Tree Diversity in The Shola Forests of Brahmagiri Wildlife Sanctuary, Karnataka, India
1
Department of Ecology and Environmental Sciences,
Pondicherry University,
Puducherry,
India
2
Department of Environmental Sciences,
Bangalore University,
Jnanabharathi Campus, Bengaluru,
Karnataka
India
Corresponding author Email: smspandian65@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.1.6
Copy the following to cite this article:
Revathy U, Nagaraja B. C, Sundarapandian S. Tree Diversity in The Shola Forests of Brahmagiri Wildlife Sanctuary, Karnataka, India. Curr World Environ 2023;18(1). DOI:http://dx.doi.org/10.12944/CWE.18.1.6
Copy the following to cite this URL:
Revathy U, Nagaraja B. C, Sundarapandian S. Tree Diversity in The Shola Forests of Brahmagiri Wildlife Sanctuary, Karnataka, India. Curr World Environ 2023;18(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-12-09 |
|---|---|
| Accepted: | 2023-03-14 |
| Reviewed by: | 
 Shaimaa Fatih Ali
Shaimaa Fatih Ali
|
| Second Review by: |

 Aleksandar Racz
Aleksandar Racz
|
| Final Approval by: | Dr. Hemant Kumar |
Introduction
The Western Ghats are classified as one of the eight hottest hotspots globally and are famous for their unique habitats and forest types along with their endemic biological diversity. It has faced great anthropogenic pressure in recent years owing to deforestation and degradation1.From river Tapti in the north, to Kanyakumari in the south, these hill ranges run the length of India's west coast. Despite occupying an area of 180,000 km2 and making up 6% of India's total land area, the home to over 30% of the country's plant, herpeto-fauna, mammal, fish, and bird species. The southern Western Ghats cover an area of 7000 km², passing through the states of Tamil Nadu, Karnataka, Kerala and harbor an excellent endemic and endangered biodiversity. Most of the areas in these states are protected regions. The Western Ghats are home to a wide variety of systems, from tropical wet evergreen forests to grasslands with a tremendous variety of fauna and flora. They also comprise the distinctive shola forest ecosystem, which is made up of patches of evergreen forest scattered among montane grasslands. The Tropical Montane Forests or Shola forests are a peculiar kind of forest belonging to the Western Ghats, characterized by the presence of persistent cloud cover. The most distinguishing feature of the shola forests is “their ability to capture moisture by the condensation of cloud-borne moisture on the vegetation”2. The sholas are restricted to a meagre 0.14% of the Earth found between 30º N and 30º S latitudes2. The term ‘Shola’ is connected to the Tamil word ‘Cholai’, which is glorified in the ancient Tamil literature and culture, meaning ‘grove’.
Shola forests can be found at elevations ranging from above 1500 meters to as low as 1050 meters. These habitats, which are small pockets of forest, are mostly found in valleys or depressions because there they experience the least amount of fog and mist and are surrounded by grasslands. They are found in the high-elevation hilly regions of the Southern Western Ghats. The shola trees never grow on the mountain tops. They are generally slow growing and need more time to establish themselves and are sensitive to climatic conditions. Due to their low fire and frost tolerance, shola trees typically have stunted growth and cannot regrow in open spaces. The shola trees generally show stunted growth and due to their low fire and frost tolerance, they cannot regenerate in open areas3. Most of the trees are clothed with epiphytes. The shola forest soils are the best at holding water than any other soil. The monsoon rains are absorbed by these forests, and the water that has been retained is slowly released throughout the year in the form of little streams that combine to produce bigger streams and rivers. Most of South India's major rivers, including the Tunga, Bhadra, Nethravathi at Gangamoola (Kudremukh National Park), Cauvery at Talakaveri (Talakaveri Wildlife Sanctuary), Kumaradhara at Pushpagiri Wildlife Sanctuary and Lakshmanthirtha at Brahmagiri Wildlife Sanctuary, originate in Sholas.
Due to their unusual level of isolation and climatic conditions, the Shola forests of the southern Western Ghats are acknowledged for their high endemism and floral and faunal species richness4,5. The Shola enjoys a misty and cloudy climate throughout the year.A narrow ecotone that also contains many other species divides the sholas from the nearby grasslands3. Although these woods are situated in relatively inaccessible places, they continue to experience anthropogenic pressure, which results in habitat degradation, loss of biodiversity, and biomass6. Like any other tropical forest system, the large-scale conversion of the forests for agricultural purposes7 changes the land use pattern and leads to loss of biodiversity and invasion of alien species which make it significant for assessment and conservation. The shola grassland system harbors many fauna and flora of conservation status. The hydrological characteristics and biogeochemistry of these ecosystems are also critical. The human-induced climate change raises concern over the sustainability of these ecosystems and their endemic diversity. This signifies the need for the conservation and management of this forest area. Very few studies are available on the floristic and structural attributes of Western Ghats5,8,9,10,11,12,13,14,15. The range of shola forest ecosystems has been diminished in the last century mostly due to encroachment and utilization for plantation purposes or agricultural expansion9. Hence, it is important to carry out a floristic diversity study of shola ecosystems and their population structure as it could help in the conservation and management of shola forests of Western Ghats by understanding the species distribution patterns. As woody species are essential in determining the structural dynamics of a forest16 and provide an insight into conservation plans,17 the current study on the woody plant structure would enhance our knowledge on the stand structure diversity and species richness in the shola forest of Brahmagiri Wildlife Sanctuary, Karnataka, Western Ghats.
Materials and Methods
Study area
Brahmagiri Wildlife Sanctuary (latitude 11°55’ to 12° 19’ and longitude 75° 44’ to 76° 04’, part of the Western Ghats. It is named after the Brahmagiri Hill which is the sanctuary's highest peak. The sanctuary covers 181.29 km² in total. In 1974, Brahmagiri Wildlife Sanctuary was declared as a Protected Area.
The Sanctuary is unique in its ecosystem, with a rich diversity of animals and plants in a lush green and undulating landscape, having an elevation varying from 65 to 1600 meters above mean sea level. It acts as a lone corridor for the passage of animals between the Southern and Northern Western Ghats, particularly connecting the Rajiv Gandhi National Park, Bandipur National Park, Wayanad and Aralam sanctuary, Pushpagiri Wildlife Sanctuary, and other nearby parks. Numerous endangered and rare species, including the Lion-tailed macaque, Malabar civet, Nilgiri martin, Slender loris, and Clawless otter, call it home. The river Laxmanthirtha, a significant tributary of the river Cauvery, originates in the sanctuary, while the river Barpole obtains a river status as it passes through it. The landscape is primarily undulating, with a few steeply to extremely steeply sloping valleys and hillocks. The deep loamy soil varies in depth from place to place, while the underlying rock is gneiss in origin. Here, the cold, dry, and wet seasons are noteworthy. By the middle of February, the cold season ends and the hot season begins. Mid-November to mid-January is the coldest time of the year. The rainy season lasts from June to September, whereas the dry season lasts from March to May. The Southwest Monsoon is primarily responsible for the rains received in the sanctuary. It occasionally experiences rainfall brought by the northeast monsoon. The mean yearly rainfall ranges from 2500 mm to 6000 mm18.
Tropical wet evergreen forests, semi-evergreen forests, shola forests, moist deciduous forests and grasslands are the principal types of forests found in the sanctuary. The shola forest species are mostly evergreen and range in size from dwarf trees on the edges, which can endure strong winds blowing over the hillocks, to huge trees in the centre. At least one small, perennial watercourse serves as a mini-reservoir in each of these Sholas. These forests are crucial for the environment, and each year, they face the threat of getting shrunken due to the wildfires that occur in these high-altitude grasslands.
Methods
Twenty-five plots, of size 20 m × 20 m each, were randomly laid across the site to analyze the tree structure of the shola forests in the sanctuary. The individuals enumerated were identified and classified into three categories, which are saplings (<3 cm DBH), juveniles (DBH 3-<10cm) and adults (?10cm DBH). Data are processed and various diversity indices (Simpson index, Shannon Weiner Index, Fisher’s alpha index) were computed by using the Vegan package in R.
Results
Species richness
Sixty-five tree species from 47 genera and 35 families were documented from the tropical evergreen shola forests (1 ha) of Brahmagiri Wildlife Sanctuary, Karnataka, Western Ghats (Table 1). The Shannon-Wiener Index, Simpson and Fisher’s alpha diversity indices were 3.654, 0.960 and 15.471 respectively for the area studied. These values indicate that the area shows high species diversity. Out of the 65 species identified, 17 species were found endemic (26%) to the Western Ghats. Among those 17, four were endemic to Southern Western Ghats only. Cinnamomum malabatrum showed highest density among the endemic species, whereas Elaeocarpus munronii, Litsea floribunda and Ixora notoniana were the least. On the basis of the Hill numbers (q=0,1,2), the rarefaction and extrapolation curves are provided and are presented in figure 1 . The curves for species richness (q=0) to attain asymptote requires more number of individual enumerations whereas the curves for the Shannon and Simpson diversity (q=1 and 2) leveled off indicating that the sampling of the present study is adequate because even the extrapolation values do not exceed (asymptote occur within the sampling size).
Table 1: List of woody plant species with their quantitative characteristics of the shola forest of the Brahmagiri Wildlife Sanctuary, Karnataka, India.
| Species Name | Density (No./ ha) | Frequency | Basal area (m2/ha) | IVI |
| Actinodaphne bourdillonii Gamble | 20 | 6 | 0.27 | 4.21 |
| Actinodaphne tadulingamii Gamble | 18 | 6 | 0.25 | 3.99 |
| Ardisia elliptica Thunb. | 7 | 2 | 0.03 | 1.20 |
| Callicarpa tomentosa (L.) Murr. | 25 | 8 | 0.23 | 5.04 |
| Calophyllum austroindicum Kosterm. ex P.F.Stevens | 21 | 6 | 1.36 | 8.21 |
| Careya arborea Roxb. | 23 | 3 | 0.47 | 4.19 |
| Casearia rubescens Dalz. | 30 | 8 | 0.10 | 4.91 |
| Celtis philippensis wightii (Planch.) E. Soepadmo | 96 | 18 | 1.00 | 15.78 |
| Cinnamomum malabatrum (Burm. f.) Presl | 74 | 12 | 0.92 | 12.11 |
| Cinnamomum perrottetii Meisn. | 15 | 2 | 0.07 | 1.92 |
| Daphniphyllum neilgherrense (Wight) K.Rosenthal | 17 | 5 | 0.18 | 3.37 |
| Dillenia bracteata Wight | 49 | 9 | 3.02 | 17.02 |
| Dysoxylum binectariferum (Roxb.) Hook.f. ex Bedd. | 22 | 5 | 0.42 | 4.56 |
| Elaeocarpus munronii (Wl.) Masters | 1 | 1 | 0.03 | 0.49 |
| Elaeocarpus serratus L. | 84 | 15 | 3.24 | 22.06 |
| Euonymus crenulatus Wall. ex Wight & Arn. | 28 | 4 | 0.41 | 4.64 |
| Euonymus dichotomus Heyne ex Wall. | 5 | 2 | 0.07 | 1.21 |
| Euonymus indicus Heyne ex Roxb. | 8 | 5 | 0.11 | 2.49 |
| Fagraea ceylanica Thunb. | 4 | 1 | 0.04 | 0.72 |
| Ficus amplissima Sm. | 1 | 1 | 0.48 | 2.09 |
| Flacourtia montana J.Graham | 7 | 3 | 0.02 | 1.48 |
| Garcinia indica (Thouars) Choisy | 6 | 3 | 0.19 | 2.03 |
| Glochidion malabaricum (Müll.Arg.) Bedd. | 28 | 6 | 0.25 | 4.67 |
| Gordonia obtusa Wall. ex Wight & Arn. | 1 | 1 | 0.21 | 1.15 |
| Hydnocarpus alpina Wight | 26 | 5 | 0.45 | 4.94 |
| Hypericum mysurense Wight & Arn. | 2 | 1 | 0.00 | 0.46 |
| Ixora notoniana Wall. ex G.Don | 3 | 3 | 0.05 | 1.33 |
| Lepisanthes decifiens | 17 | 8 | 0.09 | 4.00 |
| Ligustrum perottetti var. obovatum | 24 | 4 | 0.21 | 3.64 |
| Ligustrum robustum (Roxb.) Blume | 75 | 16 | 3.02 | 20.97 |
| Litsea deccanensis Gamble | 3 | 1 | 0.06 | 0.73 |
| Litsea floribunda (Bl.) Gamble | 1 | 1 | 0.03 | 0.49 |
| Litsea glabrata (Wall. ex Nees) Hook. fil. | 10 | 3 | 0.08 | 1.91 |
| Litsea insignis (Blume) Boerl. | 9 | 3 | 0.10 | 1.92 |
| Litsea wightiana (Nees) Benth. & Hook. fil. | 6 | 3 | 0.36 | 2.66 |
| Macaranga peltata (Roxb.) Müll.Arg. | 33 | 10 | 1.07 | 9.23 |
| Mallotus philippensis (Lam.) Müll.Arg. | 4 | 2 | 0.08 | 1.19 |
| Melicope lunu-ankenda (Gaertn.) T.G. Hartley | 16 | 5 | 0.07 | 2.90 |
| Meliosma simplicifolia (Roxb.) Walp. ssp. simplicifolia | 9 | 1 | 0.47 | 2.61 |
| Memecylon malabaricum Cogn. | 12 | 5 | 0.28 | 3.40 |
| Memecylon sp. | 2 | 1 | 0.00 | 0.46 |
| Microtropis wallichiana Wight ex Thwaites | 12 | 5 | 0.11 | 2.79 |
| Murraya paniculata (L.) Jack | 13 | 3 | 0.11 | 2.22 |
| Neolitsea cassia (L.) Kosterm. | 32 | 6 | 0.50 | 5.88 |
| Neolitsea zeylanica (Nees & T. Nees) Merr. | 59 | 7 | 0.19 | 6.90 |
| Nothapodytes nimmoniana (J.Graham) Mabb. | 21 | 8 | 1.37 | 8.84 |
| Phoenix humilis (L.) Cav. | 6 | 2 | 0.05 | 1.20 |
| Phyllanthus emblica L. | 3 | 1 | 0.00 | 0.52 |
| Psychotria dalzellii Hook.f. | 32 | 5 | 0.08 | 4.04 |
| Psydrax dicoccos Gaertn. | 44 | 8 | 0.25 | 6.42 |
| Schefflera wallichiana (Wight & Arn.) Harms | 2 | 1 | 0.01 | 0.47 |
| Sterculia guttata Roxb. | 1 | 1 | 0.08 | 0.66 |
| Symplocos cochinchinensis (Lour.) S.Moore | 183 | 14 | 0.97 | 20.39 |
| Symplocos macrocarpa macrocarpa | 9 | 4 | 0.05 | 2.04 |
| Symplocos macrophylla rosea (Beddome) Nooteboom | 33 | 9 | 2.05 | 12.44 |
| Symplocos monantha Wight | 27 | 10 | 0.22 | 5.76 |
| Symplocos sp. | 19 | 5 | 0.11 | 3.24 |
| Syzygium montanum Gamble | 5 | 3 | 0.44 | 2.85 |
| Syzygium munronii (Wt.) Chandrab. | 6 | 3 | 0.02 | 1.40 |
| Toona ciliata M. Roem. | 6 | 4 | 0.23 | 2.47 |
| Tricalysia apiocarpa (Dalzell ex Hook.f.) Gamble | 5 | 3 | 0.09 | 1.60 |
| Tricalysia sphaerocarpa (Dalzell ex Hook.f.) Gamble | 3 | 3 | 0.07 | 1.38 |
| Vernonia arborea Buch.-Ham. | 20 | 4 | 0.56 | 4.64 |
| Viburnum cylindricum Buch. | 2 | 2 | 0.00 | 0.77 |
| Wendlandia thyrsoidea (Roth) Steud. | 73 | 6 | 0.51 | 8.70 |
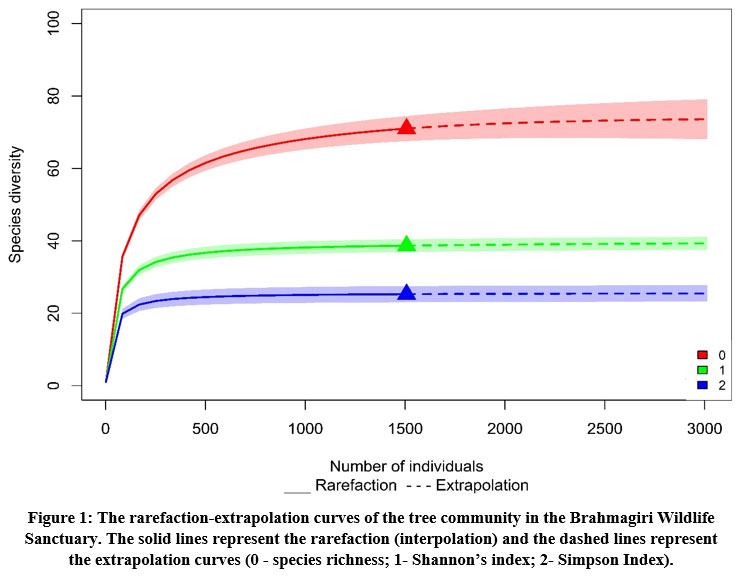 | Figure 1: The rarefaction-extrapolation curves of the tree community in the Brahmagiri Wildlife Sanctuary. The solid lines represent the rarefaction (interpolation) and the dashed lines represent the extrapolation curves (0 - species richness; 1- Shannon’s index; 2- Simpson Index).
|
Tree density, composition and occurrence
The present study in the shola forest covering 1 hectare (25 plots of 0.04 ha) yielded a total of 1507 stems. The density of the study site showed an extensive variation among the species ranging from 1 (5 species (Elaeocarpus munronii, Ficus amplissima, Gordonia obtusa, Litsea floribunda and Sterculia guttata)) to 183 stems for Symplocos cochinchinensis. Symplocos cochinchinensis is the most dominant in terms of density, followed by Celtis philippensis, Elaeocarpus serratus, Ligustrum robustum and Cinnamomum malabatrum. However, the importance value index was highest for Elaeocarpus serratus (20.36) followed by Ligustrum robustum (19.37) and Symplocos cochinchinensis (19.36). Twelve species were considered as rare (? 2 individuals in total 25 plots) for the present study. The basal area of tree species enumerated from the site was 31.19 m2 ha-1. In terms of basal area, Elaeocarpus serratus was the most dominant species followed by Dillenia bracteata and Ligustrum robustum.
Distribution pattern
The abundance to frequency analysis indicated that all the recorded species in the area showed contagious distribution patterns. Not one species was common among the 25 study plots. Celtis philippensis was distributed in more sampling units (18 study plots) followed by Ligustrum robustum (16 plots), Elaeocarpus serratus (15 plots), Symplocos cochinchinensis (14 plots), and Cinnamomum malabatrum (12 plots). However, 13 species were present in only one plot and 8 species occurred only in two plots.
Family composition
Out of 35 families, 23 families were represented by singletons and taxonomically, the most speciose family is Lauraceae with a total of 11 species, followed by Rubiaceae (6 species), Symplocaceae (5 species) and Celastraceae (4 species; Table 2). Symplocaceae was the most dominant family with regard to abundance followed by Cannabaceae, Elaeocarpaceae, Oleaceae and Lauraceae, while in terms of basal area, Elaeocarpaceae was dominated.
Table 2: Family contribution in the shola forest ecosystems at Brahmagiri Wildlife Sanctuary, Karnataka, India.
| Family | Genus | Species |
| Achariaceae | 1 | 1 |
| Araliaceae | 1 | 1 |
| Arecaceae | 1 | 1 |
| Asteraceae | 1 | 1 |
| Calophyllaceae | 1 | 1 |
| Cannabaceae | 1 | 1 |
| Celastraceae | 2 | 4 |
| Clusiaceae | 1 | 1 |
| Daphniphyllaceae | 1 | 1 |
| Dilleniaceae | 1 | 1 |
| Elaeocarpaceae | 1 | 2 |
| Euphorbiaceae | 2 | 2 |
| Flacourtiaceae | 1 | 1 |
| Gentianaceae | 1 | 1 |
| Hypericaceae | 1 | 1 |
| Icacinaceae | 1 | 1 |
| Lamiaceae | 1 | 1 |
| Lauraceae | 4 | 11 |
| Lecythidaceae | 1 | 1 |
| Malvaceae | 1 | 1 |
| Melastomataceae | 1 | 2 |
| Meliaceae | 2 | 2 |
| Moraceae | 1 | 1 |
| Myrtaceae | 1 | 2 |
| Oleaceae | 1 | 2 |
| Phyllanthaceae | 2 | 2 |
| Primulaceae | 1 | 1 |
| Rubiaceae | 5 | 6 |
| Rutaceae | 2 | 2 |
| Sabiaceae | 1 | 1 |
| Salicaceae | 1 | 1 |
| Sapindaceae | 1 | 1 |
| Symplocaceae | 1 | 5 |
| Theaceae? | 1 | 1 |
| Viburnaceae | 1 | 1 |
Stand structure
The diameter class-wise distribution of trees exposed that 67% of the forest stand has the lower diameter class (0 cm -10 cm DBH) individuals and with an increase in the diameter class, the number of individuals declined (Fig 2). The stand structure of the tree population revealed that the juveniles (637) were comparatively higher in number than adults (500) and saplings (370). In addition, diameter class-wise analysis indicated that the highest level of species richness was exhibited by the adult tree population. The diameter class-wise distribution of the top five abundant species is presented in the figure 3. Among the top five species, Symplocos cochinchinensis and Elaeocarpus serratus have more juvenile population than that of saplings and adults whereas the adult population was highest in Cinnamomum malabatrum and Ligustrum robustum. Symplocos cochinchinensis positioned in first among the top five, whose adult population diameter did not exceed the 20 cm DBH. In contrast, among the 5 top species, Elaeocarpus serratus and Ligustrum robustum species alone represented the large diameter class (?60 cm DBH) individuals. Among the adult population of all the top species, abundance decreases with an increase in the diameter class.
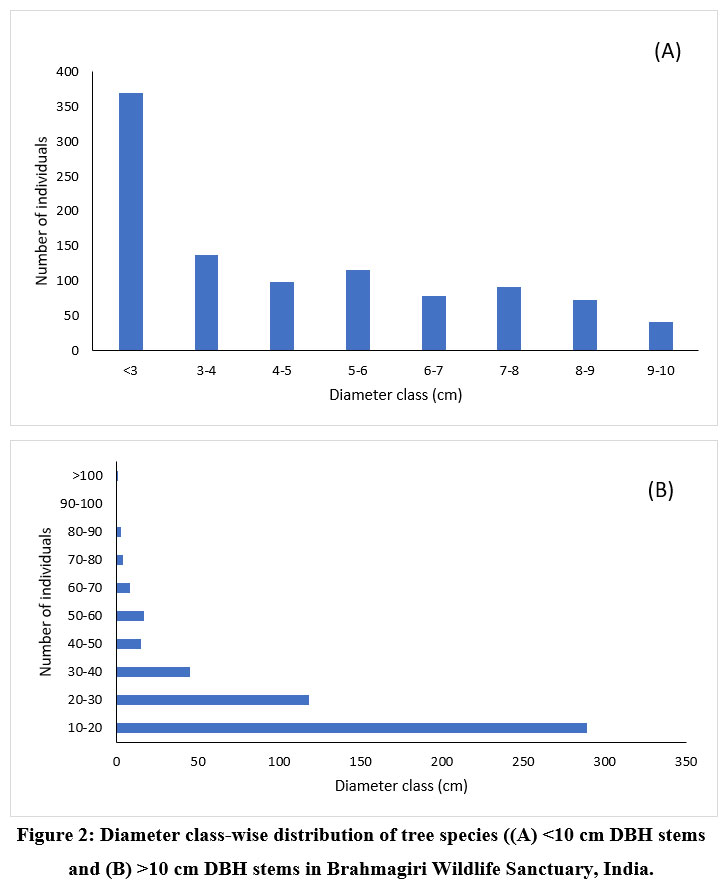 | Figure 2: Diameter class-wise distribution of tree species ((A) <10 cm DBH stems and (B) >10 cm DBH stems in Brahmagiri Wildlife Sanctuary, India.
|
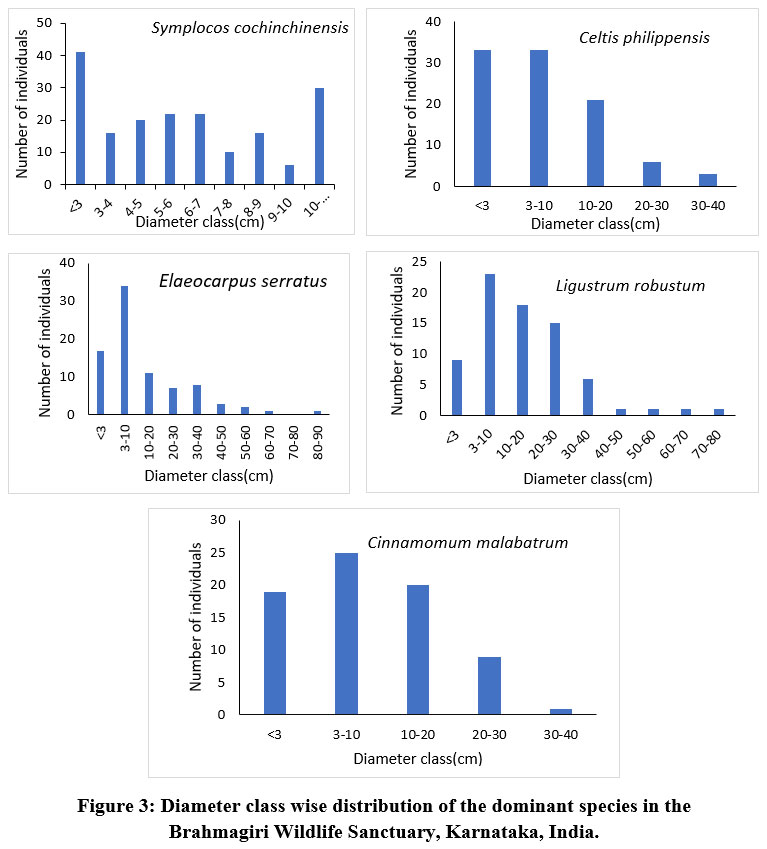 | Figure 3: Diameter class wise distribution of the dominant species in the Brahmagiri Wildlife Sanctuary, Karnataka, India.
|
Regeneration status of tree species
Among the identified species, 41 species showed all three stages of development, i.e., sapling stage, juveniles and adults (Table 3). In total, 500 adult (?10 cm DBH) stems were enumerated from the present study followed by 637 juveniles (?3 cm - <10 cm DBH) and 370 saplings (<3 cm DBH). Five species have no adult population (Hypericum mysurense, Memecylon sp., Phyllanthus emblica, Schefflera wallichiana and Viburnum cylindricum) whereas their sapling and juvenile population was found. On contrary, for nine species, the adult population was only obtained without juveniles or saplings which indicates that those species have poor regeneration potential in the study area. Only one species (Phyllanthus emblica) had only saplings recorded without any juvenile or adult population. However, the Phyllanthus emblica was documented from the plot which is located towards the periphery region of the study area. Two species had no juvenile population but the sapling stage and adults were there.
In the case of 17 endemic species, 10 species showed all three stages of development (adults, juveniles and saplings) while six species have no sapling population which indicates its poor regeneration potential in the study area. However, Elaeocarpus munronii and Litsea floribunda had an adult population alone in the study area which had no sapling and juvenile populations.
Table 3: Diameter classwise distribution of the woody plant species community in the Brahmagiri wildlife Sanctuary, Karnataka, India.
| Species Name | Saplings | Juveniles | Adults |
| Actinodaphne bourdillonii Gamble | 6 | 7 | 7 |
| Actinodaphne tadulingamii Gamble | 9 | 6 | 3 |
| Ardisia elliptica Thunb. | 4 | 2 | 1 |
| Callicarpa tomentosa (L.) Murr. | 7 | 13 | 5 |
| Calophyllum austroindicum Kosterm. ex P.F.Stevens | 1 | 7 | 13 |
| Careya arborea Roxb. | 4 | 10 | 9 |
| Casearia rubescens Dalz. | 12 | 14 | 4 |
| Celtis philippensis wightii (Planch.) E. Soepadmo | 33 | 33 | 30 |
| Cinnamomum malabatrum (Burm. f.) Presl | 19 | 25 | 30 |
| Cinnamomum perrottetii Meisn. | - | 14 | 1 |
| Daphniphyllum neilgherrense (Wight) K.Rosenthal | 6 | 6 | 5 |
| Dillenia bracteata Wight | 13 | 11 | 25 |
| Dysoxylum binectariferum (Roxb.) Hook.f. ex Bedd. | 5 | 10 | 7 |
| Elaeocarpus munronii (Wl.) Masters | - | - | 1 |
| Elaeocarpus serratus L. | 17 | 34 | 33 |
| Euonymus crenulatus Wall. ex Wight & Arn. | 4 | 15 | 9 |
| Euonymus dichotomus Heyne ex Wall. | 1 | 1 | 3 |
| Euonymus indicus Heyne ex Roxb. | 1 | 2 | 5 |
| Fagraea ceylanica Thunb. | - | 3 | 1 |
| Ficus amplissima Sm. | - | - | 1 |
| Flacourtia montana J.Graham | 6 | - | 1 |
| Garcinia indica (Thouars) Choisy | - | 1 | 5 |
| Glochidion malabaricum (Müll.Arg.) Bedd. | 11 | 10 | 7 |
| Gordonia obtusa Wall. ex Wight & Arn. | - | - | 1 |
| Hydnocarpus alpina Wight | 11 | 3 | 12 |
| Hypericum mysurense Wight & Arn. | - | 2 | - |
| Ixora notoniana Wall. ex G.Don | - | 1 | 2 |
| Lepisanthes decifiens | 1 | 12 | 4 |
| Ligustrum perottetti var. obovatum | 14 | 3 | 7 |
| Ligustrum robustum (Roxb.) Blume | 9 | 23 | 43 |
| Litsea deccanensis Gamble | - | 1 | 2 |
| Litsea floribunda (Bl.) Gamble | - | - | 1 |
| Litsea glabrata (Wall. ex Nees) Hook. fil. | - | 6 | 4 |
| Litsea insignis (Blume) Boerl. | 2 | 2 | 5 |
| Litsea wightiana (Nees) Benth. & Hook. fil. | - | 4 | 2 |
| Macaranga peltata (Roxb.) Müll.Arg. | 10 | 3 | 20 |
| Mallotus philippensis (Lam.) Müll.Arg. | - | - | 4 |
| Melicope lunu-ankenda (Gaertn.) T.G. Hartley | 1 | 13 | 2 |
| Meliosma simplicifolia (Roxb.) Walp. ssp. simplicifolia | 3 | 1 | 5 |
| Memecylon malabaricum Cogn. | 2 | 6 | 4 |
| Memecylon sp. | 1 | 1 | - |
| Microtropis wallichiana Wight ex Thwaites | 3 | 6 | 3 |
| Murraya paniculata (L.) Jack | 1 | 8 | 4 |
| Neolitsea cassia (L.) Kosterm. | 10 | 10 | 12 |
| Neolitsea zeylanica (Nees & T. Nees) Merr. | 35 | 18 | 6 |
| Nothapodytes nimmoniana (J.Graham) Mabb. | 3 | 8 | 10 |
| Phoenix humilis (L.) Cav. | - | 3 | 3 |
| Phyllanthus emblica L. | 3 | - | - |
| Psychotria dalzellii Hook.f. | 19 | 11 | 2 |
| Psydrax dicoccos Gaertn. | 1 | 39 | 4 |
| Schefflera wallichiana (Wight & Arn.) Harms | - | 2 | - |
| Sterculia guttata Roxb. | - | - | 1 |
| Symplocos cochinchinensis (Lour.) S.Moore | 41 | 112 | 30 |
| Symplocos macrocarpa macrocarpa | 1 | 5 | 3 |
| Symplocos macrophylla rosea (Beddome) Nooteboom | - | 5 | 28 |
| Symplocos monantha Wight | 5 | 17 | 5 |
| Symplocos sp. | 3 | 14 | 2 |
| Syzygium montanum Gamble | - | - | 5 |
| Syzygium munronii (Wt.) Chandrab. | 3 | 2 | 1 |
| Toona ciliata M. Roem. | 1 | 2 | 3 |
| Tricalysia apiocarpa (Dalzell ex Hook.f.) Gamble | - | 3 | 2 |
| Tricalysia sphaerocarpa (Dalzell ex Hook.f.) Gamble | - | 1 | 2 |
| Vernonia arborea Buch.-Ham. | 4 | 8 | 8 |
| Viburnum cylindricum Buch. | 1 | 1 | - |
| Wendlandia thyrsoidea (Roth) Steud. | 18 | 47 | 8 |
Discussion
The present woody plant diversity study in the tropical evergreen shola forest patches of Brahmagiri Wildlife Sanctuary, Karnataka was carried out to understand the structure and floristic composition of that forest. These forests belong to the category of montane evergreen shola forests. The study has recorded a total of 65 tree species (?1cm DBH) from 1 ha, showing greater species richness compared to 61 species in 63.17 ha15 and 54 species in 11.34 ha10. Similarly, the values obtained from the study are comparatively greater than that of those studies done in 1 ha areas of the upper montane ecosystem in Southern Brazil19 (26 species ha-1), secondary tropical evergreen forest in Cachar district of Assam20 (52 species ha-1), tropical wet evergreen forest in Nelliampathy hills, Kerala21 (30 species ha-1) and tropical wet evergreen forest in Karnataka22 (28 -38 species ha-1). The values (63 species ha-1) obtained from the Thaishola of Nilgiri mountains23 (2000 - 2200 masl) are close to the diversity values obtained from the present study. At the same time, the value is lower than that of the species diversity recorded from the montane shola forests of Kukkal, Palni hills9 (83 species ha-1) and Nilgiri Mountains, southern India14 (97 species ha-1). The variations in species diversity can be altered due to the differences in elevation24 climatic, edaphic characteristics, rainfall patterns25 and other environmental conditions26. Human interference and cattle grazing is almost nil in the present study site. The sanctuary area is well preserved, undisturbed and no encroachment reported in the vicinity of the sanctuary even though the area around is mostly utilized for coffee plantations. These could be the reasons for greater species richness in this area.
The adult tree density (?10 cm DBH) in the current study is lower compared to the values recorded from Southeast Belize27 (937 individuals ha-1), Southern Brazil19 (1208 individuals ha-1)Brazilian Amazon28 (1482 individuals ha-1), Bolivian Amazon29 (649 individuals ha-1), Ecuadorian Amazon30 (693 individuals ha-1), Mare, Central Amazonia31 (645 individuals ha-1), Brazilian Amazon32 (618-654 individuals ha-1), East Usambara Mountains, Tanzania33 (880 individuals ha-1), Borneo34 (602 individuals ha-1), Cachar district, Assam20 (2152 individuals ha-1) Nagaland35 (613 individuals ha-1) and Nilgiri Mountains, southern India14 (1224 individuals ha-1). At the same time, the values are comparable with Monteverde Cloud Forest Reserve, Costa Rica36 (555 individuals ha-1), Venezuela37 (355-563 individuals ha-1); Central Africa38 (425 individuals ha-1) and Kukkal Forest, Palni hills9 (451 individuals ha-1). However, the current study showed greater density values when compared with Namdapha Tiger Reserve, Arunachal Pradesh39 (328 individuals ha-1). These variations in stem density might be attributed to elevation, composition of species and micro climatic conditions40.
The basal area of a species indicates how dominant it is in a stand41.The current study showed a basal area of 31.19 m2 ha-1 which is much closer to the pantropical average42 (32 m2 ha-1). The basal area values from the Nilgiri Mountains, southern India14 (53.33 m2 ha-1), Kukkal, Palni hills9 (62 m2 ha-1), Kalakad, Western Ghats43 (55 -94 m2 ha-1), Nelliampathy hills, Kerala21 (61.9 m2 ha-1), Hollongapar Gibbon wildlife sanctuary, Assam44 (58 m2 ha-1) and Southern Brazil, South America17 (47.3 m2 ha-1) are all higher than the values obtained from the present study, as here the adult population (?10 cm DBH) was lower than the juveniles which could be the reason for the lower basal area. Whereas, our values are comparable with several reports (31 m2 ha-1) 31; (30.03 m2 ha-1) 45; (29.6 m2 ha-1) 38; (29 - 42 m2 ha-1)22. However, some studies28, 29, 19 & 46 reported lower values than the present study. Wide variations in the basal area across different regions would be attributed to structural variations in the forest. Only 16 large diameter (?60 cm DBH) individuals were present which belongs to 8 species (Ficus amplissima Sm., Dillenia bracteata, Ligustrum robustum, Symplocos macrophylla rosea, Elaeocarpus serratus L., Litsea wightiana, Calophyllum austroindicum, Nothapodytes nimmoniana). Sholas would have a larger proportion of adult trees with high basal area but the present study area have fewer large diameter trees recorded, at the same time, a large number (1007) of small-diameter (?10 cm DBH) trees are present in the study site. This variation in the abundance of different diameter classes could be the reason for the lower basal area compared to other shola forests.
The study resulted in a total density of 1507 individuals (?1 cm DBH) in 1 ha area of which the lower diameter class (?10cm) individuals are having higher proportion (67% (1007 individuals)) than that of the adult class (?10 cm DBH) (500 individuals). Similarly, it is observed more juvenile population than adult population and stated that the presence of more lower diameter class individuals than the high diameter class can be perceived as an indication of a regenerating forest47. In the study, 41 out of 65 species (63%) showed all three growth phases (saplings, juveniles and adults). The difference in the population structure can be due to microclimatic variations, topographic and edaphic peculiarities48 etc. Along with that, dominant adult trees species have also exhibited an adequate sapling and juvenile population too, thereby indicating that those species have a good regeneration potential49. Symplocacae family shows dominance in the juvenile population as they have exhibited a high regeneration potential in the present study area. The species that showed profuse regeneration (?10 cm DBH) in the study site were Symplocos cochinchinensis, Celtis philippensis, Neolitsea zeylanica, Wendlandia thyrsoidea, Elaeocarpus serratus and Cinnamomum malabatrum. However, 8 species occur as adult stage only with no sapling or juvenile stage of development, of which 2 species (Elaeocarpus munronii and Litsea floribunda) are endemic to Western Ghats. These results indicate that attention is required in order to sustain the population of those species for the future. Understanding the regeneration potential of each species is needed for proper forest management and species conservation measures50. In particular, studies are required to recognize the reasons for the poor regeneration potential of those endemic species in the study area for conservation in the future. The poor regeneration capacity of those species can be attributed to their inability to tolerate extreme weather conditions, less seed supply, germination potential, light inavailability, soil nutrients and other properties and the landscape nature40. In several species, the younger stage population was way higher than the adults, which is likely that the species composition and structure of these forests can be altered over time. Out of 18000 species of flowering plants documented from India, 30% belong to the Western Ghats51,52. The total number of endemic plant species in the Western Ghats was estimated as 16001 and 56% of the evergreen tree species are endemic. There were 17 species in this study that are endemic to the Western Ghats which identifies 26% of the total species (65) collected. The endemism level of the current study is comparable to the study of Davidar et al., 2007(30%). Among the identified species, three species were identified as those which grow in disturbed evergreen forests (Callicarpa tomentosa, Ficus amplissima, Garcinia indica) though there weren’t any signs of disturbance noted from the sites of the sample collection, and these three species show poor regeneration potential as well (only Callicarpa tomentosa displays all three stages of development). Eleven species have been identified as those that are found along the margins of the hills and forests.
Conclusion
The present study exposes that the vegetation structure of the Brahmagiri wildlife sanctuary retains tree diversity comparable to the pantropical average. All the dominant species in this study site have better regeneration potential. However, few species including two endemic species have poor regeneration status. This enlighten that thorough understanding of the regeneration potential of those species is warranted in order to carry out proper management plans and conservation.
Acknowledgement
The authors are thankful to the Forest Department of Karnataka state for giving permission to carry out this study and also the Madikkeri Division and Brahmagiri Wildlife Sanctuary officials for their support and help throughout the sampling period. We are grateful to Prof. N. Parthasarathy, Department of Ecology and Environmental Sciences, Pondicherry University for his help in the species identification. Also, we acknowledge the University Grants Commission (UGC), Government of India for their financial support for the study.
Conflict of interest
There is no conflict of interest.
References
- Jha C. S., Dutt, C. B. S. and Bawa K. S. Deforestation and land use changes in Western Ghats, India. Current Science 2000; 79:231–238. https://www.jstor.org/stable/24103455
- Lawrence S. Hamilton, James O. Juvik, Scatena F. N. Tropical Montane Cloud Forests; Volume 110 of Ecological Studies. Springer Science and Business Media 1995; 14-110. https://link.springer.com/book/10.1007/978-1-4612-2500-3
CrossRef - Meher-Homji V.M. Phytogeography of the South Indian Hill Stations. Bulletin of the Torrey Botanical Club 1967; 94(4)230-242. https://www.jstor.org/stable/2483901
CrossRef - Blasco F. Aspects of the flora and ecology of savannas of the south Indian Hills. Journal of Bombay Natural History Society 1970; 67:522-534.https://www.cabdirect.org/cabdirect/abstract/19729702091
- Jose S., Sreepathy A., Kumar B.M. and Venugopal V.K. Structural, floristic and edaphic attributes of the grassland-shola forests of Eravikulam in peninsular India. Forest Ecology and Management 1994; 65(2-3):279-91.https://www.sciencedirect.com/science/article/pii/0378112794901767
CrossRef - Sasmitha R., Muhammad Iqshanullah A. and Arunachalam R. Ecosystem Changes in Shola Forest-Grassland Mosaic of the Nilgiri Biosphere Reserve (NBR). Chapter 3, Environmental Issues and Sustainable Development. London, United Kingdom: Intech Open Limited 2021:29-36. https://www.intechopen.com/chapters/74357
CrossRef - Sathish B. N., Bhavya C. K., Kushalappa C. G., Nanaya K. M., Dhanush C., Devagiri G. M., and Gajendra C. V. Dynamics of native tree structure and diversity in coffee agroforest: a case study from Central Western Ghats. Agroforestry Systems 2022 96(1); 161-172. https://link.springer.com/article/10.1007/s10457-021-00713-8
CrossRef - Chittibabu C.V. and Parthasarathy N. Attenuated tree species diversity in human-impacted tropical evergreen forest sites at Kolli hills, Eastern Ghats, India. Biodiversity and Conservation 2000; 9:1493-1519.https://link.springer.com/article/10.1023/A:1008971015545
- Davidar P., Mohandass D. and Vijayan L. Floristic inventory of woody plants in a tropical montane (shola) forest in the Palni hills of the Western Ghats, India. Tropical Ecology 2007; 48:15-25. https://www.academia.edu/download/31544992/Davidar_Dass_n_Vijayan.pdf
- Mohandass D. and Davidar P. Floristic structure and diversity of a tropical montane evergreen forest (shola) of the Nilgiri Mountains, southern India. Tropical Ecology 2009; 50:219-229. https://www.academia.edu/download/33146345/Floristic_stracture.pdf
- Mohandass D. and Davidar P. The relationship between area and vegetation structure and diversity in montane forest (shola) patches in southern India. Plant Ecology and Diversity 2010; 3:67-76. https://www.tandfonline.com/doi/abs/10.1080/17550874.2010.492843
CrossRef - Sellamuthu S. and Lalitha V. Plant diversity and phenological pattern in the montane wet temperate forests of the southern Western Ghats, India. Forestry Studies in China 2010; 12(3):116–125. https://link.springer.com/article/10.1007/s11632-010-0302-0
CrossRef - Thomas B., Chandrashekara U.M. and Rajendran A. Floristic diversity along an altitudinal gradient of Mannavan Shola forest in Southern Western Ghats of Kerala. Journal of Research in Biology 2011; 1(2):101-109. http://www.jresearchbiology.com/documents/RA0025.pdf
- Mohandass D., Hughes A. C., Mackay B., Davidar P., and Chhabra T. Floristic species composition and structure of a mid-elevation tropical montane evergreen forests (sholas) of the western ghats, southern India. Tropical Ecology 2016; 57(3): 533-543. : https://www.researchgate.net/publication/294806311
- Mohandass D., Campbell M. J. and Davidar P. Impact of patch size on woody tree species richness and abundance in a tropical montane evergreen forest patches of south India. Journal of Forestry Research 2018; 29(6):1675–1687. https://link.springer.com/article/10.1007/s11676-018-0592-y
CrossRef - Tadwalkar M., Joglekar A., Mhaskar M., and Patwardhan A. Woody species diversity from proposed ecologically sensitive area of northern Western Ghats: implications for biodiversity management. Journal of Threatened Taxa 2020; 12(9), 16048-16063. https://www.threatenedtaxa.org/JoTT/article/view/5524
CrossRef - Ao A., Changkija S. and Tripathi S. K. Species diversity, population structure, and regeneration status of trees in Fakim Wildlife Sanctuary, Nagaland, Northeast India. Biodiversitas 2020; 21(6). https://doi.org/10.13057/biodiv/d210654
CrossRef - Management Plan of Brahmagiri Wildlife Sanctuary. Management Plans, Madikkeri Wildlife Division. Karnataka Forest Department- www.aranya.gov.in. 2011-12 to 2015-16;
- Suhs R. B., Hoeltgebaum M. P., Nuernberg-Silva A., Fiaschi P., Neckel-Oliveira S. and Peroni N. Species diversity, community structure and ecological traits of trees in an upper montane forest, Southern Brazil. Acta Botanica Brasilica 2019; 33(1):153–162. https://doi.org/10.1590/0102-33062018abb0250
CrossRef - Borogayary B., Das A.K. and Nath A.J. Tree species composition and population structure of a secondary tropical evergreen forest in Cachar district, Assam. Journal of Environmental Biology 2018; 39(1):67-71. 10.22438/jeb/39/1/MRN-487
CrossRef - Chandrashekara U.M. and Ramakrishnan P.S. Vegetation and gap dynamics of a tropical wet evergreen forest in the Western Ghats of Kerala, India. Journal of Tropical Ecology 1994; 10:337–354. https://doi.org/10.1017/S0266467400008014
CrossRef - Swamy S. L., Dutt C. B. S., Murthy M. S., Mishra A. and Bargali S. S. Floristics and dry matter dynamics of tropical wet evergreen forests of Western Ghats, India. Current Science 2010; 99(3): 353-364. https://www.researchgate.net/publication/276417853
- Narendran K., Indu K. Murthy, Suresh H. S., Dattaraja H. S., Ravindranath N. H. and Sukumar R. Nontimber Forest Product Extraction, Utilization and Valuation: A Case Study From The Nilgiri Biosphere Reserve, Southern India. Economic Botany 2001; 55(4):528-538. https://link.springer.com/article/10.1007/BF02871715
CrossRef - Trigas P., Panitsa M. and Tsiftsis S. Elevational gradient of vascular plant species richness and endemism in crete - the effect of post-isolation mountain uplift on a continental island system. PLoS ONE 2013; 8(3):1-13. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0059425
CrossRef - Reddy C. S., Babar S., Amamath G. and Pattanaik C. Structure and floristic composition of tree stand in tropical forest in the Eastern Ghats of northern Andhra Pradesh, India. Journal of Forestry Research 2011; 22:491-500.https://link.springer.com/article/10.1007/s11676-011-0193-5
CrossRef - Tielborgeri K., Bilton M. C., Metz J., Kigel J., Holzapfel C., Lebrija-Trejos E., Konsens I., Parag H. A. and Stemberg M. Middle-Eastern plant communities tolerate 9 years of drought in a multisite climate manipulation experiment. Nature Communications 2014;5102. https://www.nature.com/articles/ncomms6102
CrossRef - Luna-Kamyshev N. M., Lopez-Martinez J. O., Vargas-Larreta B., Islebe G. A., Villalobos-Guerrero T. F., de la Rosa A. V., Reyes-Mendoza O. F. and Trevino-Garza E. Floristic Composition, Diversity, and Biomass of a Protected Tropical Evergreen Forest Belize. Tropical Conservation Science 2020; 13:1-13. https://journals.sagepub.com/doi/pdf/10.1177/1940082920915433
CrossRef - Pires J.M., Dobzhansky T. and Black G.A. An estimate of the number of species of trees in an Amazonian forest community. Botanical Gazette 1953;114(4): 467-477. https://www.journals.uchicago.edu/doi/abs/10.1086/335790
CrossRef - Boom B. M. A Forest Inventory in Amazonian Bolivia. Biotropica 1986; 18(4):287-294. https://www.jstor.org/stable/2388571
CrossRef - Valencia R., Balslev H. and Mino G.C.P.Y. High tree alpha-diversity in Amazonian Ecuador. Biodiversity and Conservation 1994; 3:21–28. https://link.springer.com/article/10.1007/BF00115330
CrossRef - Milliken W. Structure and composition of one hectare of central Amazonian terra firme forest. Biotropica 1998; 30(4):530–537. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1744-7429.1998.tb00093.x
CrossRef - de Oliveira A. A.and Mori S. A. A central Amazonian terra firme forest. I. High tree species richness on poor soils. Biodiversity and Conservation 1999; 8:1219–1244. https://link.springer.com/article/10.1023/A:1008908615271
CrossRef - Huang W., Pohjonen V., Johansson S., Nashanda M., Katigula M.I.L. and Luukkanen O. Species diversity, forest structure and species composition in Tanzanian tropical forests. Forest Ecology and Management 2003;173(1-3):11-24. https://www.sciencedirect.com/science/article/pii/S0378112701008209
CrossRef - Slik J. W. F., Shin-Ichiro Aiba, Francis Q. Brearley, Chuck H. Cannon, Olle Forshed, Kanehiro Kitayama, Hidetoshi Nagamasu, Reuben Nilus, John Payne, Gary Paoli, Axel D. Poulsen, Niels Raes, Douglas Sheil, Kade Sidiyasa, Eizi Suzuki, Johan L. C. H. and van Valkenburg. Environmental correlates of tree biomass, basal area, wood specific gravity and stem density gradients in Borneo’s tropical forests. Global Ecology and Biogeography 2010; 19(1):50–60. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1466-8238.2009.00489.x
CrossRef - Mishra G. and Das P. K. Comparative Study on Tree Diversity and Population Structure in Two Forest Types of Nagaland, India. Proceedings of the National Academy of Sciences India Section B - Biological Sciences 2018; 89(4):1305–1310. https://link.springer.com/article/10.1007/s40011-018-1051-4
CrossRef - Nadkarni N.M., Matelson T.J. and Haber W.A. Structural characteristics and floristic composition of a neotropical cloud forest, Monteverde, Costa Rica. Journal of Tropical Ecology 1995; 11:481–495. https://doi.org/10.1017/S0266467400009020
CrossRef - Zent E. L. and Zent S. Floristic composition, structure, and diversity of four forest plots in the Sierra Maigualida, Venezuelan Guayana. Biodiversity and Conservation 2004; 13: 2453–2484. https://link.springer.com/article/10.1023/B:BIOC.0000048447.40238.f2
CrossRef - Lewis S.L., Sonke B., Sunderland T., Begne S.K., Lopez-Gonzalez G., van der Heijden G.M.F., Phillips O.L., Affum-Baffoe K., Baker T.R., Banin L., Bastin J.F., Beeckman H., Boeckx P., Bogaert J., De Canniere C., Chezeaux E., Clark C.J., Collins M., Djagbletey G., Djuikouo M.N.K., Droissart V., Doucet J.L., Ewango C.E.N., Fauset S., Feldpausch T.R., Foli E.G., Gillet J.F., Hamilton A.C., Harris D.J., Hart T.B., de Haulleville T., Hladik A., Hufkens K., Huygens D., Jeanmart P., Jeffery K.J., Kearsley E., Leal M.E., Lloyd J., Lovett J.C., Makana J.R., Malhi Y., Marshall A.R., Ojo L., Peh K.S.H., Pickavance G., Poulsen J.R., Reitsma J.M., Sheil D, Simo M., Steppe K., Taedoumg H.E., Talbot J., Taplin J.R.D., Taylor D., Thomas S.C., Toirambe B., Verbeeck H., Vleminckx J., White L.J.T., Willcock S., Woell H. and Zemagho L. Aboveground biomass and structure of 260 African tropical forests. Philosophical Transactions of the Royal Society B Biological Sciences 2013; 368(1625):1-14. https://royalsocietypublishing.org/doi/abs/10.1098/rstb.2012.0295
CrossRef - Proctor J., Haridasan K. and Smith G. W. How far north does Lowland Evergreen Tropical Rain Forest go? Global Ecology and Biogeography Letters 1987; 7(2):141–146. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1466-8238.1998.00270.x
CrossRef - Subashree K., Dar J. A., Karuppusamy S. and Sundarapandian S. Plant diversity, structure and regeneration potential in tropical forests of Western Ghats, India. Acta Ecologica Sinica 2021; 41(4):259–284. https://www.sciencedirect.com/science/article/pii/S1872203219301611
CrossRef - Vignesh A., Sivalingam R., and Vasanth K. Diversity of Woody Flora and Physico-chemical Attributes of Soil in the Nilgiri Hills, Tamil Nadu, India. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 2022; 92(1), 95-103. https://link.springer.com/article/10.1007/s40011-021-01279-2
CrossRef - Dawkins H.C. The volume increment of natural tropical high-forest and limitations on its improvements. Empire Forestry Review 1959; 38:175–180.https://www.jstor.org/stable/42600614
- Parthasarathy N. Tree diversity and distribution in undisturbed and human-impacted sites of tropical wet evergreen forest in southern Western Ghats, India. Biodiversity and Conservation 1999; 8:1365–1381.https://link.springer.com/article/10.1023/A:1008949407385
- Sarkar M. and Devi A. Assessment of diversity, population structure and regeneration status of tree species in Hollongapar Gibbon Wildlife Sanctuary, Assam, Northeast India. Tropical Plant Research 2014;1(2):26–36.https://www.tropicalplantresearch.com/download/14/5.pdf
- Shanmughavel P., Zheng Z., Liqing S. and Min C. Floristic structure and biomass distribution of a tropical seasonal rain forest in Xishuangbanna, southwest China. Biomass and Bioenergy 2001; 21(3):165-175. https://www.sciencedirect.com/science/article/pii/S096195340100023X
CrossRef - Rabha D. Species composition and structure of Sal (Shorea robusta Gaertn. f.) forests along disturbance gradients of Western Assam, Northeast India. Tropical Plant Research 2014; 1(3):16–21. https://www.tropicalplantresearch.com/vol1Issue3/pdf/3.1.pdf
- Bhat D.M., Hegde G.T., Shetti D.M., Patgar S.G., Hegde G.N., Furtado R.M., Shastri C.M., Bhat P.R. and Ravindranath N.H. Impact of disturbance on composition, structure, and floristics of tropical moist forests in Uttara Kannada district, Western Ghats, India. Ecotropica 2011; 17:1-14. https://www.soctropecol.eu/PDF/Ecotropica_2011/Bhat_et_al_2011.pdf
- Sundarapandian S. and Karoor P.J. Edge effects on plant diversity in tropical forest ecosystems at Periyar Wildlife sanctuary in the Western Ghats of India. Journal of Forest Research 2013; 24(3):403-418. https://link.springer.com/article/10.1007/s11676-013-0373-6
CrossRef - Khan M.L., Rai J.P.N. and Tripathi R.S. Regeneration and survival of tree seedlings and sprouts in tropical deciduous and sub-tropical forests of Meghalaya, India. Forest Ecology and Management 1986; 14:293-304. https://www.sciencedirect.com/science/article/pii/0378112786901751
CrossRef - Tynsong H., Dkhar M., and Tiwari B. Tree diversity and vegetation structure of the tropical evergreen forests of the southern slopes of Meghalaya, North East India. Asian Journal of Forestry 2022; 6(1). https://smujo.id/ajf/article/view/10634
- Radhamoni H. V. N., Queenborough S. A., Arietta A. A., Suresh H. S., Dattaraja H. S., Kumar S. S. and Comita L. S. Local?and landscape?scale drivers of terrestrial herbaceous plant diversity along a tropical rainfall gradient in Western Ghats, India. Journal of Ecology 2023; 00:1–16. https://doi.org/10.1111/1365-2745.14075
CrossRef - Gadgil M., Krishnan B. J., Ganeshaiah K. N., Vijayan V. S., Borges R., Sukumar R., Noronha L., Nayak V. S., Subramaniam D. K., Varma R. V., Gautam S. P., Navalgund R. R. and Subrahmanyam G. V. Report of the Western Ghats ecology expert panel 2011. Ministry of Environment and Forests, Government of India. https://www.keralabiodiversity.org/images/pdf/wgeep.pdf







