Synthesis, Characterization and Efficiency of 1-Ethyl-3-methylimidazolium tetrafluoroborate ([emim]BF4) for Degradation of Waste Nylon-66
1
Department of Chemistry,
Sardar Patel Mahvidyalaya,
Chandrapur,
Maharashtra
India
Corresponding author Email: sunilchikte78@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.3.25
Copy the following to cite this article:
Chikte S. R, Madhamshettiwar S. V. Synthesis, Characterization and Efficiency of 1-Ethyl-3-methylimidazolium tetrafluoroborate ([emim]BF4) for Degradation of Waste Nylon-66. Curr World Environ 2024;19(3). DOI:http://dx.doi.org/10.12944/CWE.19.3.25
Copy the following to cite this URL:
Chikte S. R, Madhamshettiwar S. V. Synthesis, Characterization and Efficiency of 1-Ethyl-3-methylimidazolium tetrafluoroborate ([emim]BF4) for Degradation of Waste Nylon-66. Curr World Environ 2024;19(3).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2024-07-12 |
|---|---|
| Accepted: | 2024-11-11 |
| Reviewed by: | 
 Ephsy K Davis
Ephsy K Davis
|
| Second Review by: |

 Manisha Sharma
Manisha Sharma
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Plastic is an essential part of modern living. Since the beginning of the twentieth century, when plastics were first created in significant amounts, living without plastic has been increasingly difficult.1 Plastics are very flexible substances with a wide range of applications that have greatly improved human well-being because of their molecular composition and additives. However, plastics present a major treatment and disposal difficulty in the management of urban solid waste because of their relative inertness and lack of biodegradability.2 Unquestionably, the widespread and careless use and disposal of these non-biodegradable materials has severely disrupted and harmed the environment and its biodiversity.3 The environmental impact of plastics is currently a global concern due to the limited methods of treatment and disposal along with the rising rates of manufacturing and usage. In many developing and underdeveloped countries, burning plastic garbage is a common practice due to the scarcity of well-planned and prepared landfill sites for its disposal. The hazardous and toxic gasses produced by this procedure pollute the air. Moreover, several unsafe disposal techniques, such as open dumping, uncontrolled combustion, unscientific composting, and inappropriate garbage dumps, are routinely employed in countries like India.4 Due to a lack of disposal options, just 10% of the million tons of 140, globally yearly production of synthetic polymers are recycled or repurposed.5 People from all around the world have been trying to decompose or transform plastic trash into a form that can be used by employing a variety of techniques. For example, the incineration process involves the burning of plastic garbage, but it produces toxic gases. Recycling is an additional technique for transforming plastic trash into different, usable forms. However, the recycling process, involves energy consumption to convert plastic into another form, but it stays the same. Hence proper technique is required to convert the plastic into usable form for a pollution-free environment. Degrading waste plastics into its monomers was found to be the best method to overcome these issues.6 Polymer degradation is the process that results in any physical or chemical change in the properties of polymers due to external influences (such as light, heat, moisture, etc.), chemical conditions, or biological activity. Several mechanisms contribute to the degradation of waste polymer including physical, chemical, and biological processes. Degradation of waste polymer by chemical method involves pyrolysis, hydrolysis, catalytic, and oxidative methods.7-13 For this, a variety of methods including thermolysis14 and solvolysis using sub- and supercritical fluids are frequently used.15,16 In such methodologies, the utilization of a solvent with a high boiling point or a high-pressure apparatus is typically necessary. When organic solvents are utilized in such reactions, precautionary measures must be implemented to mitigate the risk of fire. Another approach to polymer degradation involves biodegradation techniques17, 18, where microorganisms such as Bacillus Subtilis economically break down the polymer. However, not all polymers can undergo degradation through this method due to unfavorable environmental conditions and extended periods. To overcome these problems and to increase the efficiency as well as to modify the process of degradation, ionic liquids are introduced as a new reaction medium that has a unique feature compared to conventional organic solvents.
ILs gaining attention as potential replacements for volatile organic solvents. They are categorized as green solvents since they are recyclable, nonflammable, and nonvolatile. They are regarded as favorable medium candidates for chemical syntheses because of their exceptional qualities, which include their excellent solvating potential,19 thermal stability,20 and their adjustable properties by appropriate choices of cations and anions.21 Ionic liquids have demonstrated during the two decades to be extremely promising "green" solvents that provide several benefits over conventional organic solvents.22,23 Overall, the chemical industry regards them as valuable solvents because of their remarkable solvent properties, stability at high temperatures, and lack of volatility. Ionic liquids (ILs) having melting point less than 100 0C, or even room temperature (RTILs), since they only include organic cations and inorganic or organic anions.24,25 Interest in ionic liquids has grown significantly as a potential replacement for volatile organic solvents. ILs are also referred to as greener solvents because of their recyclable, nonflammable, and non-volatile properties. ILs can be used as solvents and catalysts in the degradation of waste polymers due to their solvating potential, thermal stability, and their adjustable properties by appropriate choice of cations and anions. Ionic liquids are a unique class of compounds that have generated important interest during some previous years due to some of their extraordinary properties and a very wide range of applications. Ionic liquid [Bmim][FeCl4] can used to catalyze the depolymerization of lignin to methyl p-hydroxycinnamate. Overall catalysts [FeCl4]- found an important part during the catalytic process, having two activation modes and three distinct reaction routes that can be used to achieve the complete depolymerization of the model compound lignin.26 Ionic liquid [emim]BF4, is an example of a hydrophilic ionic that was effective for the degradation of nylon-6 into caprolactam monomer. The ionic liquid was successfully separated from the obtained monomer by the simple extraction method.27 Without the use of conventional acid or base catalysts, the methanolysis of poly (lactic acid) (PLA) was accomplished with ease at lower pressures and temperatures when the ionic liquid [Bmim][Ac] was used as a catalyst.28
There has been a considerable amount of research on the breakdown of PET, polyester, lignin, cellulose, etc., but not much on the breakdown of polyamides, such as nylon-6 and nylon-66. The main objective of this research work is to identify and synthesize the ionic liquid which can degrade effectively waste nylon-66 in to its monomers. This research work includes the synthesis 1-ethyl-3-methylimidazolium tetrafluoroborate and its use for depolymerization of nylon-66 into dibenzoyl derivative of hexamethylene diamine (DBHMD) and adipic acid. The present study provides the new usage of ionic liquid as solvent as well as catalyst for degradradtion of waste nylon-66..
Materials and Methods
Materials
Waste nylon-66, 1-methylimidazole (99 % pure from Sigma-Aldrich), chloroethane, magnesium sulfate, acetonitrile, dichloromethane, m-cresol, benzoyl chloride, methanol, etc.
Synthesis of 1-ethyl-3-methylimidazolium tetrafluoroborate ([emim]BF4)
The synthesis of 1-ethyl-3-methylimidazolium tetrafluoroborate involves a two-step process. The first step is known as quaternization, which involves the formation of 1-ethtyl-3-methylimidazolium chloride by the combination of an equal molar amount of 1-methylimidazole (7.38g, .1mol) in a flask with rounded bottom and chloroethane (6.45g, .1mol) with constant stirring. An ice bath was used to control the exothermic reaction. The mixture was refluxed at around 60–70 °C using a water condenser with constant stirring for 26–28 hours. After refluxing and cooling at ambient temperature, deionized water was used to extract the reaction mixture several times to remove the byproducts and unreacted starting material. After the separation of the organic layer of 1-ethyl-3-methylimidazolium chloride, anhydrous magnesium sulfate was used for drying it, and was removed by filtration method. A rotary evaporator is used to remove some traces of solvent to obtain pure ([emim]Cl). The second step involves the anion exchange method, in which an equimolar amount of ([emim]Cl) (5.23g, .03mol.) in acetonitrile was prepared and mixed with an aqueous solution of sodium tetrafluoroborate (3.3g, .03mol) with constant stirring at room temperature. With this addition, precipitates of sodium chloride were separated by the simple filtration method. Acetonitrile and the remaining water were removed under reduced pressure using a rotary evaporator. To obtain pure 1-ethyl-3-methylimidazoliumte trafluoroborate [emim]BF4, it was repeatedly extracted with dichloromethane, followed by removing it under reduced pressure.
Pretreatment of waste nylon-66
Collected Waste nylon-66 was contaminated with dust particles and some other microorganisms. To purify waste nylon-66, it was treated with a 1 g/L solution of nonionic detergent at around 70–80 °C for 6-7 hours. Furthermore, to remove any surface impurities, it was properly cleaned with distilled water and then allowed to dry at around 60–70 °C for 3–4 hours.
Determination of molecular weight of nylon-66
By using the Mark-Houwink equation ([n] = KMa), an average molecular weight of waste nylon-66 (polyamide) can be calculated by the intrinsic viscosity of nylon-66 solution in m-cresol.
Where [n] = intrinsic viscosity. K, a = constant parameters for m-cresol solvent.
K = 2.41 × 10-3 and a = 0.61 at 28 0C
M = molecular weight.
The specific viscosity (nsp) and relative viscosity (nr) are related to each other by the following equation, (nsp) = nr-1
nr = flow time of solution/flow time of m-cresol
When polymer concentration approaches zero, the limit of the ratio (nsp/c) gives the intrinsic viscosity.
Experimental method for degradation of nylon-66 in [emim]BF4
1-ethyl-3-methylimidazoliumtetrafloroborate, which is a hydrophilic ionic liquid, was used in two different conditions for the degradation of nylon-66. The first experimental procedure involves the degradation of 0.5g of waste nylon-66 in the presence of 1.9g of [emim]BF4 ionic liquid and 0.02g of DMAP catalyst. The contents of the reaction refluxed in the nitrogen atmosphere under normal pressure at around 290–300 °C for 1 hour. Black oil was obtained. After cooling and dilution with 10 ml of distilled water, it was repeatedly extracted with ethyl acetate. By evaporating the extracting solvent, it leaves behind a crude colorless compound of adipic acid. It was recrystallized with methanol. For the recovery of hexamethylenediamine, the same reaction mixture was treated with excess of benzoyl chloride (C6H5COCl), due to which dibenzoyl derivative of hexamethylenediamine (DBHMD) precipitate out.
The second approach for the degradation of nylon-66 was hydrolysis method, in which 0.5g of waste nylon-66 was refluxed in the presence of 20 ml of 5 N HCl and 1.9g of [emim]BF4 ionic liquid. This method involves the refluxing of the reaction mixture at around 100–120 °C for 15-20 hours under normal pressure. After cooling the reaction mixture, 5 N NaOH was added to make the solution just alkaline. The slightly excess of benzoyl chloride to the reaction contents gives the benzoyl derivative of hexamethylenediamine. Filtrate was extracted multiple times with ethyl acetate yield adipic acid, which was further recrystallized with methanol. Ionic liquid [emim]BF4 was regenerated in a vacuum by using a rotary evaporator.
Result and discussion
Molecular weight of nylon-66
An average molecular weight of nylon-66 was obtained by measuring the viscosity of different concentration solutions of nylon-66 in m-cresol solvent by using Ostwald’s viscometer. Experimentally different concentrations of nylon-66 were prepared in m-cresol solvent and for each solution; specific viscosity was measured (table 1). The graph was plotted between reduced viscosity (nsp/c) or inherent viscosity (ninh) against the concentrations of nylon-66 in m-cresol solvent. The graph was extrapolated for zero concentration to obtain the intrinsic viscosity. The average molecular weight of nylon-66 can be calculated using the equation [n] = KMa.
Table 1: experimental determination of molecular weight for nylon-66
Conc. of nylon-66 (g/25 ml) | Flow time (sec.) | (nr) = n/no = t2 /t1 | nsp =nr -1 | nsp/c | ln nr | (ninh ) ln nr /c |
0.075 | 356 | 1.402 | 0.402 | 5.36 | 0.33378 | 4.504 |
0.0625 | 322 | 1.268 | 0.268 | 4.29 | 0.2374 | 3.798 |
0.05 | 302 | 1.189 | 0.189 | 3.78 | 0.1731 | 3.462 |
0.0375 | 284 | 1.118 | 0.118 | 3.15 | 0.1115 | 2.973 |
0.025 | 270 | 1.062 | 0.062 | 2.48 | 0.0601 | 2.404 |
0.0125 | 259 | 1.019 | 0.019 | 1.52 | 0.0188 | 1.504 |
m-cresol | 254 | - | - | - | - | - |
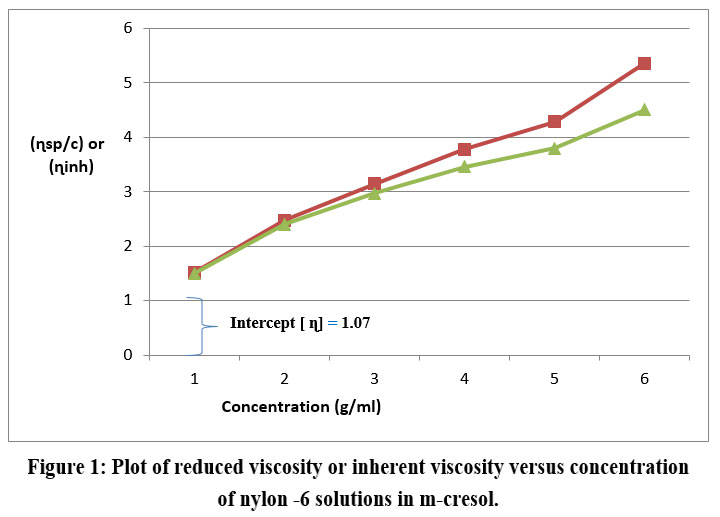 | Figure 1: Plot of reduced viscosity or inherent viscosity versus concentration of nylon -6 solutions in m-cresol.
|
From the graph, intrinsic viscosity [n] was found to be 1.5. The constant K and a depend on the solvent used. For m-cresol, K and a, are 2.41 × 10-3 and a = 0.61 at 28 0C respectively. By solving the above equation, the average molecular weight for nylon-66 was found to be 21,867 g/mol. approximately which found in the range of literature value 12,000 – 50,000 g/mol.
Synthesis of ([emim]BF4)
The synthesis of ionic liquid [emim]BF4 involves a two-step process. The first step involves the quaternization method for the synthesis of [emim]Cl as the initial product. The required IL was obtained by anion exchange with NaBF4. The faint yellow colored viscous liquid [emim]BF4 was obtained with 85.65 % yield. The crude IL was washed with acetone. 1H NMR, and 13C NMR techniques were used to check the purity of the desired IL.
1H-NMR (e ppm) (DMSO): 1.21 [t, 3H, (CH3), CH3-CH2], 3.92 [s, 3H, (CH3), N-CH3], 3.98 [q, 2H, (CH2), –N-CH2-CH3], 7.16-7.22 [d, 2H, N-CH=CH-N], 8.35 [s, 1H, (CH), N=CH-N].
The singlet at 2.97ppm was obtained due to the DMSO solvent.
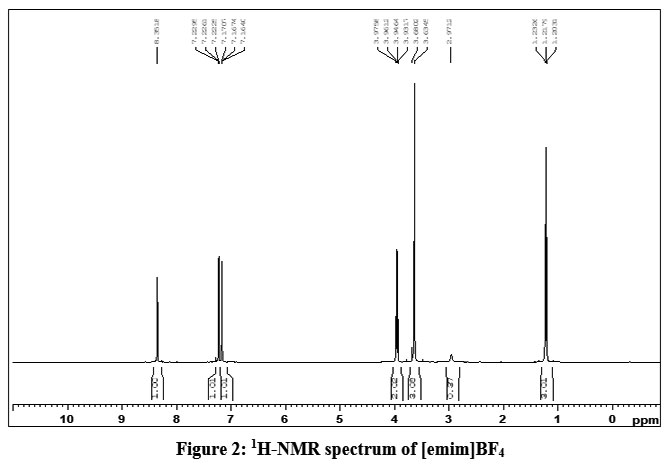 | Figure 2: 1H-NMR spectrum of [emim]BF4
|
13C-NMR (e ppm) (CDCl3): 14.65 [C atom from –CH3, CH3-CH2-N], 44.69 [C atom from –CH3, -CH3-N], 77.50 [C atom from CH2, CH3-CH2-N], 135.54 [C atom from CH, N-CH-N], 121.81 and 123.41[two sp2 C atoms, HC=CH].
The peak at e 35.56 was obtained due to C- atoms from methyl group in CDCl3 solvent.
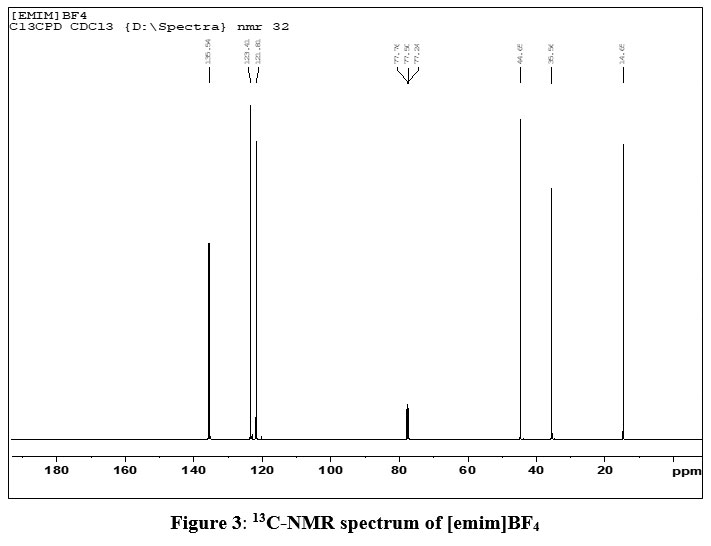 | Figure 3: 13C-NMR spectrum of [emim]BF4
|
Degradation of nylon-66 in IL [emim] BF4
IL [emim] BF4 was used for the degradation of nylon-66 into its monomers by using a DMAP catalyst. Ionic liquid [emim]BF4 provides the reaction medium as a solvent. The maximum yield of monomers was obtained at 300 0C. IL [emim]BF4 directly involves in the breaking of the amide (CO-NH) linkage in nylon-66. After dilution with distilled water and extraction with ethyl acetate, the crude product of adipic acid was obtained. The same reaction mixture was treated with benzoyl chloride. Benzoylation takes place at the nitrogen atom by consuming its lone pair of electrons, and the removal of the hydrogen atom from both -NH2 groups of hexamethylenediamine yields DBHMD. The yield of DBHMD and adipic acid was found to be 4.325g and 0.157g respectively. In this method IL [emim]BF4, provides the reaction medium as a solvent for degradation of waste nylon-66.
In the hydrolysis method, 0.5 g of waste nylon-66, 0.1mol [emim]BF4, and 5N HCl were refluxed at around 100–120 °C for several hours. For benzoylation, the solution was made just alkaline (pH~ 7.5) with the addition of a slight excess of 5N sodium hydroxide. The obtained adipic acid was recrystallized with methanol. The yield of DBHMD and adipic acid was found to be 8.408g and 0.245g respectively. In hydrolysis method, the ionic liquid [emim]BF4 plays the role of catalyst and catalyzed the hydrolysis of nylon-66. In this method [emim]BF4 does not directly break the amide linkage, but it initiate the interaction between water and amide linkages of nylon-66. IL [emim]BF4 enhance the penetration of water molecule in to the nylon-66 matrix and catalyze the hydrolysis of nylon-66. As [emim]BF4 is water soluble, less viscous, it allows the water to diffuse easily and thus swells and solubilize the nylon-66 and resulting the degradation of waste nylon-66.
The monomers DBHMD and adipic acid were characterized by FT-IR. In both methods, the ionic liquid was recovered in a vacuum by using a rotary evaporator.
FT-IR (DBHMD) cm-1
3305.37 (N-H stretching), 1698 (C=O stretching), 1627, 1530, 1479, 1374 (C=C stretching in benzoyl group), 1416 (C-C stretching in CH2 groups), The two peaks at 788 and 861 corresponds to mono substituted benzene ring from fig.4. Due to symmetrical structure of DBHMD only one peak for N-H and C=O stretching was obtained.
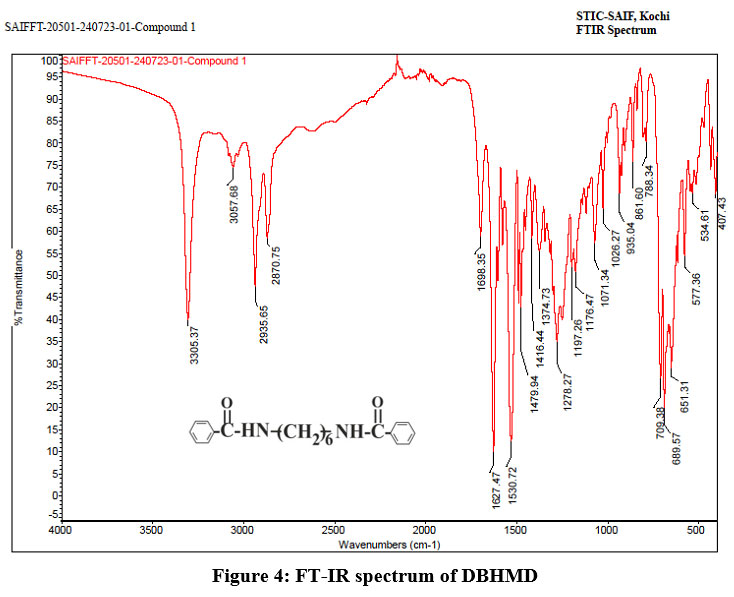 | Figure 4: FT-IR spectrum of DBHMD
|
FT-IR (Adipic acid) cm-1
Adipic acid shows the broad absorption band in the region of 2500-3000 cm-1 due to -OH stretching in the carboxylic group (-COOH).
2909 (-OH stretching in –COOH), 2944-3003 (C-H stretching in >CH2), 1532, 1440 are due to C-O and C-C stretching respectively.
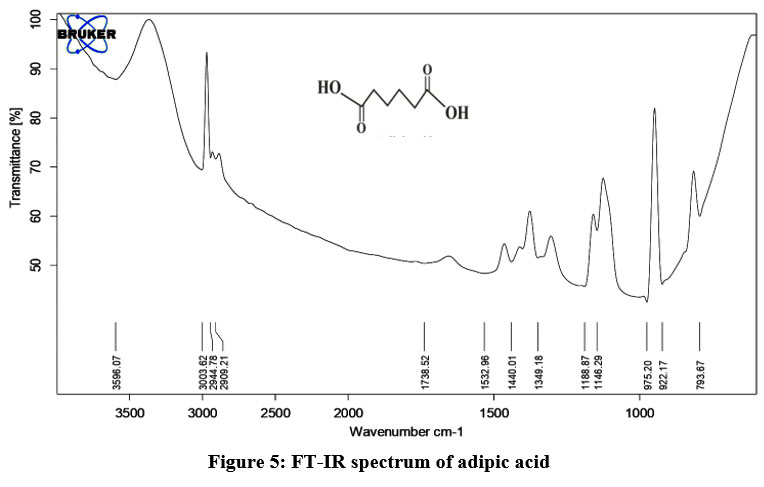 | Figure 5: FT-IR spectrum of adipic acid
|
Recovered [emim]BF4 ionic liquid was characterized by FT-IR (fig. 6). It was observed that, after recovery of IL, there was no change in the internal structure of [emim]BF4 and its IR spectra completely matched with the original IR spectra. It can be used 4-5 times without changing its efficiency. The BF4- anion showed a strong absorption peak at 1017 cm-1 corresponding to B-F str.
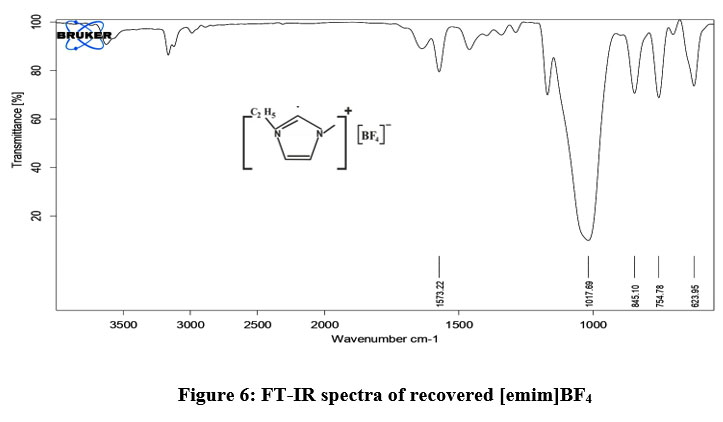 | Figure 6: FT-IR spectra of recovered [emim]BF4
|
Conclusion
The degradation of nylon-66 using 1-ethyl-3-methylimidazoliumtetrafluoroborate ([emim]BF4) provides valuable insight into a novel approach for the breakdown of polyamide and the disposal of this widely used synthetic polymer. Ionic liquids ([emim]BF4) have shown high potential in the degradation of nylon-66 into benzoyl derivatives of hexamethylene diammine and adipic acid, which were confirmed by FT-IR spectra. This method offers a feasible solution to overcome the environmental pollution caused by the disposal of waste nylon-66, which is durable and persistent in the environment. Ionic liquid ([emim]BF4) has shown efficiency as a solvent as well as a catalyst during the hydrolysis of nylon-66 into useful products without generating any harmful by-products. Different parameters like reaction time and temperature can optimize the degradation process. Because of the minimal volatility and repetitive usability, ILs do not create any additional burden on the environment. It is regenerated at the end of the reaction without changing its efficiency, and it can be used four to five times. The potential use of ionic liquids shows a promising alternative solvent instead of commercially hazardous solvents. In the future, there is a need to enhance the process to increase the effectiveness of the degradation of nylon-66 and evaluate the economic feasibility of industrial-scale application. Additionally, sustainable practices also require investigating the reusability and recyclability of ionic liquids.
Acknowledgement
We are thankful to the Principal, Sardar Patel Mahavidyalaya, Chandrapur (M.S.), for providing the facilities to perform the experiments. We are also thankful to SAIF, Punjab University, Chandigarh, SAIF, STIC, Kochi University, Kochi, Nagpur University, Nagpur for 1H, 13C-NMR, and FT-IR analyses of the samples.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this research article.
Conflict of Interest
The author(s) do not have any conflict of interest.
Data Availability Statement
Data is generated by author undertaken study.
Ethics Statement
This research does not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement- This study did not involve human participants, and therefore, informed consent was not required.
Author Contributions
Both the authors equally involved in performing experimental work and preparing the manuscript.
References
- Barnes, D. K. A., Galgani, F., Thompson, R. C., & Barlaz, M. Biological Sciences, 364(1526), 1985–1998. (2009) https://doi.org/10.1098/rstb.2008.0205
CrossRef - Priyanka, N., &Archana, T. Journal of Environmental & Analytical Toxicology, 01(02) (2011).https://doi.org/10.4172/2161-0525.1000111
CrossRef - Barnes, D. K. A., Galgani, F., Thompson, R. C., & Barlaz, M. Biological Sciences, 364(1526), 1985–1998 (2009).. https://doi.org/10.1098/rstb.2008.0205
CrossRef - Kandakatla, P., Mahto, B., & Goel, S. In Int. J. Environment and Waste Management 11 (4) (2013).
CrossRef - Masayuki, S. Current opinion in Biotechnology, 12(3), 242-247 (2001).
CrossRef - Karaduman, A. Energy Sources, 24(7), 667–674 (2002). https://doi.org/10.1080/00908310290086590
CrossRef - Patil, D. B., & Madhamshettiwar, S. V. Journal of Applied Chemistry, 2014(1), 286709 (2014).
CrossRef - Wang, J., Han, W., Li, C., Luo, H., & Zhao, J. Green Chemistry, 21(8), 1914–1921 (2019).
- Wu, W., Zhang, Y., Wang, D., & Zhao, M. Journal of Hazardous Materials, 415, 1255–1285 (2021).
- Hu, X. Industrial & Engineering Chemistry Research, 55(39), 1352–1359 (2016).
- Li, Y. Fuel Processing Technology, 181, 246–253 (2018).
- Yang, X., Zhang, J., & Qi, H. Journal of Cleaner Production, 256, 1202–1269 (2020).
- Rajarathnam, D., Rao, K. M., & Chandramouli, R. Journal of Polymers and the Environment, 28(6), 1712–1721 (2020).
- Holland, B. J., & Hay, J. N. (2000). PolymerInternational, 49(9), 943-948.
CrossRef - Kamimura, A., Oishi, Y., Kaiso, K., Sugimoto, T., & Kashiwagi, K. ChemSusChem: Chemistry & Sustainability Energy & Materials, 1(1?2), 82-84 (2008).
CrossRef - Goto, M. J. Supercrit. Fluids, 47, 500–507 (2009).
CrossRef - Eubeler, J. P., Bernhard, M., & Knepper, T. P. TrAC - Trends Anal. Chem., 29, 84–100 (2010).
CrossRef - Xu, R. et al. Bioresour. Technol., 269, 557–566 (2018).
CrossRef - Plechkova, N. V., & Seddon, K. R. In Chemical Society Reviews, 37(1), 123–150 (2008).
CrossRef - Meine, N., Benedito, F., & Rinaldi, R. Green Chemistry, 12(10), 1711–1714 (2010). https://doi.org/10.1039/c0gc00091d
CrossRef - Zhang, W., & Cue, B. W. (Eds.). (2018).
- Rogers, R. D., & Seddon, K. R. (Eds.). (2003).
- Hallett, J. P., & Welton, T. In Chemical Reviews, 111(5), 3508–3576 (2011). https://doi.org/10.1021/cr1003248
CrossRef - Zhang, W., & Cue, B. W. (Eds.). (2018).
- Rogers, R. D., & Seddon, K. R. Science, 302(5646), 792-793 (2003).
CrossRef - Zhang, T., Zhang, Y., Wang, Y., Huo, F., Li, Z., Zeng, Q., & Li, X. Frontiers in Chemistry, 7, 446 (2019).
CrossRef - Kamimura, A., Shiramatsu, Y., & Kawamoto, T. Green Energy and Environment, 4(2), 166–170 (2019).. https://doi.org/10.1016/j.gee.2019.01.002.
CrossRef - Song, X., Zhang, X., Wang, H., Liu, F., Yu, S., & Liu, S. Polymer Degradation and Stability, 98(12), 2760–2764 (2013).. https://doi.org/10.1016/j.polymdegradstab.2013.10.012
CrossRef






