A Review of Defluoridation Techniques of Global and Indian Prominence
DOI: http://dx.doi.org/10.12944/CWE.17.1.5
Copy the following to cite this article:
Sudhakar V, Naik S. S. Pretreatment, Hydrolysis And Fermentation of Lignocellulosic Biomass for Bioethanol. Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.5
Copy the following to cite this URL:
Sudhakar V, Naik S. S. Pretreatment, Hydrolysis And Fermentation of Lignocellulosic Biomass for Bioethanol. Curr World Environ 2022;17(1). Available From:
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 13-07-2021 |
|---|---|
| Accepted: | 14-03-2022 |
| Reviewed by: | 
 Ghanim Hassan
Ghanim Hassan
|
| Second Review by: |

 Grigorios Kyriakopoulos
Grigorios Kyriakopoulos
|
| Final Approval by: | Dr. Rui Alexandre Castanho |
Introduction
Fluoride due to its significant effect on human wellbeing has drawn worldwide attention. Exposure to the high dose of fluoride over a long period result in various health symptoms like dental fluorosis (stained and chipped teeth), skeletal fluorosis (deformed and stiff bones) and other non-skeletal symptoms, ranging from gastric to reproductive systems. The population of poor and underdeveloped regions like Africa and Asia represent the major population suffering from fluorosis. Besides, millions of people living in these countries are exposed to fluoride-rich water due to the unavailability of infrastructure and resources resulting in morbidities and sufferings. Since fluorosisis prevalent in almost all parts of the world and over the years, there has been limited success in the treatment of the health effects and avoiding exposure to this hazardous substance remains the only viable approach. Therefore, there is an imperative need to find a solution to reduce fluoride contamination in groundwater.There are many sources of fluoride exposure for humans however groundwater has been a major exposure route due to the global spread and dependence of people on groundwater, especially in the rural areas.Due to excessive fluoride presence in groundwater, public health is at stakeglobally. Since drinking water is the major route of fluoride exposure, so to control harmful health effects of fluoride it is necessary to control fluoride in the drinking water (Table 1).
Table 1: Concentration of Fluoride in Drinking Water and Its Effect on Health.
|
Concentration of Fluoride (mg/L) |
Impact on Health |
|
0.0 − 0.5 |
Limited growth and fertility, dental caries |
|
0.5 − 1.5 |
Promotes dental health, prevents tooth decay |
|
1.5 − 4.0 |
Dental fluorosis (mottling of teeth) |
|
4.0 − 10.0 |
Dental & skeletal fluorosis (pain in back and neck bones) |
|
> 10. |
Crippling fluorosis |
Besides groundwater, fluoride is also contributed by many industrial processes which release a large amount of wastewater that needs to be treated before discharging it into streams and rivers (Fig. 1).
Removal of fluoride ion from drinking water (defluoridation) is one of the major global challenges that the world is facing today. Though efforts are being made at individual levels to reduce fluoride contamination in potable water, the real challenge is to find a technology or process to remove it effectively and in cost effective manner as this problem is specifically concentrated among poor and marginalized people living in undeveloped regions of the world or in areas where there is no easily available alternative source.
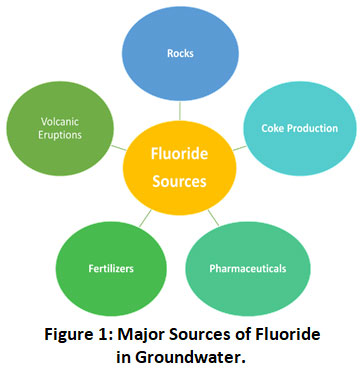 |
Figure 1: Major Sources of Fluoride in Groundwater. Click here to view Figure |
The present review discusses about th edefluoridation techniques being used in various countries of the world with focus on the advantages and disadvantages of each technique.
Earlier Defluoridation was Largely Classified into adsorptive and additive methods.1
In adsorptive method, surface interaction between adsorbent coated surface and water containing fluoride results in adsorption of fluoride on to adsorbent while water passes through the surface. Whereas in later method certain chemicals or substances are added to fluoride-containing water that reacts with each other under specific conditions and forms removable insoluble complexes. However, various new methods and techniques in addition to these two have been developed in recent decades with different advantages and shortcomings such as membrane filtration, electrodialysis, advanced oxidation processes (AOPs), membrane distillation and so on.2-6These fluoride removal techniques can be categorized as adsorption, ion-exchange, coagulation-precipitation, membrane process and biological methods.7All these fluoride removal methods have their own limitations and advantages under different working conditions.
Adsorption
Adsorption is a process that can be characterized as the transfer of ions from the solution to the solid phase by different mechanisms (Fig.2). It includes physical adsorption or chemisorption by different processes such as chelation, complexation, ion exchange etc.8
Materials with the porous structure are capable of adsorption and hence are used for adsorption. However, generally those materials are preferred which are extremely microporous and can be controlled such as activated carbon, aluminium, silica, zeolites etc. having pores of desired quality and quantity.9 Besides, properties similar to higher adsorption capacity and ease of regeneration are also desired in an adsorbent.8 Due to its simplistic design, ease of operation, cost-effectiveness, efficiency and reusability, adsorption is one of the most widely considered water defluoridation technique especially for small communities or even for household applications.10 In addition, the use of indigenous materials makes the process even more economical. The efficiency of adsorption depends on various factors such as physico-chemical properties of adsorbent, the dose of the adsorbent, affinity to fluoride ions, initial fluoride concentration and loading capacity.11,12
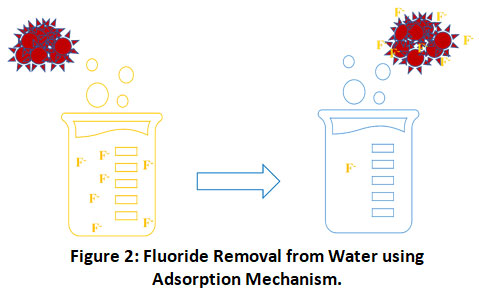 |
Figure 2: Fluoride Removal from Water using Adsorption Mechanism. Click here to view Figure |
However, it has also been observed that over the time adsorbent gets saturated and fluoride removal capacity reduces with every regeneration cycle. This can be attributed to inefficiency to completely remove previously adsorbed material during the desorption process.13 Different types of adsorbents have been tested and are modified to get the desired results. These adsorbents can be broadly classified as oxides and hydroxides, biosorbents, geomaterials, carbonaceous materials, industrial products, and by-products. Table 2 reviews some of the recent studies related to these different adsorption materials and their analysis conditions.
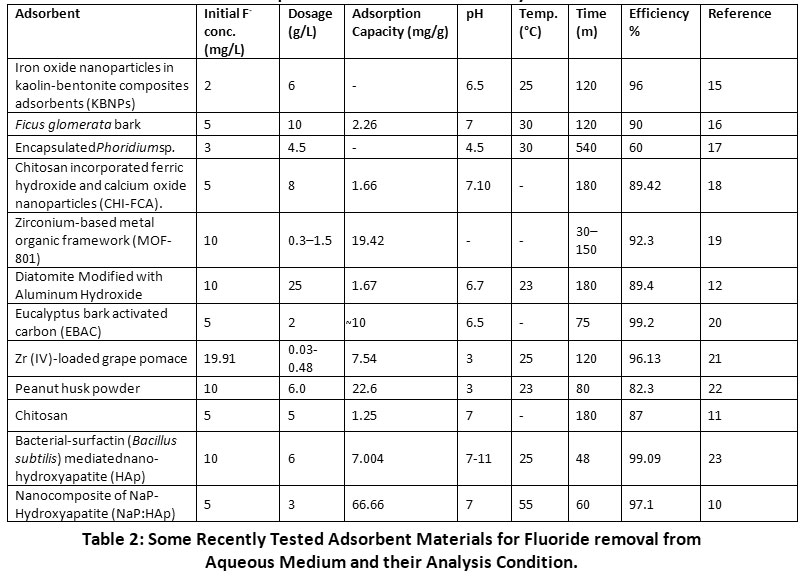 |
Table 2: Some Recently Tested Adsorbent Materials for Fluoride removal from Aqueous Medium and their Analysis Condition. Click here to view Table |
By using secondary factors such as magnetic field, electric fields, irradiation, and ultrasonic waves adsorption process is further being improved. Aigbe14 utilized anticlockwise rotating magnetic field with magnetic nanocomposite for stimulating fluoride adsorption in aqueous solution. Fluoride ion removal was significantly improved, besides with varying magnetic field intensity and different magnetic exposure time, removal of fluoride varied considerably. Nanoparticles with nanoscale properties such as catalytic surface, high reactivity, many active sites, ease of separation and very high surface to volume ratio prove as excellent adsorbents for fluoride removal from water than the conventional adsorbents. Borgohain24 used porous MgO nanostructures as an adsorbent for defluoridation and kinetic studies and observed 90% of fluoride elimination within five minutes only. Highest adsorption capacity (29131 mg/g @ 313 K) for any adsorbent till date was observed in the study. Besides, recycling capacity up to five cycles was observed without losing any performance. Zawar25 established high defluoridation efficiency of CaCO3 nanoparticle (as adsorbent) in drinking water (98-100%) at low cost and in a short time. The adsorbent was prepared along with defluoridation by co-precipitation of calcium chloride and sodium carbonate. Adsorbent showed high adsorption capacity of 725.21 mg/g and that too at room temperatures without any rigid conditions. Several other nano adsorbent materials have been extensively used for fluoride removal such as Al (III) modified calcium hydroxyapatite26, γ-alumina27, nanoporous biochar-supported magnesium oxide.7
Recently adsorption using biological agents has been used in various studies, which utilizes both physicochemical and metabolic pathways for removal of fluoride and other metal ions17,23 Due to micron level size of these microbial cells, surface area for adsorption is very high removing organic and inorganic fluoride species effectively from aqueous solution.28 Halder29 investigated fluoride removal of wastewater using powdered activated carbon developed from the stem of Eichhornia crassipes and observed that it can be used as an economical defluoridation treatment. Use of biological adsorbents like microbial cells is an eco-friendly and cost-effective technique for fluoride removal. It also offers the advantage of easy availability, regeneration of biosorbent, producing very minimal sludge and in many cases metal recovery also.30,31
Ion-exchange
Ion exchange is a water treatment technique that can remove objectionable ions like fluoride along with some other ions such as chloride (having the same charge) which are not harmful or less objectionable (Fig. 3).
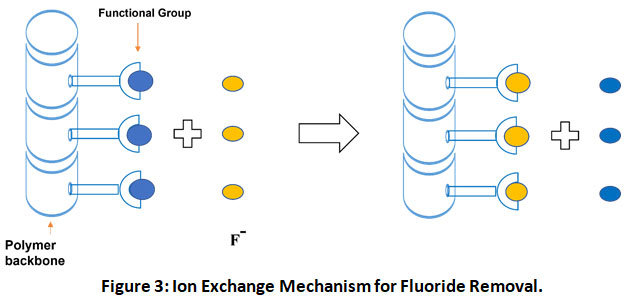 |
Figure 3: Ion Exchange Mechanism for Fluoride Removal. Click here to view Figure |
It has been a conventional fluoride removal process for many years. Ion exchange materials are insoluble in water and hold the replaceable ions loosely which are used for exchanging ions from solution.9 Ion exchange materials can be classified as natural and synthetic. Natural materials include cellulose, living cells, proteins, and certain soil particles whereas synthetic materials can be further classified as membranes and beaded polymer resins. Depending on the functional group attached to the matrix, ion exchange resins can be categorized into anionic and cationic types. Anion exchangers such as inorganic metallic oxides exchange negatively charged ions (like fluoride) whereas cation exchangers such as zeolites exchange positively charged ions from the solution.32 These resins have small porous beads and are insoluble in most organic solvents and water.
Due to weak binding force exchanged ion is weakly attached with the base and can be easily reversed by another chosen ion, passing through a functional group.33 Water flows down through ion exchange packed column, which attaches the desired ions to be removed and as the resin gets saturated, it is back washed with mild acid or alkali solution. In case of fluoride anion exchange resins with quaternary ammonium, functional groups are used which replace fluoride with chloride attached to these functional groups and on saturation the resin is back washed with supersaturated sodium chloride salt.34 The replacement of chloride in resin with fluoride of the solution is caused by stronger electronegativity of fluoride ions.35Indion FR 10, a commercial ion exchanges resin and an anion exchanger, Ceralite IRA 400, for replacing chloride ions has been found to have efficiency up to 95% .36 Ion exchange has been an effective process for fluoride removal due to its simplicity in removing ionic contaminants. Strong anion-exchange resins have been known to remove up to 95% of fluoride ions from aqueous solutions. However, ion exchange resins are exhaustive, require longer reaction time and need frequent regeneration and generate a large volume of wastewater which makes it unattractive.37 Besides, requirement of large volume of regenerate for regeneration of ion exchange resin also limits usage of this technique.
Precipitation - Coagulation
Precipitation by coagulation is another economical and efficient method for water defluoridation in which charged particles of suspension are neutralized and agglomerate to settle down (Fig. 4). pH and temperature of the solution are the key aspects of the precipitation process and therefore addition of certain chemicals or reducing solution temperature makes solution unstable and aids in precipitation.38 Chemicals as ferric chloride, ferrous sulfate, lime, potash alum and sodium bicarbonates are commonly used chemicals for precipitation.39 However, for fluoride removal, conventionally alum (Al2 (SO4)3.18H2O) and lime (Ca(OH)2) have been the most extensively used coagulants.40 But its major disadvantage is the inability to reduce fluoride under WHO prescribed permissible limits.8 However, today various modification and new technologies for coagulation are developing which have shown good results similar to the use of plant-based coagulants, electrocoagulation, metal ion-assisted electrocoagulation.41 It has also been observed that due to high chemical demand, operational cost is generally very high and the process generates lots of aluminium-containing toxic sludge.32 The Nalgonda technique developed in India is one of the best examples of this technique. These days’ natural coagulants like plant-based materials are also examined for their sludge free and eco-friendly nature. Gandhi and Srisha42examined the fluoride removal using a natural coagulant Passiflora foetida fruits. High fluoride removal efficiency was observed at acidic and neutral pH mediums which gradually decreased with increasing alkalinity. It was also observed that the coagulant worked best for low initial fluoride concentrations.
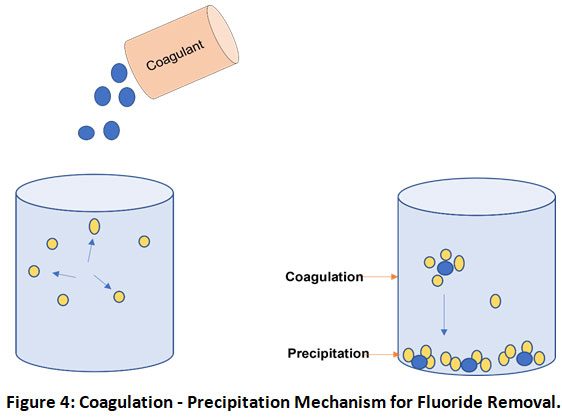 |
Figure 4: Coagulation - Precipitation Mechanism for Fluoride Removal. Click here to view Figure |
Suspended and dissolved solids from a liquid can also be removed by passing an electric current through the solution, which destabilizes the solids and aids in settling.43 Electrodes used in electrocoagulation are made of sacrificial metals such as aluminium for fluoride which produce flocs of trivalent aluminium hydroxide on supplying electricity.44 Unlike other coagulating methods producing lots of sludge, electrical conductivity (EC) generates minimal sludge, besides there is no need to add chemicals which make it a favorable defluoridation option. Graca7 used aluminium electrodes for fluoride removal by continuous electrocoagulation process and proved that the setup can remove 97% of fluoride from the water. All the operating variables like flow rate, current intensity, electrodes configuration etc. were observed to have a significant effect on fluoride removal. In another study, Mena45 effectively reduced fluoride-rich groundwater from 6.02- 8.98 mg/L to well below permissible limits of 1.5 mg/L using EC from a volcanic source at a low operating cost of 0.20 and 0.26 €/m3. However, scale formation on the electrode and alkalization of treated water were observed as the shortcomings of the EC.
Membrane Process
Membrane processes providing pure and ultrapure water are highly efficient advanced defluoridation technologies using a semi-permeable membrane between adjacent phases for removing water contaminants such as fluoride (Fig. 5).46 These membranes can be categorized as natural (cellulose acetate, cellulose triacetate) and synthetic (polysulfone, polyamide) depending on the type of material used. Membrane’s pore size and material depend upon the material to be segregated. Based on techniques used to separate fluoride using membranes the process can be further divided into subtypes such as reverse osmosis, nanofiltration, ultrafiltration, dialysis, electrodialysis.
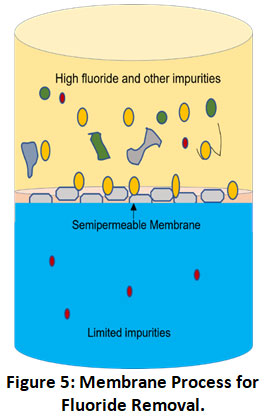 |
Figure 5: Membrane Process for Fluoride Removal. Click here to view Figure |
Reverse Osmosis (RO) is a physical method in which the water is forced under pressure to pass through a selectively sized semi-permeable membrane to separate the dissolved solids and other unwanted impurities from water for household and industrial purposes. RO has also been used for defluoridation in drinking and industrial water. Membranes used in reverse osmosis vary depending on the type of water to be treated, economic considerations, and working conditions such as temperature, pressure and membrane recovery.46Also, there is maintenance issue in membrane filtration because of fouling by particulate matterand plugging issues.However, RO is affected by various parameters like ionic strength, type of ionic exchange membrane used, pH, presence of co-existing anions and applied potential etc.47 Nevertheless, RO is not affected by initial concentration in water as up to 90% of fluoride can be removed using reverse osmosis.48
Nanofiltration is another type of convenient membrane process operating comparatively at lesser pressure and capacity. Nanofilteration membranes have more permeability than RO. Other advantages include cost-effective installation, operation and maintenance.49 Nevertheless, it proves inefficient with high fluoride waters besides membrane concentrate disposal remains a major concern.50 Separation of fluoride using RO and NF is controlled by size exclusion (steric effect) instead of charge as observed with electrodialysis. Besides, the pore size of nano-filters is larger as compared to those in membranes of RO and accordingly, less pressure is required for nanofiltration and many essential minerals are retained to some extent.48Nasr2examined the performance of NF5 and NF9 two commercial nano-filter membranes and these were observed to effectively remove fluoride ions over chloride ions. It was also observed that certain ions act as interference in fluoride removal in groundwater filtration. Similarly, Emamjomeh51investigated the efficiency of FILMTEC-NF90-4040 membrane under different temperature and pressure conditions in fluoride removal from groundwater. It was observed as an efficient fluoride removal membrane, besides, it was also observed that temperature and pressure considerably affect the efficiency of nanofiltration by this membrane. Despite many advantages of RO and nanofiltration over other fluoride removal methods, membrane in these methods suffer from fouling.
Donnan Dialysis (DD) is one of the simplest membrane separation method of ions from dilute solutions using concentration gradient or concentration difference as the driving force. It is simple, economical, and energy-efficient process although operates very slowly compared to other methods like RO and electrodialysis. Besides, it does not require regeneration and can operate in continuity for a longer duration. Boubakri52, attained an efficiency of 75.52% fluoride separation at optimum conditions using this technique. However, this technique is expensive and has reduced efficiency in saline waters.
Electrodialysis (ED) is direct current (electricity) driven electrochemical membrane technology, for separating ions (like fluoride) instead of using pressure as in reverse osmosis.53 Depending on the charge of permeating ions, membranes used in ED can be classified as anion and cation exchange membranes. In a constant electric field, ions are separated through ion exchange membranes as anions migrate to the anode and can pass through anion exchange membrane; however, they cannot transport through cation exchange membranes and vice versa for cations, forming dilute and concentrate streams.54Many workers have used electrodialysis for fluoride removal from groundwater and effluents.3,4,54Ahmed53carried out fluoride removal using conventional electrodialysis in fluoride-rich (0.8 and 4 mg/L) water of Tunisia. Very high defluoridation rate up to 92% was observed using batch recirculation mode, besides, it was also efficient in reducing the salinity of the water.
In general membrane processes suffer from the issue of brine and sludge disposal, interference with other ions, and are economically unsuitable.55 However, compared to reverse osmosis and nano-filtration, electrodialysis is energy efficient, causes minimum water wastage, generates minimal residues and due to lesser fouling has a long membrane life.47 Besides, it is simple, requires minimum chemicals and has an average installation cost.56
Biological Defluoridation
Biological defluoridation includes the use of living organisms like bacteria, algae, fungi and even higher plants for fluoride removal from water, sludge and polluted soil to acceptable levels (Fig. 6).57
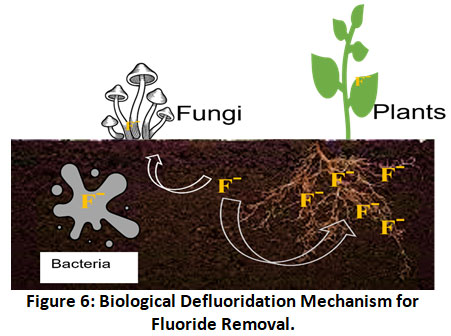 |
Figure 6: Biological Defluoridation Mechanism for Fluoride Removal. Click here to view Figure |
Out of these, bacterial and phytoremediation are most used techniques of biological remediation. Unlike conventional methods of defluoridation biological methods are eco-friendly, economical, and most sustainable.58 Like humans and other plants and animals, fluoride affects bacterial cells in various ways and fluoride toxicity eliminate up to 80% of bacterial biomass from soil.59 However, various fluoride-resistant bacteria species are reported to metabolize fluoride and other heavy metals from soil and water.60 Many of these bacterial species have been utilized in various studies for effectively removing fluoride from water and soil (Table 3).
Table 3: Comparative Illustration of Defluoridation by Different Bacteria and their Operational Conditions.
|
Species |
pH |
Temp. |
Incubation |
Accumulated Fluoride % |
References |
|
Pseudomonas putida |
7 |
37 |
24 hours |
93.4 |
5 |
|
Paenibacillussp. |
8 |
40 |
8 days |
73.3 |
61 |
|
Staphylococcus lentus |
7 |
35 |
24 hours |
91.8 |
62 |
|
Bacillus flexus |
7 |
35.5 |
- |
82-86 |
63 |
|
Acinetobacter RH5 |
7 |
30 |
8 days |
25.7 |
64 |
For the restoration of contaminated environments use of plants (phytoremediation) is another most promising and environmentally sustainable solution.65 It can be applied for remediation of large-scale areas where other techniques are unproductive and uneconomical as it does not require sophisticated equipment and trained personnel.66 Moreover, it is an emerging and sustainable technology with long-term applicability.
However, very limited studies of fluoride removal by phytoremediation have been undertaken. Generally, hyper-accumulator plants showing appreciable fluoride accumulation with little toxicity are preferred for fluoride removal.67Besides, plants with a fibrous root system and higher biomass are best suited for phytoremediation e.g. trees over herbs and shrubs. They accumulate through their roots and transport the contaminants to other aboveground parts for storage.68 Khandare69 attempted fluoride removal using garden ornamentals like Neriumoleander, Portulaca oleracea and Pogonatherumcrinitum and observed positive results especially with Nerium oleander (92%) compared to other plant species. In a comparative study by Karmakar70 on the efficiency of three aquatic plants for fluoride removal at low fluoride contamination, maximum efficiency was observed in Pistia stratiotes (19.87%) followed by Spirodelapolyrhiza (19.23%) and Eichhornia crassipes (12.71%). Similarly, Baunthiyal and Sharma71 evaluated defluoridation potential of some hydrophytes in aquatic environment viz. Cladophora glomerata, Hydrilla verticillata and Chara coralline. Out of these, Chara coralline proves better compared to other macrophytes.
Integrated Approaches
To treat fluoride-rich groundwater and industrial effluents instead of single technique a combination of treatments provides promising results. Biological treatments are generally combined with electrocoagulation as it acts as a pretreatment by detoxifying the wastewater and removing the inhibiting dissolved materials, besides, it augments the microbial activity increasing the overall efficiency and performance of treatment.72 An integrated approach of fluoride removal using simultaneous adsorption (Citrus limetta and leaves of Ficus religiosa) and bioaccumulation (Gram-Negative Bacteria Shewanellaputrefaciens) revealed that removal efficiency of simultaneous treatment was maximum followed by bioaccumulation and adsorption.73 Similarly, Mohammad and Kumar74 tested immobilized Actinobacter on the surface of sweet lemon peel to examine the simultaneous effect of adsorption and bio-accumulation process for fluoride removal. It was observed that fluoride removal was mainly caused by bioaccumulation (Actinobacter) than adsorption (lemon peel) but it was also observed that bioremoval is a comparatively slower process. Chee75 observed greater fluoride removal using natural (Moringa oleifera seed and eggshell) and chemical (Ecogent F-Loc) coagulants in combination as compared to individual coagulants. Similarly, Jadhao76 examined the combined effect of chemical precipitation (gypsum plaster) and electrocoagulation in defluoridation of wastewater. The efficiency of fluoride removal was proved to be enhanced due to the formation of Ca-F bonds.
Defluoridation Methods Adopted around the World
Several defluoridation methods have been adopted for remediating fluoride contaminated groundwater in different countries. One of the most extensively used defluoridation method employed for fluoride removal in underground mine water of South Africa during the 1980s was use of activated alumina. The technique was successful to reduce fluoride levels down to 1mg/L from 8mg/L.77Knowledge of fluoride treatment using alum in Egypt dates to 1500 BC, however, its use in Ethiopia, Kenya and Tanzania were reported during the 1980s only after learning from experiences of the USA (1930s) and India (1970s-1980s). Two community units of Nalgonda technique were set up in central Ethiopia, which was functional for almost 40 years. However, their efficiency was low (60%) owing to ageing and maintenance, besides activated alumina which was imported from overseas was not economical.78Besides, due to certain shortcomings in the Nalgonda technique and easy availability of bone char defluoridation in these countries focus shifted to bone char technology.79However, bone char is considered to be unhygienic as it harbours bacteria and is less acceptable due to religious and cultural objections.In rural areas of the United Republic of Tanzania (Arusha region), contact precipitation-based plants installed in various schools and households have shown good results. In this technique, fluoride is precipitated in presence of bone char, after the addition of compounds of calcium and phosphate.80 The USA, from 1940 to 1960 also adopted defluoridation on the field using the bone charcoal technique which is one of the earliest practiced defluoridation techniques.81 One such defluoridation plant was set up in Britton, SD, with an exchange capacity of 102 g fluoride/m3 with waters having an initial fluoride concentration of 5 mg/L.82
In Thailand, reverse osmosis-based systems have been installed in more than twelve hundred villages for fluoride removal. However, owing to the high installation and maintenance costs, these are not sustainable and many rural areas are still exposed to high fluoride waters.83Besides, bone char-based systems were also tried successfully in Thailand.8 Clay column filters (based on up-flow) have been used successfully for defluoridation in Sri Lanka. About fourteen hundred of such clay brick let filled domestic filters have been installed in around sixty villages and almost eighty percent were found in operational condition for more than two years.84
Recently, nanofiltration has developed as one of the well-established water treatment processes and it has also been instrumental for water defluoridation. Finland successfully implemented a nanofiltration plant with a capacity of 380–600 m3/day for fluoride removal (efficiency-76%) from groundwater.85 Many other techniques for fluoride removal are practiced successfully around the globe at different scales however the required information is not available due to the limited literature published.
Defluoridation Methods Adopted in India
India is one of the severely affected countries with fluoride and there has been a constant demand for economical and effective defluoridation technique. Indian workers have also contributed to the development of many novel techniques which have shown promising results as discussed above and can be modified according to India conditions and materials for even better results. However, very limited approaches have been translated to the field, which have shown positive results. These include:
Nalgonda Technique
It is a precipitation-coagulation-based technique developed in 1961 by the National Environment Engineering Research Institute, Nagpur after testing many materials. It has been successfully applied to the field from the laboratory and has been used both at community and household levels nationally as well as internationally. It involves the addition of lime, alum and bleaching powder followed by brisk mixing, flocculation, sedimentation, and filtration. Addition of alum and lime helps in the formation and settling of flocs of aluminium hydroxide and bleaching powder is used for disinfection. Entire operation requires about 2-3 hours and many batches can be purified in a day.8Over the years this technique has been widely used and modified and chemicals required are also cheaply and easily available. However, due to the need for regular mixing, it is highly work-intensive. Besides, there are complaints of undesirable taste in water and risk of exposure of aluminium, having very low (0.2 mg/L) permissible limits which can cause adverse health effects such as dementia.86 At the community level, it was first started in Kadri town (Nalgonda) of Andhra Pradesh.
Algona Technique
Algona technique was developed in India by National Environmental Engineering Research Institute (NEERI) by using polymeric aluminum hydroxide and calcium salts. The Algona technique has an advantage over Nalgonda as less concentration of aluminium hydroxide is required and the further modification of this technique by using poly aluminum chloride increases its efficiency and is more cost-effective.
KRASS Technique
It has been established by PHE (Public Health Engineering) of Rajasthan and CSIR (Council of Scientific & Industrial Research). In this process, contaminated water is passed through specially designed filter media to remove fluoride from water. It is an adsorption-based process and 10% alum solution is used for recharging the column. One kilogram of alum is found to remove about 4 grams of fluoride using this technique. Besides, the technique is found to work best at the fluoride concentration of 11-12 mg/L and 7-8 pH range.87
Prasanti Technique (Activated Alumina)
This technique has been in use for defluoridation in many Indian villages. It was developed by Satya Sai University (Bioscience Department) at Prasanti Nilayam, Andhra Pradesh.88 In Udaipur (Rajasthan), activated alumina [(Al2O)3; a porous and solid form of aluminium oxide] has been used by Sarita Sansthan supported by UNICEF, which are providing buckets with micro-filters and activated aluminium for fluoride removal. However, it has also some limitations like need to have trained people for reactivation of micro-filters, generation of aluminium byproducts as residue and costly operational and maintenance cost.87
IISc Method
Developed by the Indian Institute of Science (IISc), Bangalore it is a precipitation-based fluoride removal technique that uses chemicals such as magnesium oxide, sodium bisulphate and lime. Fluoride in water reacts with magnesium oxide, forming insoluble magnesium fluoride which precipitates out of the solution. Sodium bisulphate adjusts the pH, disturbed by the addition of magnesium oxide and lime removes bicarbonate interference of sodium bisulphate.86
Due to the huge population and scattered habitations, it is very difficult to address the fluoride menace through community-based systems in India. However, the Government of India (GoI) has also been striving for providing fluoride-free water to the public. In 1986, GoI launched ‘Control of Fluorosis’, a technology mission on safe drinking water. Similarly, in rural areas, under Rajiv Gandhi Drinking Water Mission, many hand pump attached-fill and draw (F&D) plants have been developed (by NEERI, Nagpur) based on Nalgonda technique.89 Besides, various projects have been launched from time to time in different states of India such as Project SARITA in Dungarpur, Rajasthan which is based on Nalgonda technique; fluorosis mitigation in Nalgonda, Andhra Pradesh based on bone char based defluoridation techniques; Hogenakkal water supply and fluorosis mitigation project etc. Similar projects have been launched in Odisha, Karnataka, Madhya Pradesh and Uttar Pradesh also.90 In a significant move, GoI has recently launched Jal Jeevan Mission which aims to provide safe and adequate piped water connections to all households in rural areas by 2024.
Conclusion and Recommendations
This literature review clearly shows that fluoride contamination in groundwater is a global challenge and today fluorosis is ubiquitous in almost all parts of the world affecting millions of people directly or indirectly. There has been tremendous progress in the fluoride removal technologies over the years using different approaches in different countries of the world. Although all the discussed methods have proved to be efficient for fluoride removal in a particular setup but till date there has not been a single technique developed for defluoridation that can claim to be practically viable solution for fluoride reduction throughout. In India and some other countries, precipitation-coagulation-based techniques such as the Nalgonda technique have proved somewhat promising especially in rural set-up and decentralized water sources in some communities. However, these methods need to be customized for better results and to be applied throughout the country. Thus, the development of efficient and appropriate defluoridation techniques which can be applied universallyis the need of the hour and requires extensive research in this field.
Acknowledgments
The authors sincerely thank the support extended by Head, the Department of Environmental Science, University of Jammu for providing the necessary facilities for the present work.
Funding Source
Financial assistance provided by Science and Engineering Research Board (SERB) under Grant no. EMR/2016/006400 is gratefully acknowledged.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Ingle N. A., Dubey H. V., Kaur N., Sharma I. Defluoridation techniques: Which one to choose. Journal of Health Research 2014;1(1).
- Nasr A. B., Charcosset C., Amar R. B., Walh K. Defluoridation of water by nanofiltration. Journal of Fluorine Chemistry 2013;150: 92–97.
- Gmar S., Ben Salah Sayadi I., Helali N., Tlili M., Amor M. B. Desalination and defluoridation of tap water by electrodialysis. Environmental Processes 2015;2: 209–222. https://doi.org/10.1007/s40710-015-0112-4
- Belkada F. D., Kitous O., Drouiche N., Aoudj S., Bouchelaghem O., Abdi N., Grib H., Mameri N. Electrodialysis for fluoride and nitrate removal from synthesized photovoltaic industry wastewater. Separation and Purification Technology2018;204:108–115. https://doi.org/10.1016/j.seppur.2018.04.068
- Chandana Lakshmi M. V. V., Poornima D., Karishma N. S., VardhanY., Jyothi P. S. Removal of fluoride using Pseudomonas Putida. Journal of Biotech Research & Biochemistry 2019;2, 3.
- Graca N. S., Ribeiro A. M., RodriguesA. E. Removal of fluoride from water by a continuous electrocoagulation process. Industrial & Engineering Chemistry Research 2019;58: 5314-5321.
- Wan S., Lin J., Tao W., Yang Y., Li Y., He F. Enhanced fluoride removal from water by nanoporous biochar-supported magnesium oxide. Industrial & Engineering Chemistry Research2019;58(23): 9988–9996. https://doi.org/10.1021/acs.iecr.9b01368
- Ayoob S., Gupta A. K., Venugopal T. B. A conceptual overview on sustainable technologies for the defluoridation of drinking water. Critical Reviews in Environmental Science and Technology 2008;38(6): 401-470.
- Sharma S., BhattacharyaA. Drinking water contamination and treatment techniques. Applied Water Science2017; 7: 1043–1067. https://doi.org/10.1007/s13201-016-0455-7
- Zendehdel M., Shoshtari-Yeganeh B., Khanmohamadi H., Cruciani G. Removal of fluoride from aqueous solution by adsorption on NaP:HAp nanocomposite using response surface methodology. Process Safety and Environmental Protection 2017;109:172–191. https://doi.org/10.1016/j.psep.2017.03.028
- Akbari H., Jorfi S., Mahvi A. H., Yousefi M., Balarake D.Adsorption of fluoride on chitosan in aqueous solutions: Determination of adsorption kinetics. Fluoride2018;51:319–327.
- Akafu T., ChimdiA., Gomoro K. Removal of Fluoride from Drinking Water by Sorption Using Diatomite Modified with Aluminium Hydroxide. Journal of Analytical Methods in Chemistry2019; 1–11.
- Vivek Vardhan C. M., Srimurali M. Removal of fluoride from water using novel sorbent lanthanum-impregnated bauxite. SpringerPlus 2016; 5(1):14-26. https://doi.org/10.1186/s40064-016-3112-6
- Aigbe U. O., Onyancha R. B., Ukhurebor K. E., ObodoK. O. Removal of fluoride ions using a polypyrrole magnetic nanocomposite influenced by a rotating magnetic field.Royal Society of Chemistry, Advances2020;10(1); 595–609. https://doi.org/10.1039/c9ra07379e.
- Annan E., Nyankson E., Agyei-Tuffour B., Armah S. K., Nkrumah-Buandoh G., HodasiJ. A. M., Oteng-Peprah M. Synthesis and Characterization of Modified Kaolin-Bentonite Composites for Enhanced Fluoride Removal from Drinking Water. Advances in Materials Science and Engineering2021: 1-12.
- George A. M., Tembhurkar, A. R.Taguchi.Experimental design for adsorptive removal of fluoride from water using novel Ficus glomerata Bark-developed biosorbent. International Journal of Environmental Science and Technology2020;https://doi.org/10.1007/s13762-020-02787-w
- Mittal Y., Srivastava P., Kumar N., Yadav A. K. Remediation of fluoride contaminated water using encapsulated active growing algae. Environmental Technology & Innovation 2020;100855. https://doi.org/10.1016/j.eti.2020.100855
- SenguptaP., SahaS., Banerjee S., Dey A., Sarkar P. Removal of fluoride ion from drinking water by a new Fe(OH)3/ nano CaO impregnated chitosan composite adsorbent. Polymer-Plastics Technology and Materials2020;1191-1203. https://doi.org/10.1080/25740881.2020.1725567
- Tan T. L., Krusnamurthy P. A., Nakajima H., Rashid S. A. Adsorptive, kinetics and regeneration studies of fluoride removal from water using zirconium-based metal organic frameworks. Royal Society of Chemistry, Advances 2020;10(32):18740–18752. https://doi.org/10.1039/d0ra01268h..
- Mahvi A. H., Mostafapour F. K., Balarak D. Adsorption of fluoride from aqueous solution by eucalyptus bark activated carbon: Thermodynamic analysis. Fluoride 2019; 52: 562–568.
- Zhang, Y., & Huang, K. Grape pomace as a biosorbent for fluoride removal from groundwater. Royal Society of Chemistry, Advances 2019;9(14):7767–7776. https://doi.org/10.1039/c9ra00109c
- Abdisa G. J. Preparation and evaluation of adsorption effectiveness of peanut husk for the removal of fluoride ion from aqueous solution. Modern Chemistry &Applications2018; 6(3):261-266.
- Maity J. P., Hsu C. M., Lin T. J., Lee W. C., Bhattacharya P., Bundschuh J., Chen C. Y. Removal of fluoride from water through bacterial-surfactin mediated novel hydroxyapatite nanoparticle and its efficiency assessment: Adsorption isotherm, adsorption kinetic and adsorption thermodynamics. Environmental Nanotechnology, Monitoring & Management 2018;9: 18–28. https://doi.org/10.1016/j.enmm.2017.11.001
- Borgohain X., Boruah A., Sarma G. K., Rashid M. H. Rapid and extremely high adsorption performance of porous MgO nanostructures for fluoride removal from water. Journal of Molecular Liquids 2020;112799. https://doi.org/10.1016/j.molliq.2020.112799
- ZawarM., Nazir R., Hamid A., Lima E. C., Shah M. R. Rapid defluoridation of drinking water by calcium carbonate nanoadsorbent: characterization, adsorption studies and application to real samples’ treatment. Water supply2020; 20: 667-678.
- Nie Y., Hu C., Kong C. Enhanced fluoride adsorption using Al (III) modified calcium hydroxyapatite. Journal of Hazardous Materials 2012;233: 194–199.
- Chinnakoti P., Chunduri A. L. A., Vankayala R. K., Patnaik S., Kamisetti V. Enhanced fluoride adsorption by nano crystalline γ-alumina: adsorption kinetics, isotherm modeling and thermodynamic studies. Applied Water Science2017;7(5): 2413–2423. https://doi.org/10.1007/s13201-016-0437-9
- Aksu Z., Tatli A. Í., Tunç Ö. A comparative adsorption/biosorption study of Acid Blue 161: Effect of temperature on equilibrium and kinetic parameters. Chemical Engineering Journal 2008;142: 23–39. https://doi.org/10.1016/j.cej.2007.11.005
- Halder G. Sinha K., Dhawane S. Defluoridation of wastewater using powdered activated carbon developed from Eichhornia crassipes stem: Optimization by response surface methodology. Desalination and Water Treatment2014;1-14. https://doi.org/10.1080/19443994.2014.942375
- AhalyaN., Ramachandra T. V., Kanamadi R. D.Biosorption of heavy metals. Research Journal of Chemistry & Environment2003;7: 20–30.
- Mohan S. V., Ramanaiah S. V., Rajkumar B., Sarma P. N. Removal of fluoride from aqueous phase by biosorption onto algal biosorbent Spirogyra sp.-IO2: sorption mechanism elucidation. Journal of Hazardous Materials2007;141: 465–474.
- Yadav K. K., Gupta N., Kumar V., Khan S. A., Kumar A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environment International 2018;111:80–108. https://doi.org/10.1016/j.envint.2017.11.014
- Neumann S., Fatula P. Principles of ion exchange in wastewater treatment. In Asian Water. Techno Focus. 2009; March 18. Retrieved July 17, 2020, from https://www.academia.edu/5390275/090316_asian_water_principles_of_ion_exchange_neumann_03_09
- Raghav, S., Nair, M., & Kumar, D. Tetragonal prism shaped Ni-Al bimetallic adsorbent for study of adsorptive removal of fluoride and role of ion-exchange. Applied Surface Science 2019;143785. https://doi.org/10.1016/j.apsusc.2019.143785
- Razbe N., Kumar R., Kumar P. R. Various options for removal of fluoride from drinking water. Journal of Applied Physics2013;3:40-47.
- WangJ., Lin X., Luo X., Long Y. A sorbent of carboxymethyl cellulose loaded with zirconium for the removal of fluoride from aqueous solution. Chemical Engineering 2014; 252:415–422. https ://doi.org/10.1016/j.cej.2014.05.008
- Kumar A., Balouch A., Abdullah.Remediation of toxic fluoride from aqueous media by various techniques. International Journal of Environmental Analytical Chemistry2019;1–24. https://doi.org/10.1080/03067319.2019.1669580
- Adhikari S., Mandal S., Kim D. H., Mishra A. K. An overview of treatment technologies for the removal of emerging and nanomaterials contaminants from municipal and industrial wastewater. In A. K. Mishra, M. D. A. Hossain, N. Drouiche (Eds.), Emerging and Nanomaterial Contaminants in Wastewater: Advanced Treatment Technologies (pp. 3–40). Elsevier; 2019.
- Aziz H. A., AdlanM. N., Ariffin K. S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresource Technology2008; 99(6):1578–1583. https://doi.org/10.1016/j.biortech.2007.04.007
- Nawlakhe W. G., Kulkarni D. N., Pathak B. N., Bulusu K. R. Defluoridation of water by Nalgonda technique. Indian Journal of Environmental Health 1975;17:26-65.
- Govindan K., Raja M., Uma Maheshwari S., NoelM., Oren Y. Comparison and understanding of fluoride removal mechanism in Ca2+, Mg2+ and Al3+ ion assisted electrocoagulation process using Fe and Al electrodes. Journal of Environmental ChemicalEngineering2015;3(3): 1784–1793. https://doi.org/10.1016/j.jece.2015.06.014
- Gandhi N., Sirisha D. Removal of Fluoride by using Passiflora foetidafruits as Natural Coagulant. Discovery Nature2019;13: 44-61.
- Emamjomeh M. M., Sivakumar M., VaryaniA. S. Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation (ECF) process. Desalination2011;275(1-3): 102–106. https://doi.org/10.1016/j.desal.2011.02.032
- Mouedhen G., Feki M., Wery M. D. P., Ayedi H. F. Behavior of aluminium electrodes in electrocoagulation process. Journal of Hazardous Materials 2008;150:124–35.
- Mena V. F., Betancor-Abreu A., González S., Delgado S., Souto R. M., Santana J. J. Fluoride removal from natural volcanic underground water by an electrocoagulation process: Parametric and cost evaluations. Journal of Environmental Management 2019;246: 472–483. https://doi.org/10.1016/j.jenvman.2019.05.147
- Velazquez-Jimenez L. H., Vences-Alvarez E., Flores-Arciniega J. L., Flores-Zuniga H., Rangel-Mendez J. R. Water defluoridation with special emphasis on adsorbents-containing metal oxides and/or hydroxides: A review. Separation and Purification Technology 2015;150: 292–307.
- Kabay N., Arar O., Samatya S., Yuksel U., Yuksel M. Separation of fluoride from aqueous solution by electrodialysis; Effect of process parameters and other ionic species. Journal of Hazardous Materials2008;153: 107-113.
- Waghmare S. S., Arfin T.Fluoride removal from water by various techniques: ReviewInt. Journal of Innovative Science and Engineering Technology 2015; 2(9): 560.
- Diawara C. K. Nanofiltration process efficiency in water desalination. Separation and Purification Technology 2008;37: 302-324.
- Ayala L. I. M., Paquet M., Janowska K., Jamard P., QuistJensen C. A., Bosio G. N., Martire D. O., Fabbri D., Boffa V. Water ? Defluoridation: Nanofiltration vs Membrane Distillation. Industrial & Engineering Chemistry Research2018;57:14740−14748.
- Emamjomeh M. M., Varyani A. S., AlijaniM. H., AzimanY. H., Tari K. Effect of temperature and pressure on removal of fluoride from groundwater using nanofiltration. Journal of Mazandaran University of Medical Sciences2018;27: 166–176.
- Boubakri A., Helali N., Tlili M., Amor M. B. Fluoride removal from diluted solutions by Donnan dialysis using full factorial design. Korean Journal of Chemical Engineering2014;31(3): 461–466. https://doi.org/10.1007/s11814-013-0263-9
- Ahmed A., Grzegorzek M., Majewska-Nowak K. The effect of nitrate on fluoride removal by batch electrodialysis. Environment Protection Engineering2019;45: 87-101.
- Grzegorzek M., Majewska-Nowak K. M. Use of the electrodialysis process for fluoride ion and salt removal from multi-constituent aqueous solutions. Architecture Civil Engineering Environment 2016;9: 107-113.
- Jadhav S. V., Bringas E., Yadav G. D., Rathod V. K., Ortiz I., Marathe K. V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. Journal of Environmental Management2015;162:306–325. https://doi.org/10.1016/j.jenvman.2015.07.020
- Zeni M., Riveros R., Melo K., Primieri R., Lorenzini S. Study on fluoride reduction in artesian well-water from electrodialysis process. Desalination 2005; 185: 241–244.
- Akpor O. B., Muchie M.Remediation of heavy metals in drinking water and wastewater treatment systems: Processes and applications. International Journal of Physical Science2010; 5:1807–1817.
- Katiyar P., Pandey N., Sahu K. K. Biological approaches of fluoride remediation: potential for environmental clean-up. Environmental Science and Pollution Research 2020;https://doi.org/10.1007/s11356-020-08224-2
- Tscherko D., Kandeler E. Ecotoxicological effects of fluorine deposits on microbial biomass and enzyme activities in grasslands. European J. of Soil Sci. 1997; 48:329–335.
- Juwarkar A., Yadav S. Bioaccumulation and biotransformation of heavy metals. Biorem Technologies2010;9: 266–284.
- Sharma S., Upadhyay D., Singh B., Shrivastava D., Kulshreshtha N. M. Defluoridation of water using autochthonous bacterial isolates. Environmental Monitoring and Assessment2019; 191:1-13.
- MukherjeeS., Sahu P., Halder G. Comparative assessment of the fluoride removal capability of immobilized and dead cells of Staphylococcus lentus (KX941098) isolated from contaminated groundwater. Env. Progress & Sustainable Energy2018;37: 5.
- Thesai A. S, Rajakumar S., Ayyasamy P. M. Removal of fluoride in aqueous medium under the optimum conditions through intracellular accumulation in Bacillus flexus (PN4). Environmental Technology2020; 41(9):1185–1198. https://doi.org/10.1080/09593330.2018.1523951
- Mukherjee S., Sahu P., Halder G. Microbial remediation of fluoride-contaminated water via a novel bacterium Providencia vermicola (KX926492). Journal of Environmental Management 2017;204(1): 413–423.
- Capuana M.A.Review of the performance of woody and herbaceous ornamental plants for phytoremediation in urban areas. Forest 2020;13:139-151.
- LeguizamoM. A. O., Gómez W. D. F., Sarmiento M. C. G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere2017;168: 1230–1247.
- Baunthiyal M., Ranghar S. Accumulation of fluoride by plants: Potential for phytoremediation. CLEAN - Soil, Air, Water 2013; 43(1): 127–132.
- SharmaS., Singh B., Manchanda V. K. Phytoremediation: role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environmental Science and Pollution Research 2015; 22(2): 946–962. https://doi.org/10.1007/s11356-014-3635-8
- Khandare R. V., Desai S. B., Bhujbal S. S., WatharkarA. D., Biradar S. P., Pawar P. K., Govindwar S. P. Phytoremediation of fluoride with garden ornamentals Nerium oleander, Portulaca oleracea, and Pogonatherumcrinitum. Environmental Science and Pollution Research2017;24(7): 6833-6839.
- Karmakar S., Mukherjee J., Mukherje S. Removal of fluoride contamination in water by three aquatic plants. International Journal of Phytoremediation2016;18(3): 222- 227. https://doi.org/10.1080/15226514.2015.1073676.
- Baunthiyal M., Sharma V. Phytoremediation potential of selected hydrophytes for fluoride in aquatic environment. World Review of Science, Technology and Sustainable Development2017; 13(2): 133–144.
- Al-Qodah Z., Al-Qudah Y., Omar W.On the performance of electrocoagulation-assisted biological treatment processes: A review on the state of the art. Environmental Science and Pollution Research2019;26: 28689–28713. https://doi.org/10.1007/s11356-019-06053-6 .
- Dwivedi S., Mondal P., Balomajumder C. Bioremoval of fluoride from synthetic water using gram-negative bacteria Shewanellaputrefaciens. Journal of Hazardous, Toxic, and Radioactive Waste 2017;21(2): 04016023. https://doi.org/10.1061/(asce)hz.2153-5515.0000341
- Mohammad A., Kumar S. Adsorptive and bioremoval of fluoride from synthetic wastewater by using Actinobacter immobilized on the surface of sweet lemon peel International Journal of Engineering Research and Technology 2019;8(06):132-137.
- CheeV. L., Chong C. H., Choo C. M., Choong T. S. Y. Combined natural and chemical coagulants to remove fluoride from wastewater. IOP Conference Series: Materials Science and Engineering 2020;778, 012129. https://doi.org/10.1088/1757-899x/778/1/012129.
- Jadhao V. K., Kodape S., Junghare K. Optimization of electrocoagulation process for fluoride removal: A blending approach using gypsum plaster rich wastewater. Sustainable Environment Research2019;29(6). https://doi.org/10.1186/s42834-019-0002-y
- Schoeman J. J., Botha G. R. An evaluation of the activated alumina process for fluoride removal from drinking water and some factors influencing its performance. Water SA, 1985; 11(1): 25–32.
- Mueller K., Johnson C. A., Meierhofer R., Wegelin M. Fluoride removal in developing countries: State of the art of defluoridation techniques in East Africa.2006.
- Dahi E. Africa's U-turn in defluoridation policy: From the Nalgonda technique to bone char. Fluoride 2016; 49(4).
- Dahi E. Contact precipitation for defluoridation of water. In 22nd WEDC Conference:Reaching the unreached: Challenges for the 21st century (pp. 262-265). WEDC.1996.
- American Water Works Association(AWWA). Defluoridation of water. In: Water Quality and Treatment. 3rd Edition, McGraw-Hill, 1971; 436–440.
- Maier F. J. Defluoridation of municipal water supplies. Journal of American Water Work Association 1953; 45: 879–888.
- Mutchimadilok Y., Smittakorn S., Mongkolnchai-arunya S., Durnford, D. Defluoridation with locally produced thai bone char. Advances in Environmental Chemistry 2014; 1:1-9.
- Chibi C., Haarhoff J. A promising approach to fluoride removal in rural drinking water supplies. Proceedings of WISA. Biennial Conference, Sun City, South Africa.2000
- KettunenR., Keskitalo P. Combination of membrane technology and limestone filtration to control drinking water quality. Desalination 2000; 131: 271–283.
- Karunanithi M., Agarwal R., Qanungo K. A review of fluoride removal from groundwater. PeriodicaPolytechnica Chemical Engineering 2019;63(3): 425–437. https://doi.org/10.3311/PPch.12076.
- Agarwal K. C., Gupta S. K., Gupta A. B. Development of new low cost defluoridation technology (KRASS). Water Science and Technology 1999;40(2):167-73.
- Rao V. K. Defluoridation of drinking water by Prasanthi technique. National Workshop on defluoridation technologies for Fluorosis control. 1997
- Biradar S. V. Deflouridation – A Review. Asian Journal of Pharmaceutical Technology & Innovation 2018;6(27): 01-08.
- Mumtaz N., Pandey G. A. Study on integrated fluorosis mitigation plan for endemic fluorosis region – An Indian Perspective. International Journal of Civil Engineering and Technology2017;8(4): 84–91.







