in the environment43. Also, the high concentration of contaminants in the leachate sample referred to the landfill has need to be closed because of its weakening capacity 44.
Likewise, Vanadium (V) in collected leachate samples in the pre-monsoon season was found in a concentration of 0.9mg/L, in the monsoon season, concentration was observed 1.3mg/L, and Post monsoon season concentration of vanadium was 1.5 mg/L. The presence of Vanadium (V) in landfill leachate may be due to steel-making wastes such as slay and spent refractory liners disposed of in a landfill. Vanadium (V) is a toxic metal that becomes flexible in alkaline leachate liberated during weathering of steel slag and slag has a detrimental effect on water bodies because of toxicity 45,46.
During pre-monsoon, monsoon some of the other metals analyzed from the collected leachate sample was Selenium (Se) which was found in the concentration of 0.026mg/L, and 0.020mg/which was found below the detection limit in the post-monsoon season. According to 47,48,49 in electronic items Selenium (Se) is mostly used because having semiconducting and photosensitive characteristics, the main cause of selenium occurrence in landfill leachate was the disposal of huge quantity electronic items such as a printer, toners, rectifiers, extra from electronic producer facilities and along with disposal of their solid waste in landfill. These results revealed a need to observe and analysis of the landfill leachate for contamination of selenium.
Antimony (Sb) from collected leachate samples was observed in a concentration of 0.05 mg/l in the monsoon season. And found BDL (Below the detection limit) in pre-monsoon and post-monsoon seasons. According to 50,51 the main source of Sb in residential waste in Japan, Germany, and Canada were plastic, textiles, polyester fibers, cathode ray tube (CRT) of television, and their plastic cover. Antimony (Sb) concentration in residential waste is low as compared to industrial waste. No such studies like Antimony content in household waste were found in India.
Heavy metals are non-biodegradable inorganic and also alkaline nature of leachate give inhibitory dissolution of metal in landfill at ages52. Heavy metal was observed in the highest concentration at the time of the pre-monsoon season and concentration slightly declined in post-monsoon and great reduction at the time of rainfall season 30. Occurrences of chromium in the samples of leachates may be assigned to the presence of Lead-Chromium batteries, colored plastic bags, unwanted polybags, and void paint containers in the dumping area. In agricultural practices, fertilizers and pesticides which are commonly called an agrochemical are the major sources of Zinc (Zn)53.
Variations of heavy metal in landfill leachates may be due to types of waste generation from households at different seasons, climate (dry and rainy seasons), and landfill management practices at the landfill.
Seasonal Variation of Sub-Indices of LPI and Overall LPI
Seasonal variation of LPI value of the present study area landfill leachate in the three seasons were presented in Tables 2, 3, and 4 and its comparison with set standard mentioned in SWM Rule 2016 are shown in Table 5 and Fig 3. The calculated overall LPI of the Okhla landfill was found 32.5 in the pre-monsoon period, 28.2 in the monsoon, and 32 .0 in the post-monsoon monitoring period. This value showed that the contamination potential of landfill leachate varies according to the season of the year because of the dryness and wetness of the season. Rainfall plays important role in the reduction of the concentration of pollutants occurring in leachate, during monsoon season almost all the pollutant concentration go on decrease because of dilution. In all the seasons calculated LPI value exceeded the calculated treated leachate standard value as shown in Fig.3.
In pre and post-monsoon the calculated overall LPI indicates that landfill leachate was more concentrated and high contamination potential than the calculated overall LPI of monsoon season because of high temperature and more evaporation. The overall LPI value of the collected leachate sample from the Okhla landfill in three seasons depicts the value of LPI beyond the standard set i.e 7.3 same was reported by Somani et al.35 LPI value of the present study area i.e Okhla landfill site denotes that the waste settled is polluted therefore, all values determined at the time of all the three seasons detected beyond the set standards.
As shown in Table 5 and Fig.3 the calculated overall LPI of Okhla landfill was found in order of LPI pre-monsoon>LPI post-monsoon>LPI monsoon as these values were compared with calculated treated leachate standard which is 6.5 which revealed that Okhla landfill leachate is highly concentrated and high contamination potential and this toxicity may reach in an aquifer and finally in groundwater of place close to the landfill 54. Leachate contamination potential is lower in the rainy season as compared with pre and post-monsoon seasons.
Seasonal variation of the LPI value of Okhla landfill has been also reported by 36 observed that LPI value were 62.32 in the hot and 44.14 in the cold seasons subsequently and was beyond its standard value of 7.3 which is the thresholds limit for the leachate discharges set by the Government of India (SWM Rule 2016)22. The LPI value of 7.3 is the standard and permissible limit for any treated leachate if discharge inland, sewer, or subsurface needs meet this standard before discharge (SWM Rule 2016) The LPI values of Okhla landfill was observed at 42.1 has been calculated by Kumar and Alappat 15 and reported that LPI for Okhla landfill is significantly high and also calculated treated leachate standard LPI value which was obtained as 7.3 in their studies
Presently studied a seasonal variation of overall LPI from the study area was found greater than the recommended standard of 7.3 and needs to reduce the concentration of leachate and a suitable treatment method urgently require. The overall LPI values of all the studied seasons exceeded the standard LPI value of 7.3 15 anticipating a grave complicated environmental impact that exceeds the surrounding soil and water resources. LPI for the Okhla landfill was 37.91 and reported that reason for the high LPI of the landfill was because of the attached from its environment, lack of bottom liner, leachate migrating freely, and inadequate leachate treatment &collection system, 37.
Table 2: Sub-indices and overall LPI of Okhla landfill during Pre-monsoon season.
| Index | Parameter | Weight factor (Wi) | Concentration | Sub-index value (Pi) | Wi*pi |
LPI ORGANIC (LPI or) | COD | 0.267 | 59136 | 95 | 25.36 |
| BOD | 0.263 | 36311 | 85 | 22.35 | |
| TCB | 0.224 | 433.3 | 63 | 14.11 | |
Summation LPI or | 0.754 | 61.8 82.0 | |||
LPI INORGANIC (LPI in) | pH
| 0.214
| 6.63 | 6 | 1.28 |
| Ammonia nitrogen | 0.198 | 1525 | 100 | 19.8 | |
| Cl | 0.187 | 1330 | 100 | 18.7 | |
| TKN | 0.206 | 760 | 23 | 4.73 | |
| TDS | 0.195 | 62821 | 100 | 19.5 | |
Summation LPI in | 1 | 40.58 40.58 | |||
LPI HEAVY METAL (LPI hm) | Cr | 0.125 | 0.4148 | 6 | 0.75 |
| Pb | 0.123 | 1.442 | 10 | 1.25 | |
| As | 0.119 | 0.093 | 5 | 0.59 | |
| Zn | 0.110 | 1.239 | 8 | 0.88 | |
| Fe | 0.088 | 306.68 | 9 | 0.79 | |
| Cu | 0.098 | 0.399 | 6 | 0.588 | |
| Ni | 0.102 | 1.172 | 7 | 0.714 | |
| Hg | 0.121 | 0.01 | 5 | 0.60 | |
Summation LPI hm | 0.886 | 6.15 6.94 | |||
Overall LPI
| Equation (3) | 32.57 | |||
Table 3: Sub-indices and overall LPI of Okhla landfill during Monsoon season.
| Index | Parameter | Weight factor Wi | Concentration | Sub-index Pi | Wi*pi |
LPI ORGANIC (LPIor) | COD | 0.267 | 42856.3 | 90 | 24.03 |
| BOD | 0.263 | 18960.7 | 68 | 17.88 | |
| TCB | 0.224 | 9480 | 87 | 19.48 | |
Summation LPI or | 0.754 |
|
| 61.38 81.4 | |
LPI INORGANIC (LPI in) | pH
| 0.214
| 7.34 | 8 | 1.712 |
| Ammonia nitrogen | 0.198 | 776 | 83 | 16.43 | |
| Cl | 0.187 | 1030 | 92 | 17.2 | |
| TKN | 0.206 | 332 | 12 | 2.473 | |
| TDS | 0.195 | 15933.3 | 40 | 7.8 | |
Summation LPI in | 1 |
|
| 25.94 25.94 | |
LPI HEAVY METAL (LPI hm) | Cr | 0.125 | 0.0432 | 5 | 0.62 |
| Pb | 0.123 | 0 | 5 | 0.61 | |
| As | 0.119 | 0 | 5 | 0.59 | |
| Zn | 0.110 | 0.766 | 5 | 0.55 | |
| Fe | 0.886 | 62.35 | 5 | 0.44 | |
| Cu | 0.098 | 0.474 | 7 | 0.68 | |
| Ni | 0.102 | 0.195 | 5.5 | 0.56 | |
| Hg | 0.121 | 0.001 | 5 | 0.60 | |
Summation LPI hm | 0.886 |
|
| 4.65 5.24 | |
| Overall LPI | Equation (3) | 28.21 | |||
Table 4: Sub-indices and overall LPI of Okhla landfill during Post- monsoon season.
Index | Parameter | Weight factor (Wi) | Concentration | Sub-index value (Pi) | Wi*pi |
LPI ORGANIC (LPIor) | COD | 0.267 | 47485.3 | 95 | 25.36 |
BOD | 0.263 | 25202.3 | 78 | 20.51 | |
TCB | 0.224 | 50484.2 | 100 | 22.4 | |
Summation LPI or | 0.754 |
|
| 68.27 90.54 | |
LPI INORGANIC (LPI in) | pH
| 0.214
| 7.95 | 8 | 1.71 |
Ammonia nitrogen | 0.198 | 1043.33 | 98 | 19.40 | |
Cl | 0.187 | 1300 | 100 | 18.7 | |
TKN | 0.206 | 520 | 15 | 3.06 | |
TDS | 0.195 | 20220.3 | 48 | 9.36 | |
Summation LPI in | 1 |
|
| 30.47 30.47 | |
LPI HEAVY METAL (LPI hm) | Cr | 0.125 | 0.983 | 8 | 1 |
Pb | 0.123 | 0.995 | 9 | 1.10 | |
As | 0.119 | 0.449 | 5.5 | 0.65 | |
Zn | 0.110 | 0.739 | 5.5 | 0.65 | |
Fe | 0.088 | 15.437 | 5.2 | 0.45 | |
Cu | 0.098 | 0.496 | 6 | 0.58 | |
Ni | 0.102 | 0.392 | 5.4 | 0.55 | |
Hg | 0.121 | 0.001 | 5 | 0.60 | |
Summation LPIhm | 0.886 |
|
| 5.58 6.29 | |
Overall LPI |
| Equation (3) | 32.0 | ||
Table 5: Sub-indices and overall LPI of Treated leachate standard.
Index | Parameter | Weight factor (Wi) | Concentration | Sub-index value of treated leachate (Ti)
| Wi*Ti |
LPI ORGANIC (LPI or) | COD | 0.267 | 250 | 10 | 2.67 |
BOD | 0.263 | 30 | 6 | 1.57 | |
TCB | 0.224 | --- | --- | -- | |
Summation LPI or. | 0.754 |
|
| 4.24 5.62 | |
LPI INORGANIC (LPI in) | pH
| 0.214
| 5.5-9 | 5 | 1.07 |
Ammonia nitrogen | 0.198 | 50 | 7 | 1.38 | |
Cl | 0.187 | 1000 | 8 | 1.49 | |
TKN | 0.206 | 100 | 6 | 1.23 | |
TDS | 0.195 | 2100 | 7 | 1.36 | |
Summation LPI in. | 1 |
|
|
3.81 3.81 | |
LPI HEAVY METAL (LPI hm)
| Cr | 0.125 | 2.0 | 9 | 1.125 |
Pb | 0.123 | 0.1 | 5 | 0.61 | |
As | 0.119 | 0.2 | 5 | 0.59 | |
Zn | 0.110 | 5.0 | 6 | 0.66 | |
Fe | 0.088 | … | …. |
| |
Cu | 0.098 | 3.0 | 18 | 1.76 | |
Ni | 0.102 | 3.0 | 10 | 1.02 | |
hg | 0.121 | 0.01 | 6 | 0.72 | |
| Summation LPI hm | 0.886 |
|
| 6.48 7.31
|
Overall LPI |
| Equation (3) |
| 6 | |
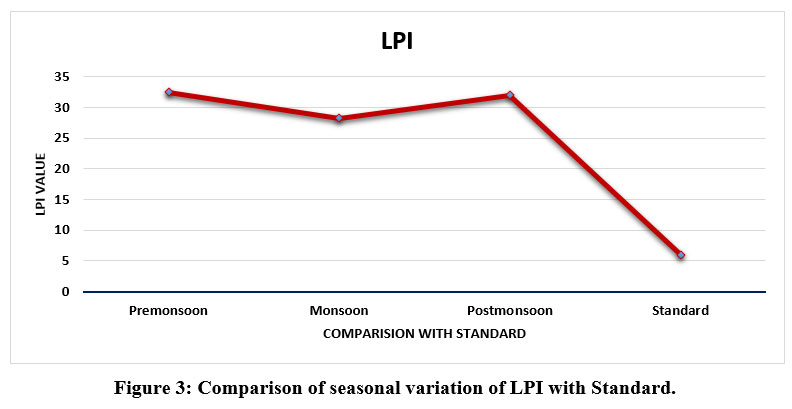 | Figure 3: Comparison of seasonal variation of LPI with Standard.
|
During all three seasons the three sub-indices of LPI (LPIorg, LPIin, and LPIhm) for the present study area was computed which are presented in Table 2, 3, 4. Calculated Sub-indices LPI for treated leachate standard was mentioned in Table 5. A comparison of the calculated seasonal variation of sub-LPI with Sub LPI of calculated treated leachate standard was shown in Fig 4. As shown in table 2 during the pre-monsoon season the sub-indices value of LPIorg was 82.0 and LPIin 40.58and then LPIhm 6.94. Table 3 represents sub-indices of LPI of monsoon season, LPIorg 81.4, LPIin 25.94, LPIhm 5.24. Sub-indices of LPI post-monsoon were presented in table 4, LPI or 90.5, LPIin 30.47, and LPIhm 6.29.
Likewise Treated leachate standard value total LPI and sub-LPI were also computed as shown in Table 5 and observed that (LPIor, LPIin, and LPIhm) were all found under standards set by authorities for leachate disposal in the public sewer, surface, and subsurface water and later compare with the standard of LPI as shown in Fig 4. The sub-indices of LPI of the study area were in order of (LPIor>LPIin>LPIhm) which means in all three seasons the calculated LPIhm value was low and come under the standard set 7.3. In all the seasons LPIorg organic pollutants dominate then LPIin inorganic and followed by LPIhm heavy metals.
The same case study was also carried out by 24 at Namso landfill, in Vietnam, and observed that the LPIor and LPIin were over than LPIhm, and found that seasonal variation of overall LPI value was very low during observing periods. The high values of LPIorg are indicative of acetogenic processes 17 and studied the Sub-indices LPI of Harewood Whin Landfill in the UK observed a value that LPIorg, LPin, LPIhm were 53.5, 17.09, and 5.5 these values revealed leachate have high in organic content and low heavy metal contamination it mean low LPIhm value.
 | Figure 4: Showing seasonal variation of three Sub-indices of LPI and its comparison with treated leachate standard.
|
In the monitoring period of three seasons the sub-indices of LPI value shows that LPI org were higher in all season followed by LPI in and then LPIhm because of high concentration of COD, TCB, and BOD pollutants in leachates. The low heavy metal pollutants LPIhm helps in the flourishing of a living organism and assist biological leachate treatment, on the other hand, low inorganic pollutants LPIin again aid in this wastewater treatment. As per Kamaruddin et al.25 the flexibility and dissolution of inorganic components were not encouraged by high pH and are probably in charge of low sub-indices LPI of LPI such as heavy metal and inorganic components. These situations recommend that the rapid methanogenic phase are working simultaneously with the acetogenic phase.
The present study area i.e Okhla landfill completed its commission date in the year 2018 but solid waste is still dumped or deposition of waste without any attention to segregation that’s why the biodegradability ratio value was high as a young landfill. In sub-indices LPI org dominates i.e COD, BOD, TCB and followed by LPIin ammonia, chloride, TDS, TKN, and in the case of LPI hm Pb then followed Zn, Cu, Ni, Fe, etc. 54 LPI is lower in case of post-monsoon as compared with pre-monsoon, the concentration of chlorides are much higher in pre-monsoon times and commonly found heavy metals were such as Pb, Zn, Cr, Fe, Cd, Ni, Zn, Mn, Cu, and Co. Singh and Mittal55 studied correlation between LPI and heavy metals of Okhla landfill and observed strong correlate were found between Copper, Zinc, Lead, Chromium, and LPI and concluded that the contribution of heavy metals is seriously more to landfill pollution.
Conclusions
Based on sub-LPI indices of the landfill leachate, a suitable treatment method may be opted for the treatment. Okhla landfill shows a high biodegradability value of 0.4-0.6, which may be the continuous deposition of fresh solid waste on old waste. The concentration of leachate varies with changes in seasons and contamination potential accordingly. Calculated sub-LPI revealed organic pollutants dominate in all the seasons i.e LPI org >LPI in>LPIhm. It is also concluded that calculated LPIhm in all three seasons comes under the standard of 7.3. The concentration of heavy metal was observed low, so biological treatment may be suitable. Seasonal variation of the overall calculated LPI value obtained was 32.5 in pre-monsoon, 28.0 in monsoon, and 32.0 in Post monsoon. In order of pre-monsoon > Post monsoon > Monsoon which are observed exceeded the calculated treated leachate standard set by MOEFCC for release of treated leachate in inland water public sewer and subsurface water. The future work of this research will be the treatment of landfill leachate more preferred biological treatment observed from the results that the concentrations of heavy metals were too low.
Acknowledgment
We acknowledge the support of the UGC (University Grant Commission) NET- JRF fellowship. We also acknowledge the CPCB and DJB, New Delhi for their Laboratory facilities and their support.
Conflict of Interest
All the authors confirmed no competing interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Eggen T., Moeder M., Arukwe A. Municipal landfill leachates: a significant source for new and emerging pollutants. Science of Total Environment. 2010; 408 (21): 5147–5157.
CrossRef - Simon E., Adianimovie S. Estimating the temporal leachate contamination potential of Yenagoa central waste dumpsite, using Leachate pollution index, Abanigi Road Etelebu, Nigeria. Asian Journal of Advanced Research and Report .2022; 16 (7):31-39. Doi: 10.9734/AJARR/2022/v16i730484.
CrossRef - Walker WH., Illinois. Groundwater pollution. Journal of American water works Association.1969; 61: pp.31-40
CrossRef - Chian E.S.K., De Walle F.B. Sanitary landfill leachates, and their treatment. Journal. Environment Engineering Division, ASCE. 1976; 102:411-431.
CrossRef - Kelley W.E, Journal of Environmental Engineering Division, ASCE. 1976;102(6), pp.1189-1199
CrossRef - Masters G.M, Introduction to Environmental Engineering and Science’, Prentice – Hall of India Pvt. Ltd., New Delhi, India. 1998.
- Kumar D., Khare M., and Alappat B.J. Threat to groundwater from the municipal landfills in Delhi’, India. Proceedings 28th WEDC Conference on Sustainable Environmental Sanitation and Water Services, Kolkata, India. 2002; 377-380
- Naveen B.P., Malik R.K. Assessment of Leachate Pollution Index for the Delhi Landfill Sites, India Open Access Journal of Science and Engineering. 2017; 2 (9|):98-101
- B. P. Naveen and R. K Malik. Assessment of Contamination Potential of Leachate from Municipal Solid Waste Landfill Sites for Metropolitan Cities in India. Pollution;5(2): 313-322, 2019 ; DOI: 10.22059/poll.2018.266991.527
- Samal B., Mani S., Madguni O. Open Dumping of Waste and Its Impact on Our Water Resources and Health—A Case of New Delhi, India. Recent Developments in Waste. 2020; Springer
CrossRef - Arbastan H.G., Gitipour S. Evaluating the consequences of household hazardous waste diversion on public health and ecological risk of leachate exposure. International Journal of Environmental Science and Technology.2022;19:4407-4420.http: //doi.org.10.1007/s13762-022-04063-5
CrossRef - Gotvajn A.Z., Tisler T., Koncan T. Comparison of different treatment strategies for industrial landfill leachate Journal Hazardous Material. 2009; 62, 1446-1456.
CrossRef - Ghosh P., Gupta A., I. S Thakur I.S. Combined chemical and toxicological evaluation of leachate from municipal solid waste landfill sites of Delhi, India. Environmental Science Pollution Research. 2015; 22(12), 9148- 9158. https://doi.org/10.1007/s11356-015-4077-7.
CrossRef - Adesogan S.O., Omonigho B.O. Assessment of leachate contamination potential of landfills in Ibadan, Nigeria. African Journal of Environmental Science and Technology. 2021;15(5), pp. 179-187.DOI: 10.5897/AJEST2021.3009
CrossRef - Kumar D., B.J. Alappat B.J. Analysis of leachate contamination potential of a municipal landfill using leachate pollution index. Workshop on Sustainable Landfill Management, Delhi. 2003; 147–153.
- Rafizul I.M., Alamgir M., Sharif S.M.S. Analysis and Selection of Appropriate Aggregation Function for Calculating of Leachate Pollution Index of Landfill Lysimetric. Iranica Journal of Energy & Environment.2012; 3 (4): 370-379.
CrossRef - Kumar D., Alappat B.J. Analysis of leachate pollution index and formulation of sub-leachate pollution indices. Waste Management Research. 2005; 23(3):230–239.
CrossRef - Manimekalai B., Vijayalakshmi P. Analysis of Leachate Contamination Potential of a Municipal Landfill Using Leachate Pollution Index. Journal of Environmental Science, Toxicology and Food Technology.2012; 2 (1): PP 16-39.
CrossRef - Angmo S., Shah S. Impact of Okhla, Bhalswa and Ghazipur Municipal Waste Dumpsites (Landfill) on Groundwater Quality in Delhi. Current World Environment. 2021; 16 (1)
CrossRef - Angmo S., Shah S. Assessment of Waste to Energy Generation Potential of Municipal Solid Waste: A Case Study of South Delhi. Journal of Science and Technology.5 (5). 2020; DOI: https://doi.org/10.46243/jst.2020.v5.i5.pp162-170
CrossRef - American Publication on Public Health (APHA). Standard Methods for Examination of Water and Wastewater”, 23rd edition, American Public Health Association, Washington, DC. Brock T.D. and Madigan M.T. 2017: 641 – 644
- Solid Waste Management Rule (SWM Rule). Ministry of Environment Forest and Climate Change, Notification, New Delhi. 2016; 1-91
- Jorstad LB., Jankowski J., Acworth R.I. Analysis of the distribution of inorganic constituents in landfill leachate contaminated aquifers Astrolabe Park, Sydney, Australia. Environ Geology 2004 ;46(2): 263–272
CrossRef - Hoai S.T., Lan H.N., Viet N.T.N.,Hoang G.N., Kawamoto, K. Characterizing Seasonal Variation in Landfill Leachate Using Leachate Pollution Index (LPI) at Nam Son Solid Waste Land?ll in Hanoi, Vietnam. Environments, 2021; 8(17). https://doi.org/10.3390/environments8030017
CrossRef - Kamaruddin M.A., Yusoff M.S., Rui L.M., Isa A. M., Zawawi M. H., R. Alrozi. An overview of municipal solid waste management and landfill leachate treatment: Malaysia and Asian perspectives. Environment Science Pollution Research. 2017; 24:26988–27020
CrossRef - Fatta D., Papadopoulos A., Loizidou M. A study on the landfill leachate and its impact on the groundwater quality of the greater area. Environmental Geochemistry and Health.1999; 21(2) :175–190
CrossRef - Mishra S., Singh A.L., Tiwari D. Studies of the physicochemical status of the ponds at Varanasi Holy City under anthropogenic influences. International Journal of Environmental Research and Development. 2014; 4(3): pp. 261–268.
- Leu D ., Xu J ., Fang Y., Du Y., Hu L., Fang C., Shen D., Long Y. Effect of air and water the release of chlorine from semi-aerobic landfill. Environmental Technology. 2021; (14), 2197-2206. htps://doi.org/10.1080/09593330.2020.1869838
CrossRef - Koshy L., Jones T., Berube K. Bioreactivity of municipal solid waste landfill leachates-Hormesis and DNA damage. Water Research. 2008; 42 (8-9):2177–2183.
CrossRef - Adesogan S.O., Omonigho B.O. Assessment of leachate contamination potential of landfills in Ibadan, Nigeria. African Journal of Environmental Science and Technology. 2021; 15(5), pp. 179-187. DOI: 10.5897/AJEST2021.3009
CrossRef - Cameron R.D., Koch F.A. Toxicity of Landfill Leachates. Journal of World Pollution Federation.1980; 52(4):760-69
- Afsar S.S., Kumar S., Alam. Characterization of Leachate at Various landfill Sites Delhi, India. International Journal Advance Technology Engineering Science. 2015; 3(1), 552–558
- Bhalla B., Saini M.S., Jha M.K. Effect of Age and Seasonal Variations on Leachate Characteristics of Municipal Solid Waste Landfill. International Journal of Research in Engineering and Technology.2013; 2(8):223-232.
CrossRef - Somani M., Datta M., Gupta S.K., Sreekrishnan T.R., Ramana G.V. Comprehensive assessment of the leachate quality and its pollution potential from six municipal waste dumpsites of India Bioresource Technology Reports. 2019; 198–206
CrossRef - Samal B., Madguni O. Assessing Leachate Pollution Index and Its Variability across Various Seasons– A Case of Okhla Landfill Site Octa Journal of Environmental. 2018;6(1): 041-045.
- Purwar A .K. Seasonal variation analysis of leachate contamination potential from landfill using leachate pollution index. International Journal Research Applied Science Engineering Technology.2018; 6(4):270–274
CrossRef - Ghosh P., Gupta A, and Thakur I.S. (2015) Combined chemical and toxicological evaluation of leachate from 405 municipal solid waste landfill sites of Delhi, India. Environmental Science Pollution Research 22(12), 9148 406 9158.https://doi.org/10.1007/s11356-015-4077-7.
CrossRef - Sharma A., Meesa S., Pant S., Alappat B.J., Kumar D. Formulation of a landfill pollution potential index to compare pollution potential of uncontrolled landfills. Waste Management Research .2008; 26(5):474–483
CrossRef - Cohen R.D., Bowser D.H., Costa M., Chang LW, Magos L, Suzuki T, Carcinogenicity and Genotoxicity of lead, beryllium, and other metals. In: Chang LW, Magos L, Suzuki T (eds Toxicology of metals. CRC/Lewis Publishers, Boca Raton, FL. 1996; 253–284.
- Al-Abed S.R., Jegadeesan G. Arsenic Sources, and Assessment. Presented at Arsenic And Landfills: Protecting Water Workshop Organized By Superfund Basic Research Program (SBRP) in NIEHS, Boston, MA,2006
- WHO. Arsenic. https://www.who.int/news-room/fact-sheets/detail/arsenic. 2018
- Chaudhary R., Nain P., Kumar A. Temporal variation of leachate pollution index of Indian landfill sites and associated human health risk. Environmental Science and Pollution Research .2022; https://doi.org/10.1007/s11356-021-12383-1
CrossRef - Baderna D., Maggioni E., Gemma S., Moltenic M.,Lamberdo A., Colombo A., Bordonali S.,Rotella G., Lodi M., Benfeneta S., A combined approach to investigate the toxicity of an industrial landfills leachate: chemical analyses, risk, assessment and invitro assays. Environment Research. 2011; 111(4):603-13.Doi: 10.1016/j.envres.2011.01.015.
CrossRef - Talalaj I.A. Mineral and organic compounds in leachate from landfill with concentrate recirculation. Environmental Science and Pollution Research. 2015; 22(4): pp.2622–2633.
CrossRef - Hobson A.J., Stewart D. I., Mortimer R.J.G., Mayes W.M., Rogerson M.T. Leaching behavior of co-disposed steel making wastes: Effects of aeration on leachate chemistry and vanadium mobilization, Waste Management. 2018a .,81:1-10.https://doi.org/10.1016/j.wasman.2018.09.046
CrossRef - Hobson A.J., Stewart D.I., Bray A.W., Mortimer R.J.G., Mayes W.M., Riley A.L, Rogerson M.I., Burke I.T. Behavior and the fate of vanadium during the aerobic neutralization of hyper alkaline slag leachate. Science of the Total Environment. 2018b 643:1191-1199. DOI: 10.1016/j.scitotenv.2018.06.272
CrossRef - Sharma, S., Singh R. Selenium in soil, plant, and animal systems. Critical review Environmental science and technology. 1983; 13(1), 23–50
CrossRef - Minnesota Pollution Control Agency (MPCA). Environmental monitoring data for municipal landfill leachate, twin cities region, January –May. Technical Report, Minnesota pollution control agency, Groundwater and solid waste Division, St. Paul, MN. 2000
- Lemly A.D. Aquatic selenium pollution is a global environmental safety issue. Ecotoxicology and Environmental safety issue. 2004; 59:44-56
CrossRef - Nakamura K., Kinoshita, Takatsuki H. The origin and behavior of lead cadmium and antimony in residue after incineration of municipal waste.Waste Management.1996; 16(5-6):509-17.
CrossRef - Paoletti F., Seifert H., Vehlow., P. Sirini P. Oxyanion forming elements in waste composition-partitioning of Antimony Waste Management & Research. 2000;18(2):141-50
CrossRef - Kulikowska D., Klimiuk E. The effect of landfill age on municipal leachate composition Bioresource Technology. 2008; 99(13): 5981–5985.
CrossRef - Bag S., Dubey R., Mondal N. A study on estimating the leachate pollution index at the Ghazipur landfill site. Engineering Technology Journal. 2016 ;(2):62–69 ;DOI: 10.18535/etj/v1i2.04
- Ahamad A., Raju N.J., Madhav, S ., W. Gossel W., Wycisk P. Impact of non-engineered Bhalswa landfill on groundwater from quaternary alluvium in Yamuna flood plain and potential human health risk, New Delhi, India Quaternary International.2019; 507:352–369
CrossRef - Singh V., Mittal A. Toxicity analysis and public health aspects of municipal landfill leachate: a case study of Okhla Landfill, Delhi. 8th World Wide Workshop for Young Environmental Scientists WWW-YES 2009: Urban waters: resource or risks, Arcueil, France.hal0059310







