Arsenic Contamination and Its Impact on the Human
Corresponding author Email: drpkarak@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.1.6
Copy the following to cite this article:
Karak P. Arsenic Contamination and Its Impact on the Human. Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.6
Copy the following to cite this URL:
Karak P. Arsenic Contamination and Its Impact on the Human. Curr World Environ 2022;17(1). Available From:
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 07-12-2021 |
|---|---|
| Accepted: | 23-03-2022 |
| Reviewed by: | 
 Y. Vasudeva Rao
Y. Vasudeva Rao
|
| Second Review by: |

 Rohit Joshi
Rohit Joshi
|
| Final Approval by: | Dr. Wilkister Nyaora Moturi |
Introduction
Arsenic (AS) in high concentration is one of the dangerous toxicants for human health. Ground water and other heavy metals contamination are the main sources of AS pollution and have serious consequences on the human health and its effects, bio-accumulative nature, and persistence in the environment worsen the problem. In biological systems, heavy metals affect the cellular DNA and nuclear proteins leading to alteration of cell cycle, carcinogenesis or apoptosis.
Increased permissible level of AS in groundwater was found in more than 30 countries. Arsenic affected countries include Argentina, USA, China, Chile, Mexico, Hungary, Vietnam1&2. According to current data AS affects more than nineteen states of India2&3. More than 200 million people worldwide use higher recommended limit of AS contaminated drinking ground water4&5. Ground water is major source, which is used in various purposes like domestic, industrial and agricultural purposes.
Affected districts of WB are North 24-Parganas, South 24-Parganas, Nadia, Hooghly, Malda and Murshidabad where AS concentrations were found to be more than 50μg/l6. Globally around 26 million people are potentially at risk for exposed to AS contaminated ground water (>50μg/l)7.
Sources and Distribution of Arsenic
In soil occurrence of arsenic at an average concentration of 2-5mg/kg8.. Volcanic activity is the most important natural sources of AS, which can release huge amounts of AS into the atmosphere. Other sources of AS include the erosion of the rock or soil, forest fires and the other anthropogenic sources. Anthropogenic sources aggravate and accelerate the release of naturally occurring AS9.
More than 320-mineral species containing AS are found in the nature10. In nature, the ores are the most abundant source of AS. The most important ores containing AS are niceolite, realger, orpiment, lobaltile, arsenopyrite, and tennanites. Arsenopyrite (FeAsS) is the most common mineral contains AS11. After weathering of AS mineral, the AS ions adsorbs onto the Fe (III), and Mn (IV) oxide-hydroxide phases12. The AS bearing minerals constitute the source of the AS in natural water.
Arsenic trioxide is the by-product from dust and residues produced during gold and copper smelting operations. Anthropogenic industrial sources contribute to the release of arsenic directly into the environment 13.
Forms of Arsenic
Out of three allotropic forms, most stable form is silver-gray brittle crystalline solid14. The metallic AS is brittle, odourless and tasteless, tarnishes rapidly in air and when heated it rapidly oxidized to form arsenic-trioxide. Non-metallic form is less reactive and dissolves when heated with acids and alkalis. Normally AS is found in four valence states −3, 0, +3, and +5. AS is a naturally occurring metalloid, (i.e., metalloid is a substance that is not a metal but shares many qualities with metals) component of the earth’s crust. Naturally occurring AS react with oxygen or other molecules present in the air, water, or soil to form various reactive compounds15.
Arsenic occurs in two forms, inorganic and organic forms. Inorganic forms are most pentavalent state than the organic form. Inorganic AS is prevalent in two forms such as trivalent or the ASIII (arsenic tri- oxide, arsenic-trichloride and arsenites) and pentavalent or ASV (Arsenic-pentoxide). The ASIII occur in the nature at low levels mostly combined with oxygen, chlorine, and sulphur is called inorganic AS compounds. In the soil it is present in sulphide form and arsenate minerals16. It also found in the copper and lead ore deposits, in water, and in industry it is found as gas, which is very much toxic when inhaled. Inorganic forms are much more poisonous to most animals, plants, and humans than the organic AS and disturb the cellular metabolic function.
Combination of the element AS with organic compounds is often known as arsenical organic compounds. Organic compounds of AS and trimethyl arsonium salts are marine in origin. Most often organic AS has commercial application. It is used in making glass (H3AsO4), insecticides [(NaCH3HAsO3)3, (Na2CH3AsO3)3], weed killers and other compounds. Organic AS is not poisonous for humans, but it may be poisonous when it consumed at high concentrations. During the 18th to 20th centuries, many AS compounds were in use as medicines and green pigment. During World War I, many organic forms were developed17 and few forms like arsenobetaine or arsenocholine are found in seafood18.
Toxicokinetics and Metabolism
Permissible Limit of Arsenic Consumption
WHO recommended permissible value for total AS concentration is 0.01 mg/L19 and for USEPA is 0.05 mg/L. The permissible limit of AS in India and Bangladesh is at 0.05 mg/L based on an earlier report of WHO20-22 are presented in Table-1.
Table 1: Maximum Permissible Level of Arsenic in Drinking Water.
|
Organization/Region |
Maximum Permissible Value of AS (µg/L) |
Effective Year |
|
WHO |
50 |
1981 |
|
WHO |
10 |
1993 |
|
US EPA |
50 |
1942 |
|
US EPA |
10 |
2006 |
|
India |
50 |
1950 |
|
India |
10 |
2012 |
|
Bangladesh |
50 |
1950 |
|
Australia |
7 |
1996 |
|
Canada |
10 |
2006 |
|
Mexico |
50 |
1994 |
|
German Drinking Water Ordinance |
10 |
1996 |
|
UK |
50 |
1981 |
Exposure, Absorption and Metabolism of Arsenic
About 97% exposure to AS occurs through our oral intake of contaminated ground water and food. Absorption of ingested and inhaled AS occurs mostly in the GI tract (45-75)%, lungs and skin. In the GI tract, small intestine is the major site for absorption of AS.
Absorbed AS binds with haemoglobin (Hb) of red blood cells (RBC), after binding with Hb, it is transported to most of the tissues, especially in the liver, kidney, lungs, testis, and skin where it is methylated23. Compounds of AS can crosses the blood-brain and the placental barrier. Mainly accumulation of AS occur in the keratin and sulfhydryl groups rich tissues. Accumulation of inorganic AS compounds takes place in the epididymis, thyroid gland and lenses of the eyes.
The AS is eliminated mainly through urine as mixture of inorganic, mono-methylated, and di-methylated form. Lesser amounts of elimination of AS occurs via skin, hair, nails, breast milk.
Presence of intestinal bacteria and pH can influence the amount of absorbed AS which undergoes hepatic biomethylation (Fig.1). Metabolism plays an important role for AS toxicity. AS metabolised by reduction and methylation reactions, catalyzed by glutathione-S-transferase w-1 (GSTO1) and arsenite methyl-transferase (As3MT). In this methylation process, it is enzymatically converted to methylated arsenicals, which is the end product of the AS metabolism and the biomarker of the chronic AS exposure24. MMA and DMA are happened before it is expelled through the urine. Variation in AS toxicity depends on its oxidative or reductive state. The monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV) are eliminated through urine but MMAIII remains inside the cell as an intermediate product. MMAIII is highly toxic form of AS compared to the others26.
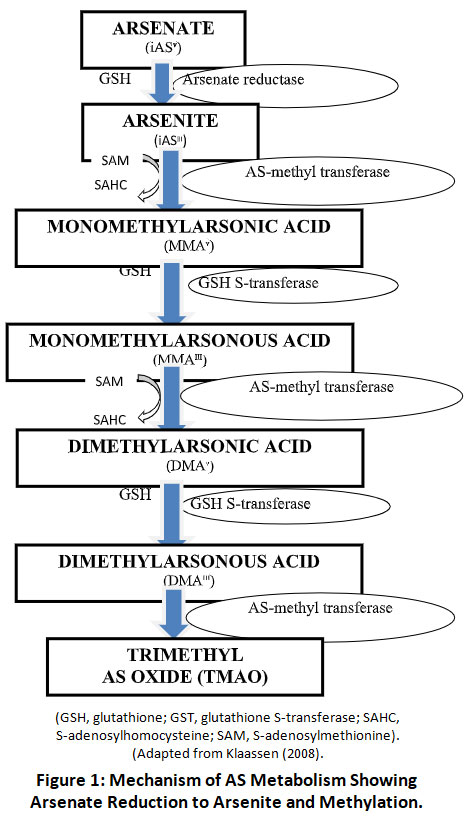 |
Figure 1: Mechanism of AS Metabolism Showing Arsenate Reduction to Arsenite and Methylation. Click here to view Figure |
Arsenic Toxicity
Toxicity of AS on human health mainly depend on its valency, state of oxidation (either as arsenite or arsenate), rate of absorption, frequency and route of intake, exposure time and bioavailability. ASIII, MMAIII, or DMAIII are higher toxic than ASV, MMAV, or DMAV. Sodium arsenite (LD50, is 15-44 mg/kg) is about 4-5 times more toxic than Sodium arsenate (LD50 is 112-175 mg/kg). LD50 value of pentavalent organic arsenicals is MMA (960 mg/kg), DMA (650 mg/kg) and 40-100 times less toxic than arsenite27&28. The toxic mechanisms of AS is much complex29-31. Toxicity is affected by its solubility, oxidation state, as well as other internal and external factors. The molecular toxic capacity of AS is usually coupled with its biotransformation mechanisms (Fig. 2). The formation of methylated products of arsenite ASIII causes increased genotoxicity and production of reactive oxygen and/or nitrogen species32-35.
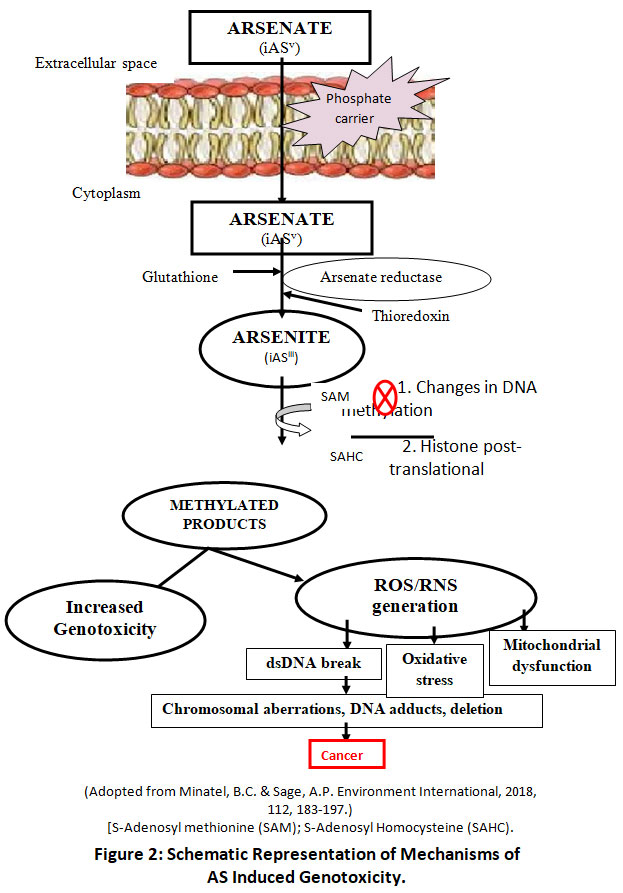 |
Figure 2: Schematic Representation of Mechanisms of AS Induced Genotoxicity. Click here to view Figure |
Biological Effects of Arsenic on Plants and Human
Arsenic is widely present in the soil, groundwater and plants. Biological effects of AS on livings organisms occur through bioaccumulation and biomagnifications (Fig.3).
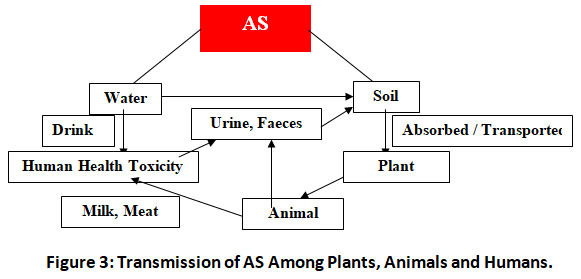 |
Figure 3: Transmission of AS Among Plants, Animals and Humans. Click here to view Figure |
Effects of Arsenic on Plants
AS is not an essential element for plant growth but it can accumulate in plants to toxic levels. Normally, AS uptake by plant depends on the total concentration and form of AS in the soil36-38. In general, plants take up the ASV through the roots via phosphate (Pi) transporters. Inside the plant tissue, it produces oxidative stress by generation of reactive oxygen species (ROS) like superoxide radical (O2−), hydroxyl radical (OH), and hydrogen peroxide (H2O2). ROS is dangerous for cellular metabolism and leads to the irreparable damage to DNA, proteins and lipids39 and leads to change in morphological, physiological, biochemical, molecular and cellular functions40-42 (Table 2).
Table 2: The Effects of AS Toxicity on Plants 43&44
|
|
Effect of Arsenic on Plants |
||
|
ROOT |
STEM |
LEAVES |
|
|
Morphology of Plant |
|
|
|
|
Growth and development |
|
|
|
|
Functional development |
|
|
|
Effects of Arsenic on Human Health
Arsenic toxicity depends on the amount and form of AS intake. Inorganic AS is highly toxic carcinogen, and is the most significant contaminant in drinking-water globally50&51.
The AS can cause various adverse impact on different organ systems of the body. Such organ systems are integumentary, cardiovascular, respiratory, gastrointestinal, endocrine, reproductive, neurological, developmental abnormalities and cancerous52. Depending on the level of exposure, its toxic effects may be acute or chronic. Acute AS toxicity occurs when a single large dose of AS cause severe symptoms. Initial symptoms of acute AS poisoning are muscular pain and weakness following severe nausea, vomiting, abdominal pain, diarrhoea, cyanosis, cardiac arrhythmia, confusion and hallucinations53. Other acute poising include bone marrow depression leading to anaemia and leucopenia, cold clammy skin, renal failure, encephalopathy and peripheral neuropathy like impaired sensation, movement of gland or organ function are also reported54.
Inhalation of AS gas leads to acute problems like cough, bronchitis, shortness of breath. AS ingestion for a prolonged period leads to an accumulation of AS in the body causing chronic AS toxicity with various clinical manifestations collectively called arsenicosis. Arsenicosis leads to keratosis, pigmentation (hyperpigmentation / hypopigmentation) of feet, hands, fingers. Chronic AS toxicity results in multiorgan system disease and the most serious consequences being the malignancy55. AS increase the incidence of carcinogenicity in organs like skin, lung, kidney, urinary bladder, prostate, and liver. Various organ systems of the body are affected by chronic arsenic exposure with inorganic AS. This article mainly focuses to explain the effect of arsenic on human health on two headings- cancerous and non-cancerous effects.
Cancer Effects
Carcinogenic property of AS compounds was first identified more than a century ago56. Since 1980, AS was listed as a human carcinogen57. AS is a unique carcinogen for which the carcinogenic risk arises from both the ingestion and inhalation58. People exposed to AS contaminated drinking water for more than 40-years develops urinary tract cancer more often than people with less than 40-years of exposure59. There is a large gap between the exposures of AS on clinical manifestation of cancer. This gap period is called the latent phase60. Ingestion of iAS causes lung cancer, and long term ingestion of iAS is linked risk of urinary bladder cancer, skin cancer and also GI trcat, kidney and prostate61-62 (Table-3).
Table 3: Epidemiologic Studies of AS Exposure and Cancers.
|
Target Organ |
Source |
Result |
Population Location |
Reference Number |
|
Skin Cancer
|
Well water |
Increased mortality |
Taiwan |
66 |
|
Drinking water |
Increased mortality |
South-West Taiwan |
67 |
|
|
Drinking water |
Increased mortality |
Taiwan |
68 |
|
|
Drinking water |
Increased mortality |
Mexico |
69 |
|
|
Drinking water |
Increased mortality |
Chile |
70 |
|
|
Drinking water |
Increased mortality |
USA |
71 |
|
|
Drinking water |
Increased mortality |
USA |
72 |
|
|
Drinking water |
Increased mortality |
USA |
73 |
|
|
Drinking water |
Increased mortality |
China |
74 |
|
|
Lung Cancer |
AS exposed area |
Increased mortality |
South-West Taiwan |
77 |
|
Drinking water |
Increased mortality |
Taiwan |
78 |
|
|
Drinking water |
Increased mortality |
Northern Chile |
61 |
|
|
Drinking water |
Increased mortality |
Chile |
70 |
|
|
Drinking water |
Increased mortality |
U.S.A |
79 |
|
|
Local water/soil |
Increased incidence |
Victoria, Australia |
80 |
|
|
Liver Cancer |
Drinking water |
Increased mortality |
South-West Taiwan |
67 |
|
Drinking water |
Increased mortality |
South-West Taiwan |
87 |
|
|
Drinking water |
Increased mortality |
South-West Taiwan |
85 |
|
|
AS contaminated region |
Increased mortality |
Northern Chile |
88 |
|
|
Drinking water |
Increased mortality |
Chile |
16 |
|
|
Local water/soil |
Increased incidence |
Victoria, Australia |
80 |
|
|
Drinking water |
Increased mortality |
West Bengal, India |
89 |
|
|
Bladder Cancer |
Drinking water |
Increased mortality |
South-West Taiwan |
67 |
|
Drinking water |
Increased mortality |
South-West Taiwan |
87 |
|
|
Drinking water |
Increased mortality |
Taiwan |
92 |
|
|
AS exposed area |
Increased Mortality |
South-West Taiwan |
77 |
|
|
AS contaminated region |
Increased mortality |
Northern Chile |
88 |
|
|
Local water/soil |
Increased incidence |
Victoria, Australia |
80 |
|
|
Drinking water |
Increased mortality |
USA |
71 |
|
|
Prostate Cancer |
Drinking water |
Increased mortality |
Southwest Taiwan |
67 |
|
Drinking water |
Increased mortality |
Southwest Taiwan |
87 |
|
|
Drinking water |
Increased mortality |
Taiwan |
92 |
|
|
Drinking water |
Increased mortality |
Southwest Taiwan |
77 |
|
|
Drinking water |
Increased mortality |
Utah, USA |
95 |
|
|
Local water/soil |
Increased incidence |
Victoria, Australia |
80 |
Skin Cancer
There is a positive link between AS exposure and skin cancer like Bowen’s disease, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)63 Arsenite has an important role on UV-induced skin cancer64. Skin cancer probably occurs through the effect on DNA repair and DNA methylation. The AS interacts with Fas/Fas ligand pathway (also known as Apo1 or CD95), results alterations in the transcription factor NF-kB (Nuclear Factor kappa-light-chain-enhancer of activated B cell) and AP-1 (Activator protein 1) activity. These transcription factors produce various inflammatory cytokines. NFAT (Nuclear factor of activated T-cells) transcription factor is linked with cancer64. Epidemiological evidence suggest that the association between AS and cancers of skin and internal organs65 (Table-3).
Lung Cancer
Lung cancer is one of the serious forms of cancer associated with AS ingestion75. Patients who were previously suffering from either hyper pigmentation or hyperkeratosis or Bowen’s disease are found to be more prone to have lung cancer. Lung cancer is also associated with occupational exposure to AS via inhalation, very little research data is available showing the association between ingested Arsenic and lung cancer. Lung adenocarcinoma is the most common and also AS-induced lung tumors are observed76 (Table 3).
Liver Cancer
Liver is one of the important internal organs affected by chronic exposure to arsenic. Direct association between AS exposure and human liver cancers was established. Epidemiological studies reveal that chronic AS exposure causes liver diseases including hepatomegaly81, hepatoportal sclerosis82, liver fibrosis and cirrhosis of liver83, and liver cancer84. Liver is the target organ for AS-induced carcinogenesis and develops hepatocellular carcinoma (HCC). Chronic exposure to AS is also linked with an increased prevalence of liver hepatitis/cirrhosis85. The potential mechanism of AS induced carcinogenesis is oxidative DNA damage, acquired tolerance to apoptosis and enhanced cell proliferation, altered DNA methylation and genomic instability86.
Bladder Cancer
Chronic intake of high levels of inorganic AS shows the strong associations and dose-response relationships between bladder cancer90 and urothelial carcinoma of the bladder (UCB)90. Chronic AS exposure leads to the expression of inflammatory enzyme cyclooxygenase-2 (COX2) and the COX2-derived prostaglandin E2 (PGE2). COX2 and PGE2 pathway causes the multistep development of cancer. Majority of UCB occurs due to expression of COX291.
Prostate Cancer
Little research study shows the strong linked between ground water arsenic toxicity and prostate cancer 92-93. In vitro study shows that AS can also causes prostate cancer cell progression. It also proved that there is a positive relation in dose-response relationships between arsenic level and age-adjusted prostate cancer mortality94.
Non-Cancer Effects
Effect on Skin
Chronic AS poisoning cause damages to many organ systems of the body. Symptoms of AS toxicity are first manifested in the skin. The first observed effects of chronic AS exposures were non-malignant skin manifestations, which include hyperpigmentation, palmar and solar keratosis 96&97. Skin lesions are found to be the most common features of chronic AS exposure. Lesions of skin occur usually 5-10 years after exposure. The first visible symptoms caused by prolonged ingestion of AS in drinking water is black brown skin pigmentation, called melanosis. If this exposure continues for longer duration, then fine freckles of spotted pigmentary changes are also observed, which is known as 'rain-drop’ pigmentation98.
Effect on Cardiovascular System
Chronic AS exposure has been linked to cardiovascular disease (CVD), including coronary heart disease (CHD), stroke, peripheral vascular disease (PVD), hypertension, atherosclerosis, ischemic heart disease, vascular disease mortality99. Chronic AS poisoning leads to inactivation of endothelial nitric oxide synthase and resulting reduction in generation and bioavailability of NO (Nitric oxide). Increased activity of vascular NOX (NADPH-oxidases) enzymes play a key role in pathogenic redox signalling in vascular disease and hypertension100. Arsenite stimulates NADPH oxidase to increase the generation of ROS (Reactive oxygen species)101. ROS production is the initial step in AS induced endothelial cell proliferation and apoptosis. These two mechanisms proposed for AS-related atherosclerosis102.
Effect on Respiratory System
Exposure of AS occurs through inhalation has significantly associated with the progression of non-malignant respiratory lung disorders. As a result there are occurrence of chronic bronchitis, bronchiectasis, chronic obstructive pulmonary disease (COPD), and respiratory disease mortality103. AS result the strength of the respiratory muscles decreases, vital capacity, total lung volume capacity also gets decreases. It is observed that those patients with the characteristic skin lesions of chronic AS toxicity have more susceptible for respiratory diseases.104.
Effect on Gastrointestinal System
Due to acute AS poisoning gastrointestinal symptoms are more common. The important clinical features of the acute AS poisoning are burning lips, painful swallowing, thirst, nausea, vomiting, and others abdominal colic diarrhoea105. Inorganic AS is ingested into the body by different routes and is metabolized by the liver. In liver, methylation takes place and produces dimethyl-arsenic glutathione, which is stored in the gall bladder and ejected in bile 106-107.
Effect on Excretory System
The kidneys are the main organ involved in the excretion of AS and its metabolites. The common affected sites are capillaries, tubules and glomeruli. Epidemiologic studies suggest that high AS exposure can be associated with chronic kidney disease (CKD)108. CKD is associated with reduced GFR (Glomerular Filtration Rate) and increased urinary albumin excretion 108.
Effect on Reproductive System
Globally, AS is recognized as a reproductive toxicant in humans. In males, exposure to AS cause reproductive organ dysfunctions likes reduction in weight of primary and accessory sex organs109. AS causes change in plasma concentrations of LH (Leutinizing Hormone) and FSH (Follicular Stimulating Hormone) by inhibiting hypothalmo-pituitary gonadal axis. Reduced plasma LH impairs Leydig cell functions and consequently reduction in testosterone production110.
AS toxicity involves with female reproductive system functions and jeopardize it in diverse ways. It causes a serious toxic effect on the primary sex organ of female (ovary). It is associated with suppression of ovarian steroidogenesis, and degenerates ovarian follicular and uterine cells. Sum of all these effects lead to abnormal reproductive functions and pregnancy outcome of a woman111.
Effect on Nervous System
The AS can alter the integration and co-ordination functions of the brain. The neurotoxic effects of AS appear to be most severe in the developing brain, as AS can cause cellular changes in the brain. Many research studies have been reported that AS exposure can cause impaired functions of neural pathways and causes polyneuropathy, EEG (Electroencephalogram) abnormalities and in extreme cases hallucinations, disorientation and agitation112. Cognitive functions disturbances associated with decreased intelligence, verbal coefficients, impairments in learning and memory, changes in behaviour, and confusion113&114.
Haematological Effect
Acute and chronic AS exposure may cause change in blood compositions115. The reduced RBC count may be due to inhibition of erythropoietin production or by the depression of bone marrow. AS exposure causes the depression of the bone marrow, results decreased RBC and WBC production. AS toxicity may causes changes in the morphological structure of RBC. AS induced changes occur in the RBC membrane integrity and deformability, which contribute to micro vascular occlusion and related peripheral vascular effects116.
Immunological Effect
Chronic exposure to AS potentially impair vital immunological functions. Reduced immunological functions lead to increased risk of infections and inflammatory-like diseases 117. The AS may affect lymphocyte, monocyte and macrophage activity resulting in immune-suppression. Prenatal AS exposure has been associated with reduced thymic index leads to reduced cell-mediated immune function118. This indicates that during childhood AS exposure causes arsenic-induced developmental immunotoxicity. Chronic high concentration of AS exposure has been associated with reduced Th1/Th2 secretion of interleukin-2 (IL-2)119. Both T lymphocytes and macrophages are involved in the initiation of humoral immune response. The AS suppresses T-cell dependent antibody responses120. The AS exposure can impair on antibody production and trans-placental IgG transport to neonates121.
Arsenic and Diabetes
Chronic high concentration AS exposure has role on the development of diabetes mellitus (DM)122. Epidemiological study and experimental evidences support the role of iAS in the development of diabetes. The possible mechanisms for the development of DM may be due to iAS interference with insulin-stimulated signal transduction pathway123 or during acute poisoning, arsenite inhibits pyruvate and α-ketoglutarate dehydrogenases enzymes which are essential for gluconeogenesis and glycolysis124. By other mechanism AS could also influence the development of type-2-DM by oxidative stress, inflammation and nonspecific mechanisms122.
Conclusion
AS and its compounds are known potent poisons causing widespread environmental contamination. From the above discussion and other research studies, we can conclude that AS is a potent carcinogen. The chronic high concentration AS exposure causes cancer of the skin, lung, liver, kidney, bladder, and prostate gland. Depending upon acute or chronic exposure AS toxicity varies. It increases in premature delivery and decreased birth weights of infants. The possibility of an association between chronic AS exposure and diabetes has implications for research and public health. The experimental and epidemiologic evidence suggest that AS has various toxic effects on different organ systems on human health.
Acknowledgements
The author would like to acknowledge the technical support provided by the faculty members and students of the Physiology Department of Bankura Christian College, Bankura, and West Bengal, India.
Funding Source
The author declares that there is no funding support was received.
Author Contributions
Prithviraj Karak: Data curation, Writing - Original draft preparation, Conceptualization, Methodology, Formal analysis and Investigation and Supervision, Review & Editing, and visualization and Software and Validation.
Conflict of Interests
The author declares that they have no conflict of interests.
References
- Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of As in natural waters. Applied Geochemistry. 2002;17(5):517–568. doi:10.1016/S0883-2927(02)00018-5.
- NIH and CGWB. Mitigation and Remedy of Groundwater as Menace in India: a Vision [Document] MoWR, GoI 2010, 184.
- Bhattacharya AK, Karthik DMP, Gautam A, Sharma A, Srinivas K, Singh PK. As contamination in the groundwater of India. Green and Sustainable Development. 2016; 3(17): 36–60.
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic As exposure: Update on a worldwide public health problem. Environmental Health Perspectives. 2013;121(3):295–302. doi:10.1289/ehp.1205875, PubMed: 23458756.
- World Health Organization. Guidelines for drinking water quality (4th ed) 2011.
- Mukherjee SC, Rahman MM, Chowdhury UK, Sengupta MK, Lodh, Chanda CR, Saha, KC, Chakraborti D. Neuropathy in As toxicity from groundwater As contamination in West Bengal, India. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38(1): 165-83. doi:10.1081/ese-120016887.
- IARC (International Agency for Research on Cancer) some Drinking water Disinfectants and Contaminants, including arsenic (2004) Monographs on evaluation of carcinogenic risk to humans. Lyon, France: IARC Press.2004, 84, 269–477.
- Klump S, Kipfer R, Cirpka OA, Harvey CF, Brennwald MS, Ashfaque KN. Groundwater dynamics and As mobilization in Bangladesh assessed using noble gases and tritium. Environmental Science and Technology. 2006; 40(1): 243–250. doi:10.1021/es051284w.
- U.S.E.P.A. Proposed revision to As drinking water standard, technical fact sheet: Proposed rule for As in drinking water and clarifications to compliance and new source contaminants monitoring. 2000. Retrieved from http://www.epa.gov/safewater/ars/prop_techfs.html,May.
- U.S. E.P.A. As Treatment Technologies for Soil, Waste, and Water. 2002, EPA-542-R-02004.
- Acharyya SK, Chakraborty P, Lahiri S, Raymahashay BC, Guha S. As poisoning in the Ganges delta. Nature. 1999; 401:545-547. doi:10.1038/44052.
- The Agency for Toxic Substances and Disease Registry (ATSDR): ToxFAQsTM for As 2001 July 12.
- Chung JY, Yu SD, Hong YS. Environmental Source of Arsenic Exposure. J Prev Med Public Health. 2014; 47(5): 253-257. doi: 10.3961/jpmph.14.036.
- Audi, G. The NUBASE evaluation of nuclear and decay proper-ties. Nucl Phys A (At Mass Data Cent), 2003; 729: 3-128.
- Agency for Toxic Substances and Disease Registry Toxicological profile for AS. 2007. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp2.pdf.
- Smith E, Naidu R, Alston AM. As in the soil environment: A review. Advances in Agronomy.1998; 64:149–195. doi:10.1016/S0065-2113(08)60504-0.
- Girard J. Principles of environmental chemistry. Jones and Bartlett Learning. 2010. ISBN 9780763759391.
- Hopenhayn C. As in drinking water: Impact on human health. Elements. 2006; 2(2): 103–107. doi:10.2113/gselements.2.2.103.
- World Health Organization. Guidelines for drinking water quality (4th ed). 2011.
- 20th ed. New York: American Public Health Association; 1998. APHA. Standard methods for the examination of water and waste water.
- World Health Organization Anon., World Health Organization guidelines for drinking water quality (2nd ed), II. Geneva: WHO. 1996.
- Indian Standards for Drinking water, Second revision of IS 10500, 2004.
- Healy SM, Aposhian AV. Diversity of inorganic arsenite transformation. Biological Trace Element Research. 1999; 68:249–266. doi: 10.1007/BF02783907.
- Kossoff D, Hudson-Edwards KA. Chapter 1. As in the environment. In J. M. Santini, S. M. Ward (Eds.) London: CRC Press Press.. The metabolism of arsenite, As in the Environment. 2012;1–23.
- Wexler, P. Encycl. Toxicologist. Elsevier, 1998; 2:291–292.
- Singh N, Kumar D, Sahu AP. As in the environment: Effects on human health and possible prevention. Journal of Environmental Biology. 2007; 28(2); Suppl 359–65. PMID: 17929751.
- Agency for Toxic Substances and Disease Registry Toxicological profile for As (2007) Retrieved from http://www.atsdr.cdc.gov/toxprofiles/tp2.pdf
- Cullen, W.R. & Bentley, R. The toxicity of trimethylarsine: an urban myth. J. Environ. Monit, 2005, 7, 11-15. doi: 10.1039/B413752N.
- International Agency for Research on Cancer, World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Lyon, France: International Agency for Research on Cancer (International Arctic Research Center), World Health Organization (WHO). 1999.
- EPA. Special report on ingested inorganic As. Skin cancer; Nutritional Essentiality, US Environmental Protection Agency, EPA/625/3-87/-13. 1988.
- Li JH, Rossman TG. Inhibition of DNA ligase activity by arsenite:A possible mechanism of its comutagenesis. Molecular Toxicology. 1989; 2(1):1-9. PMID: 2615768
- Roland-Hubaux R, Becker-Santos DD, Enfield KSS, Rowbotham D, Lam S, Lam WL, Martinez VD. Molecular features in As-induced lung tumors. Molecular cancers. 2013; 19: 12-20.
- Cobo JM, Castineira M. Oxidative stress, mitochondrial respiration, and glycemic control: clues from chronic supplementation with Cr3+ or As3+ to male Wistar rats. Nutrition. 1997; 13: 965-70. doi: 10.1016/s0899-9007(97)00338-9.
- Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, Waalkes M. As: health effects, mechanisms of actions, and research issues. Environ Health Perspect. 1999; 107:593-7. doi: 10.1289/ehp.99107593.
- Minatel BC, Sage AP. Christine Anderson, Roland Hubaux, Erin A. Marshall, Wan L. Lam, Victor D. Martinez. Environmental As exposure: From genetic susceptibility to pathogenesis. Environment International. 2018; 112:183-197. doi: 10.1016/j.envint.2017.12.017.
- Rafiq M, Shahid M, Abbas G, Shamshad S, Khalid S, Niazi NK, Dumat C. Comparative Effect of Calcium and EDTA on As Uptake and Physiological Attributes of Pisum sativum. Int. J. Phytoremed. 2017; 19(7): 662–669. https://doi.org/10.1080/15226514.2016.1278426
- Rafiq M, Shahid M, Shamshad S, Khalid S, Niazi NK; Abbas, G.; Saeed, M.F.; Ali, M. & Murtaza, B. A Comparative Study to Evaluate Efficiency of EDTA and Calcium in Alleviating As Toxicity to Germinating and Young Vicia faba L. Seedlings. J. Soils Sedim. 2018;18:2271–2281.
- Moreno-Jiménez E, Esteban E, Peñalosa JM. The Fate of As in Soil-Plant Systems. Rev Environ Contam Toxicol. 2012; 215:1-37. doi: 10.1007/978-1-4614-1463-6_1.
- Singh N, Ma LQ, Srivastava M, Rathinasabapathi B. Metabolic Adaptations to As-Induced Oxidative Stress in Pteris vittata L and Pteris ensiformis L. Plant Science. 2006; 170(2): 274-282. https://doi.org/10.1016/j.plantsci.2005.08.013
- Begum MC, Islam MS, Islam M, Amin R, Parvez MS, Kabir AH. Biochemical and Molecular Responses Underlying Differential As Tolerance in Rice (Oryza sativa L.) Plant Physiol. Biochem. 2016;104: 266–277. doi: 10.1016/j.plaphy.2016.03.034.
- Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L. Alteration in Growth, Leaf Gas Exchange, and Photosynthetic Pigments of Maize Plants under Combined Cadmium and As Stress. Water Air Soil Pollut. 2017; 228(1):13. DOI:10.1007/s11270-016-3187-2
- Srivastava S, Sinha P, Sharma YK. Status of Photosynthetic Pigments, Lipid Peroxidation and Anti-oxidative Enzymes in Vigna mungo in Presence of As. J. Plant Nut. 2017; 40(3): 298–306. https://doi.org/10.1080/01904167.2016.1240189
- Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M, Natasha. As Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health. 2018; 15(1):59. doi: 10.3390/ijerph15010059.
- Chandrakar V, Pandey N, Keshavkant S. Plant Responses to As Toxicity: Morphology and Physiology. Mechanisms of As Toxicity and Tolerance in Plants. Springer Nature Singapore Pte Ltd., ISBN 978-981-13-1291-5.2018: 27-48.
- Armendariz AL, Talano MA, Villasuso AL, Travaglia C, Racagni GE, Reinoso H, Agostini E. As Stress Induces Changes in Lipid Signalling and Evokes the Stomata Closure in Soybean. Plant Physiol. Biochem. 2016;103:45–52. DOI: 10.1016/j.plaphy.2016.02.041
- Niazi NK, Bibi I, Fatimah A, Shahid M, Javed MT, Wang H, Ok YS, Bashir S, Murtaza B, Saqib ZA. Phosphate-Assisted Phytoremediation of As by Brassica napus and Brassica juncea: Morphological and Physiological Response. Int. J. Phytoremed. 2017; 19(7): 670–78. doi: 10.1080/15226514.2016.1278427.
- Mehmood, T.; Bibi, I.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Wang, H.; Ok, Y.S.; Sarkar, B.; Javed, M.T. & Murtaza, G. Effect of Compost Addition on As Uptake, Morphological and Physiological Attributes of Maize Plants Grown in Contrasting Soils. J. Geochem. Explor., 2017, 178, 83–91. https://doi.org/10.1016/j.gexplo.2017.03.018
- Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L. Alteration in Growth, Leaf Gas Exchange, and Photosynthetic Pigments of Maize Plants under Combined Cadmium and As Stress. Water Air Soil Pollut. 2017;228(1):1-12. doi: 10.1007/s11270-016-3187-2.
- Zargari F, Pourakbar L, Salehi-Lisar SY, Razeghi J, Azad RM. An Assessment of Oxidative Stress and Antioxidant System Activity in Alfalfa Plants Treated with Different Forms of Mineral Arsenic. Journal of Plant Process and Function. 2020; 9(37):13-26.
- International Agency for Research on Cancer-World Health Organization. IARC Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to Man. International Agency for Research on Cancer (IARC), World Health Organization (WHO); Lyon, France: 1999.
- International Agency for Research on Cancer-World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer (IARC), World Health Organization (WHO); Lyon, France: 2001.
- Chakraborti D, Rahman MM, Das B, Chatterjee A, Das D, Nayak B, Pal A, Chowdhury UK, Ahmed S, Biswas BK, Sengupta MK, Hossain MA, Samanta G, Roy MM, Dutta RN, Saha KC, Mukherjee SC, Pati S, Kar PB, Mukherjee A, Kumar M. Groundwater As contamination and its health effects in India. Hydrogeol. J. 2017;25(4):1651-1181. doi: 10.1007/s10040-017-1556-6.
- Vahidnia A, van der Voet GB, de Wolff F. A. As neurotoxicity?: A review. Hum. Exp. Toxicol.2007; 26(10):823–32. doi: 10.1177/0960327107084539.
- Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of As poisoning and its effects on human health. Crit Rev Env. Sci Technol. 1999;29(3):281–13. https://doi.org/10.1080/10643389991259227.
- Saha KC. Chronic arsenical dermatoses from tube- well water in West Bengal during 1983-87 Indian J. Dermatol. 1995;40(1):1-12.
- Hutchinson J. Arsenic cancer. Br. Med. J. 1887;2:1280-1281.
- International Agency for Research on Cancer. As and As compounds 1980; 23: 39.
- Centeno JA, Tchounwou PB, Patlolla AK, Mullick FG, Murakata L, Meza E, Gibb H, Longfellow D, Yedjou CG. Environmental Pathology and Health Effects of As Poisoning: A Critical Review. In Managing As in the Environment: From Soil to Human Health, Edited by: Naidu, R., Smith, E., Owens, G., Bhattacharya, P. and Nadebaum. Melbourne, Australia: CSIRO Publishing. (ISBN: 0643068686). 2006; 311–327.
- Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, Chen C. J. Incidence of transitional cell carcinoma and As in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am. J. Epidemiol. 2001;153(5): 411–418. doi: 10.1093/aje/153.5.411.
- Steinmaus C, Yuan Y, Bates MN, Smith AH. Case-control study of bladder cancer and drinking water As in the western United States. Am. J. Epidemiol. 2003;158(12): 1193–1201. doi: 10.1093/aje/kwg281.
- Ferreccio C, Gonzalez C, Milosavlijevic V, Marshall G, Sancha, AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11(6): 673–679. doi: 10.1097/00001648-200011000-00010.
- Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environmental Health Perspectives. 2000;108(7):655–661. doi: 10.1289/ehp.00108655.
- Yu HS, Liao WT, Chai CY. Arsenic Carcinogenesis in the Skin. J Biomed Sci. 2006; 13(5):657–666. doi: 10.1007/s11373-006-9092-8.
- Chowdhury WK, Tisha A, Akter S, Zahur SMB, Hasan N, Mahadi AS, Rabby SMF, Mohib MM, Sagor MAT, Mohiuddin S. The Role of Arsenic on Skin Diseases, Hair Fall and Inflammation: AnImmunological Review and Case Studies. J Clin Exp Dermatol Res. 2017; 8(2):1-9. doi: 10.4172/2155-9554.100038
- Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol. Appl. Pharmacol. 2004;198(3):394–404. doi: 10.1016/j.taap.2003.10.016.
- Tseng WP, Chu HM, How SW, Fong JM, Lin CS, Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968;40(3):453-462. PMID: 5644201
- Chen CJ, Kuo TL, Wu MM. Arsenic and cancers. Lancet. 1988;1(8582):414-5. DOI: 10.1016/s0140-6736(88)91207-x
- Guo HR, Yu HS, Hu H, Monson RR. Arsenic in drinking water and skin cancer: cell-type specificity. Cancer Cause Control. 2001;12:909-916.
- Cebrián ME, Albores A, Aguilar M, Blakely E. Chronic Arsenic Poisoning in the North of Mexico. Hum. Toxicol. 1983;2(1):121-33. doi: 10.1177/096032718300200110.
- Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. American Journal of Epidemiology. 1998;147(7):660–9. doi: 10.1093/oxfordjournals.aje.a009507.
- Karagas MR, Tosteson TD, Morris JS, Demidenko E, Mott LA, Heaney J, Schned A. Incidence of Transitional Cell Carcinoma of the Bladder and Arsenic Exposure in New Hampshire. Cancer Causes & Control. 2004;15:465-472.
- Beane Freeman LE, Dennis LK, Lynch CF, Thorne PS, Just CL. Toenail Arsenic Content and Cutaneous Melanoma in Iowa. American Journal of Epidemiology. 2004;160(7): 679-687. doi: 10.1093/aje/kwh267.
- Knobeloch LM, Zierold KM, Anderson HA. Association of Arsenic-Contaminated Drinking-Water With Prevalence of Skin Cancer in Wisconsin's Fox River Valley. J Health Popul Nutr. 2006;24(2):206-13. PMID: 17195561
- Lamm SH, Engel A. Cancer Risks Associated with Arsenic: Lamm et al. Respond. Environ Health Perspect. 2007;115(7):A340-A341. doi: 10.1289/ehp.9927R
- Mead MN. Arsenic: in search of an antidote to a global poison. Environ Health Perspect. 2005;113(6):A378–A386. doi: 10.1289/ehp.113-a378
- Guo HR, Wang NS, Hu H, Monson RR. Cell type specificity of lung cancer associated with arsenic ingestion. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13:638-643.
- Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Enivron Health. 1999;54(3):186–193. doi: 10.1080/00039899909602258.
- Guo HR. Arsenic Level in Drinking Water and Mortality of Lung Cancer (Taiwan). Cancer Causes Control. 2004;15(2):71-7. doi: 10.1023/B:CACO.0000019503.02851.b0.
- Andrew AS, Mason RA, Memoli V, Duell EJ. Arsenic activates EGFR pathway signaling in the lung. Toxicological Sciences. 2009;109(2):350-357. https://doi.org/10.1093/toxsci/kfp015.
- Hinwood AL, Jolley DJ, Sim MR. Cancer incidence and high environmental arsenic concentrations in rural populations: results of an ecological study. Int J Environ Heal Research. 1999;9:131–141. https://doi.org/10.1080/09603129973272.
- Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol Appl Pharmacol. 2005; 206(2):169–175. doi: 10.1016/j.taap.2004.08.025.
- Nevens F, Fevery J, Van Steenbergen W, Sciot R, Desmet V, De Groote J. Arsenic and non-cirrhotic portal hypertension. A report of eight cases. J. Hepatol. 1990;11(1):80–85. doi:https://doi.org/10.1016/0168-8278(90)90276-W.
- Dhawan D, Narang AP, Datta DV. Levels of arsenic in liver cirrhosis. Toxicol. Lett. 1983;15(2-3):105–8. doi: 10.1016/0378-4274(83)90201-1.
- Liu J, Waalkes MP. Liver is a Target of Arsenic Carcinogenesis. Toxicol Sci. 2008; 105(1):24–32. doi: 10.1093/toxsci/kfn120.
- Hsu LI, Wang YH, Hsieh FI, Yang TY, Jeng RWJ, Liu CT, Chen CL, Hsu KH, Chiou HY, Wu MM, Chen CJ. Effects of Arsenic in Drinking Water on Risk of Hepatitis or Cirrhosis in Persons With and Without Chronic Viral Hepatitis. Clin Gastroenterol Hepatol. 2016;14(9):1347–1355. doi: 10.1016/j.cgh.2016.03.043.
- Arteel GE, Guo L, Schlierf T, Beier JI, Kaiser JP, Chen TS, Liu M, Conklin DP, Miller HL, Montfort C, States JC. Subhepatotoxic exposure to arsenic enhances lipopolysaccharide-induced liver injury in mice. Toxicol Appl Pharmacol. 2008;226:128-139. 10.1016/j.taap.2007.08.020.
- Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130(6):1123–1132. doi: 10.1093/oxfordjournals.aje.a115439.
- Rivara MI, Cebrián M, Corey G, Hernández M, Romieu I. Cancer risk in an arsenic-contaminated area of Chile. Toxicol Ind Health. 1997;13(2-3):321–338. doi: 10.1177/074823379701300217.
- Das N, Paul S, Chatterjee D, Banerjee N, Majumder NS, Sarma N, Sau TJ, Basu S, Banerjee S, Majumder P, Bandyopadhyay AK, States JC, Giri AK. Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health. 2012;12:639.
- Jacques NS, Parker L, Brown P, Dummer TJB. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44. doi: 10.1186/1476-069X-13-44.
- Ooki A, Begum A, Marchionni L, Bussche CJV, Mao S, Kates M, Hoqu MO. Arsenic promotes the COX2/PGE2–SOX2 axis to increase the malignant stemness properties of urothelial cells. Int. J. Cancer. 2018;143:113–126. doi: 10.1002/ijc.31290.
- Chen CJ, Wang CJ. Ecological correlation between arsenic levels in well water and age adjusted mortality from malignant neoplasms. Cancer Res. 1990;50(17):5470–5474. PMID: 2386951.
- Bulkaa CM, Jones RM, Turyk ME, Stayner LT, Argos M. Arsenic in drinking water and prostate cancer in Illinois counties: An ecologic study. Environmental Research. 2016;148: 450-456. doi: 10.1016/j.envres.2016.04.030.
- Tallaa LB, Waalkes MP. Inorganic Arsenic and Human Prostate Cancer. Environ Health Perspect. 2008;116(2):158–164. doi: 10.1289/ehp.10423.
- Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107(5):359–365. doi: 10.1289/ehp.99107359.
- Li C, Xu J, Li F, Chaudhary SC, Weng Zl, Wen J, Elmets CA, Ahsan H, Athar M. Unfolded protein response signaling and MAP kinase pathways underlie pathogenesis of As-induced cutaneous inflammation. Cancer Prev. Res. (Phila.). 2011;4(12):2101–2109. doi: 10.1158/1940-6207.CAPR-11-0343.
- Melkonian S, Argos M, Chen Y, Parvez F, Pierce B, Ahmed A, Islam T, Ahsan H. Intakes of several nutrients are associated with incidence of As-related keratotic skin lesions in Bangladesh. J. Nutr. 2012;142(12):2128–2134. doi: 10.3945/jn.112.165720.
- Tay CH. Cutaneous manifestations of As poisoning due to certain Chinese herbal medicine. Australas J Dermatol. 1974;15:121-31. doi: 10.1111/j.1440-0960.1974.tb00546.x.
- Moon K, Guallar E, Navas–Acien A. As Exposure and Cardiovascular Disease: An Updated Systematic Review. Curr Atheroscler Rep. 2012;14(6):542–555. doi: 10.1007/s11883-012-0280-x.
- Lee, M.Y. & Griendling, K.K. Redox signaling, vascular function, and hypertension. Antioxid. Redox. Signal, 2008, 10(6), 1045–1059. doi: 10.1089/ars.2007.1986.
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27(11-12):1405–12. doi: 10.1016/s0891-5849(99)00186-0.
- Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. As Exposure and Cardiovascular Disease: A Systematic Review of the Epidemiologic Evidence. Am J Epidemiol. 2005;162(11):1037–1049. doi: 10.1093/aje/kwi330.
- Parvez F, Chen Y, Yunus M, Olopade C, Segers S, Slavkovich V, Argos M, Hasan R, Ahmed A, Islam T, Akter MM, Graziano JH, Ahsan H. As Exposure and Impaired Lung Function. Findings from a Large Population-based Prospective Cohort Study. Am J Respir Crit Care Med. 2013;188(7):813–819. doi: 10.1164/rccm.201212-2282OC.
- Mazumder DN, Haque R, Ghosh N, De BK, Santra A, Chakraborti D, Smith AH. As in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol.. 2000;29(6):1047–52. doi: 10.1093/ije/29.6.1047.
- Campbell JP, Alvares JA. Acute As intoxication. Am. Fam. Physician. 1989;40:93-97. PMID: 2686377.
- Choinierea J, Li Wang. Exposure to inorganic As can lead to gut microbe perturbations and hepatocellular carcinoma. Acta Pharm Sin B. 2016;6(5):426–429. doi: 10.1016/j.apsb.2016.07.011.
- Watanabe T, Hirano S. Metabolism of As and its toxicological relevance. Arch Toxicol. 2013;87(6):969–979. doi: 10.1007/s00204-012-0904-5.
- Zheng L, Kuo CC, Fadrowski J, Agnew J, Weaver VM, Navas-Acien A. As and Chronic Kidney Disease: A Systematic Review. Curr Environ Health Rep. 2014;1(3):192–207. doi: 10.1007/s40572-014-0024-x.
- Renu K, Madhyastha H, Madhyastha R, Maruyama M, Vinayagam SK, Gopalakrishnan AV. Review on molecular and biochemical insights of As-mediated male reproductive toxicity. Life Sciences. 2018; 212(1):37-58. doi: 10.1016/j.lfs.2018.09.045.
- Kim YJ, Kim JM. As Toxicity in Male Reproduction and Development. Dev. Reprod.2015; 19(4):167–180. doi: 10.12717/DR.2015.19.4.167.
- Mondal S, Mukherjee S, Chaudhuri K, Kabir SN, Mukhopadhyay PK. Prevention of As-mediated reproductive toxicity in adult female rats by high protein diet. Pharm Biol. 2013;51(11):1363–1371. doi: 10.3109/13880209.2013.792846.
- Rodr? VM, Jiménez-Capdeville ME, Giordanoa M. The effects of As exposure on the nervous system. Toxicol Lett. 2003;145(1):1-18. doi: 10.1016/s0378-4274(03)00262-5.
- Ramos-Chávez LA, Rendón-López CRR, Zepeda A, Silva-Adaya D, Del Razo LM, Gonsebatt ME. Neurological effects of inorganic As exposure: altered cysteine/glutamate transport, NMDA expression and spatial memory impairment. Front Cell Neurosci. 2015;9: 21. https://doi.org/10.3389/fncel.2015.00021.
- Tolins M, Ruchirawat M, Landrigan P. The Developmental Neurotoxicity of As: Cognitive and Behavioral Consequences of Early Life Exposure. Annals of Global Health. 2014;80(4):303-314. doi: 10.1016/j.aogh.2014.09.005.
- Kumar R, Banerjee TK. Arsenic induced hematological and biochemical responses in nutritionally important catfish Clarias batrachus (L.). Toxicol Rep. 2016;3:148-152. doi: 10.1016/j.toxrep.2016.01.001.
- Winski SL, Carter DE, Arsenate toxicity in human erythrocytes: characterization of morphologic changes and determination of the mechanism of damage. J Toxicol Environ Health. 1998;53(5):345-355. doi: 10.1080/009841098159213.
- Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ Health. 2013;12:73.
- Ahmed S, Moore SE, Kippler M, Gardner R, Hawlader MDH, Wagatsuma Y, Raqib R, Vahter M. Arsenic Exposure and Cell-Mediated Immunity in Pre-School Children in Rural Bangladesh. Toxicol Sci. 2014; 141(1):166–175. doi: 10.1093/toxsci/kfu113.
- Conde P, Acosta-Saavedra LC, Goytia-Acevedo RC, Calderon-Aranda ES. Sodium arsenite-induced inhibition of cell proliferation is related to inhibition of IL-2 mRNA expression in mouse activated T cells. Arch Toxicol. 2007;81(4):251–9.
- Raqib R, Ahmed S, Ahsan KB, Kippler M, Akhtar E, Roy AK, Lu Y, Arifeen SE, Wagatsuma Y, Vahter M. Humoral Immunity in Arsenic-Exposed Children in Rural Bangladesh: Total Immunoglobulins and Vaccine-Specific Antibodies. Environ Health Perspect. 2017;125(6):067006. doi: 10.1289/EHP318.
- Attreed SE, Acien AN, Heaney CD. Arsenic and Immune Response to Infection During Pregnancy and Early Life. Curr Environ Health Rep. 2017;4(2):229–243. doi: 10.1007/s40572-017-0141-4.
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic Exposure and Type 2 Diabetes: A Systematic Review of the Experimental and Epidemiologic Evidence. Environ. Health Perspect. 2006;114(5):641–648. doi: 10.1289/ehp.8551.
- Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicology and Applied Pharmacology. 2004;198(3):424–433. doi: 10.1016/j.taap.2003.10.026.
- Aposhian HV. Biochemical toxicology of arsenic. In: Reviews in Biochemical Toxicology (Hodgson E, Bend JR, Philpot RM, eds). Amsterdam: Elsevier. 1989, 10, 265–289.






