Emerging Approach to Uncover Cyanotoxins in Aquatic Environment: A Concise Review
Tijjani Sabiu Imam1
 , Muhammad Haruna Tsagero1,2
, Muhammad Haruna Tsagero1,2
 , Hadiza Abdullahi Ari3,5
, Saudat Bashir Aminu6
and Adamu Yunusa Ugya3,4
*
, Hadiza Abdullahi Ari3,5
, Saudat Bashir Aminu6
and Adamu Yunusa Ugya3,4
*

1
Department of Biological Sciences,
Bayero University,
Kano,
Nigeria
2
Biological Sciences Department,
Al-Qalam University Katsina,
Katsina State
Nigeria
3
Key Lab of Groundwater Resources and Environment of Ministry of Education,
Key Lab of Water Resources and Aquatic Environment of Jilin Province,
College of New Energy and Environment, Jilin University,
Changchun,
130012
China
4
Department of Environmental Management,
Kaduna State University,
Kaduna,
Nigeria
5
Faculty of Sciences,
National Open University of Nigeria,
Nigeria
6
Department of Biological Sciences,
Kaduna State University,
Kaduna State
Nigeria
DOI: http://dx.doi.org/10.12944/CWE.15.1.13
Copy the following to cite this article:
Imam T. S, Ari H. A, Tsagero M. H, Aminu S. B, Ugya A. Y. Emerging Approach to Uncover Cyanotoxins in Aquatic Environment: A Concise Review. Curr World Environ 2020; 15(1). DOI:http://dx.doi.org/10.12944/CWE.15.1.13
Copy the following to cite this URL:
Imam T. S, Ari H. A, Tsagero M. H, Aminu S. B, Ugya A. Y. Emerging Approach to Uncover Cyanotoxins in Aquatic Environment: A Concise Review. Curr World Environ 2020; 15(1). Available from: https://bit.ly/3eJ2Wg6
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 23-11-2019 |
|---|---|
| Accepted: | 10-04-2020 |
| Reviewed by: | 
 Dr.Pankaj Mehta
Dr.Pankaj Mehta
|
| Second Review by: |

 Saurabh Gupta
Saurabh Gupta
|
| Final Approval by: | Dr Gopal Krishan |
Introduction
The algal blooms produced by cyanobacteria have shading effect on the lower layers, resulting in more respiratory activities below the surface and suffocation of fauna.1,2 Some freshwater algae e.g. Microcystics, Chlorella, Trichodesmus and Phormidium are harmful as they produce biomass which generate bad smell, causing de-oxidation and damage to aquatic life.3 The excessive growth of some species microalgae especially those of Microcysticsa nd Lyngbya lead to anoxia (lack of oxygen) in water. Phormidium bloom is known to spoil salt by imparting red colour and bad odour on brine, It also gelatinizes brine resulting to inability of brine solution to crystallize into salt.4 In agriculture, such water cannot be used for irrigation, drinking source for farm animals and crop processing.5,6 The blooms also render the water useless for domestic use and recreation by imparting unpleasant odour by producing a substance known as geosmin.7 The blue-green algae are often associated with off-flavour problems in fish because of the geosmin and methyl isoborneal (MIB) which impart undesirable flavours in fish.8,9 The blooms are also associated with the production of harmful small molecules known as cyanotoxins. Cyanotoxins bioaccumulate in the aquatic food chain and also pose serious threat to aquatic organisms across the trophic level.10 The exposure of man and other terrestrial organisms to cyanotoxins is as a result of ingestion of drinking water contaminated with these toxins although man can also be exposed to cyanotoxins after feeding on sea food that contain elevated amount of cyanotoxin accumulated in their tissues. Studies conducted by Hoagland et al., (2002) shows that the microtoxins produce by cyanobacteria can bio accumulate in sea food and subsequently causes poisoning in man when they eventually feed on the sea food.11,12 The studies further shows that different cyanobacteria produce different cyanotoxins that have different effect on human beings among which include Aphanizomenon flos-aquae which produce a cyanotoxin known as saxitoxin which is responsible for paralytic shellfish poisoning in humans.13,14 Anabaena flos-aquae produces a neuro toxin called anatoxin which affect the nerve synapses.15 Lyngby amajuscula produce a toxin known as debromaophysia toxin which is responsible for swimmer’s itch: a disease characterized by inflammation and swelling of the mucous membranes of the eyes and nose.15 Some other toxins produced by cyanobacteria are of medical importance which include nodularins, microscystins, cylindrospermopsina, lyngbyatoxin, lipopolysaccharides and aphasiatoxins because their target organs in mammals include liver, gastro-intestinal tracts, skin and any exposed tissues.16-18
The incessant discharge of pollutants into aquatic environment as a result of continuous adoption of industry-based lifestyle is leading to eutrophication thus encouraging the growth of cyanobacteria into inland water which are the important sources of drinking water on a global scale.14,19 The continuous awareness on the effect of cyanotoxins in recreational and drinking water has led to so many studies on the development of an effective method for the detection of cyanotoxins in aquatic environment.20-22 Numerous methods are available for the uncovering of cyanotoxins in aquatic environment but most of the methods are not easily accessible and require sophisticated laboratory expertise for usage.22 Quite a number of biological are available for the uncovering of cyanotoxins from aquatic environment but most of these methods have limitations for the effective detection of cyanotoxins from aquatic environment.23,24 This mini-review is aimed at summarizing the emerging approach use to uncover cyanotoxins in aquatic environment and how effective the method is in the performances of the task.
Bio-analytical Method of Cyanotoxin Detection in Aquatic Environment
Bioanalytical methods in cyanobacteria are referred to as the methods used to collect, store, process or analyses material from cyanobacteria.25 This method is used in the detection of cyanotoxins in aquatic environment.25 The two major bioanalytical methods used for the detection of cyanotoxins are enzyme linked immunosorbent assay (ELISA) and protein phosphate inhibition assay (PPIA). The enzyme linked immunosorbent assay comprises of the use of antibodies to identify cyanotoxins in aquatic environment.26 This method is easily accessible and do not involve the use of sophisticated laboratory equipment and is effective in the detection of cyanotoxins in the environment. ELISA can be used to detect a very low concentration of cyanotoxins (µ/l) in the environment. The principle of cyanotoxin detection using ELISA involves the mixing of the water sample containing cyanotoxin with liquid reagent in a reaction chamber refers to as well (Fig 1). The well contain the reactant and prevent the spill over resulting from the biochemical reactions between the reactant which produce a signal which is used to determine the concentration of the cyanotoxins in the sample collected from aquatic bodies using a spectrophotometer at a wavelength of 450 nm after series of incubating and washing.27 The limitation of the use of ELISA in the detection of cyanotoxins in aquatic bodies is the inability of the method to recognize the type of cyanotoxin in aquatic bodies and the inability of ELISA to determine the precise level of toxicity pose by cyanotoxin in aquatic environment. The protein phosphate inhibition assay is a biological investigating procedure that was developed to study the potentiality of microcystin to bind protein phosphate 1 (PP1) and protein phosphate 2A (PP2A) but now useful in the detection of the effect of microcytins and nodularin in the environment sample.28 The PPIA method of cyanotoxins detection in aquatic environment is effective and can detect cynotoxin concentration as low as 0.1µg/l, the method has been shown to be very fast and easy due to the sensitivity of the method but there is no available kit for PPIA.29 The PPIA method is highly effective and can detect the presences of toxins beyond the detection limit of ELISA and liquid chromatography-mass spectrometry (LC-MS/MS).29 The data on cyanotoxin detection using PPIA have shown positive correlation with the usage of high-performance liquid chromatography (HPLC). The limitation of the use of PPIA to identify cyanotoxins is the inability of some variant to react with protein phosphate enzymes leading to the overestimation of toxin concentration in the environmental sample or sometimes the inability to detect the present of such a variant.30
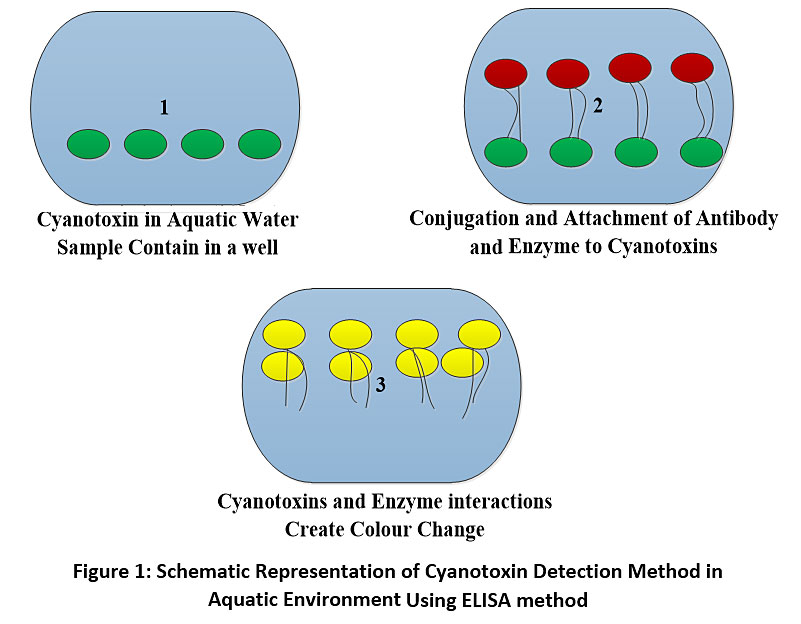 |
Figure 1: Schematic Representation of Cyanotoxin Detection Method in Aquatic Environment Using ELISA method Click here to view Figure |
Molecular Method of Cyanotoxin Detection in Aquatic Environment
Molecular method is the type of method that involves the examination and manipulation of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), protein and lipids of cyanobacteria. This mwthod of cyanotoxins detection in aquatic environment is based on hybridization and polymerase chain reaction (PCR). Hybridization method involves the measurement of the genetic similarities that exist between the DNA sequences of cyanobacterial cells forming a bloom. This method is effective in the distinguishing of the different varieties of cyanotoxins in produce by cyanobacteria but have not been used worldwide due to the fact it is time consuming and difficult to implement under simple laboratory condition. Polymerase chain reaction is another molecular method that is used in the detection of cyanotoxins in aquatic environment, this method is very sensitive because it involves the amplification of DNA sequences to generate many copies of the DNA segment. This method is effective in the investigation of the amount of cyanotoxins in water sample long before the occurrences of blooms. The limitation of molecular method of cyanotoxins detection is that it could give a misleading result particularly when the DNA of the cyanobacteria is slightly contaminated. The enzyme used for the PCR reaction are also prone to error and this can cause mutation in the PCR fragment which also contribute to the misleading result.31-33
Biological Assays of Cyanotoxin Detection in Aquatic Environment
Biological assay method is a type of cyanotoxin detection method that involves the use of living cell or tissue to determine the concentration of cyanotoxin in aquatic environment. The assay is based on the qualitative and quantitative determination of the effect of cyanotoxin on a biological tissue including invertebrate animal, vertebrate animal, plants and microorganisms. Biological assay can be used to differentiate between hepatotoxins and neurotoxin by the determination of the LD50 values of the cyanotoxins.34 These methods can be used to determine the toxicity level of an unknown cyanotoxin as such enormous number of cyanotoxins have been established for the finding of cyanotoxins in aquatic environment although the bioactivity of the toxins (potent hepatotoxicity, cytotoxicity, enzymatic activity as well as immunological interactions) is not well document due to the structural variants of toxins produce by cyanobacteria. The bioassay methods used for the detection of cyanotoxins in aquatic environment include the use of microorganisms, invertebrate animals, vertebrate animals and plant extracts.35
Bioassay Using Invertebrate Animals
However, using different experimentation techniques such as survival, feeding inhibition, population growth rate etc. the toxicity of cyanobacteria can be assigned. It is very important to break colonies or large filaments of cyanobacteria before using in daphnids based bioassay, as large colonies and filaments can present mechanical interference and feeding inadequacy to daphnids, and the mortality may not reflect the toxicity of cyanobacteria.36 Moreover, when six microcystin congeners (including MC-LR) were tested for acute toxicity and protein phosphatase inhibition with Thamnocephalus platyurus, no correlation was found between the two activities. The toxicity was highest for [D-Asp3, (E)-Dhb7] MC-RR but the protein phosphatase activity was much weaker. The study indicates that the devices other than the inhibition of protein phosphatase play in MC induced toxicity to Thamnocephalus platyurus. The consumption of mosquito adults as well as larvae has also been studied as probable bioassay systems counter to cyanobacterial toxins. Larvae of Aedes aegyptii have been found to be affected by neurotoxins and hepatotoxins from cyanobacteria. Adults of Culex pipens were found to be sensitive towards MC-LR when injected. The two mosquitoes were comparatively subtle but have not been broadly implemented owing to the complications of handling this organism. Similarly, adult houseflies (Musca sp.), diamond-backed moth (Plutella sp.) and cotton leaf worm (Spodoptera sp.) were found sensitive towards MC-LR when inserted with decontaminated toxins and natural samples gave positive results that were similar with mouse toxicity results and various insecticides. Though, the flies are problematic to handle and need microinjection, which is hard to administer.37
Bioassay Using Vertebrate Animals
Mouse bioassay has been intensively used during last two decades, and still is most preferred bioassay as far as tests for microcystins are concerned. Male Swiss albino mouse remain the most used strains for the determination of the toxicity of cyanotoxins. Toxicity is tested by intra peritoneal injection of cell lysate of cyanobacteria. Samples prepared in physiological saline solution are preferred if the amount to be injected is 0.5 ml or greater. Mice were pragmatic for 24 h then formerly sacrificed by cervical dislocation. A postmortem of liver tissue at the end of the observation period is necessary as hepatotoxins show characteristic symptoms of liver damage. These hepatotoxins are known to induce signs of hepatotoxicity characterized by degeneration and vacuolation of the hepatic parenchyma, congestion and hemorrhaging, and hepatic vacuolation. Additionally, the leakage of key hepatic enzymes i.e. glutamate pyruvate transaminase (GPT), glutamate oxalloacetate transaminase (GOT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) in serum can be investigated in cases of lack of symptoms, or if mouse survives even after the observational period. For this, blood is collected from retro orbital plexus before sacrificing the mouse and the hepatic enzymes can be investigated in serum using commercially available diagnostic kits.38 Cylindrospermopsin shows protracted symptoms resulted from gradual organ failure, especially in organs such as liver and kidneys, which necessitates longer observation period. Studies have linked acute hepatotoxicity with serious liver, kidney, and thymus damage with the Australian cylindrospermopsin-producing strain. Histological examination of the liver revealed only moderate and multifocal necrosis. The mouse toxicity is stated as LD50 mg dry weight of toxin or cyanobacteria per kg mouse body weight and a LD50 of <1000 mg dry weight is considered the cyanobacteria as non-toxic.39 The first major drawback in using mouse assay is the need of an animal house facility for rearing the animals for routine experiments. Secondly, the use of animals in toxicity studies is against scientific ethics and is actually banned in most of the countries. Moreover, where there are more than one kind of cyanotoxin present in the environment, the more rapid-acting toxin (i.e. microcystin-LR) may mask other symptoms. But the overall toxicity due to cyanobacteria can be estimated in drinking water supplies using mouse bioassays. Fishes are also affected by cyanobacterial toxins in the ways of liver damage, disturbed ionic regulation, behavioral changes and mortality. Young brown trout, Tilapia and Carp are the fishes reported to be most sensitive, and can be used as a test system against cyanobacteria. Unlike mouse bioassay, fish bioassays may not prove to be easy and sensitive. Injecting cyanobacterial extracts to fishes is a difficult task, and immersion in media containing cyanobacterial extracts might need more amounts of cyanobacterial extracts in order to get lethal effects and the oral toxicity can be subsided by the detoxification of toxin in various ways. Similar to mouse bioassay, locusts are tranquil to handle and samples can be administered by injecting low volumes (10 μl). The results, characterized by the paralytic stroke, are obtained within 90 minutes.40 The LD50 for pure saxitoxin was found to be 8 μg g-1 locust body weight, but the bioassay was not found sensitive to microcystin-LR or anatoxin-a. Moreover, relative toxicities of selected saxitoxin analogues differed from those reported in mammalian systems. Authors discussed the use of locusts as simple, ethically acceptable, broad-specificity functional bioassay, for the monitoring of saxitoxins and other paralytic shell fish toxins.41
Bioassay Using Cell Cultures
Since most of the vertebrate animals including mammals are affected by toxic cyanobacteria in various ways, bioassays using cultured mammalian cells instead of using animals have emerged as appropriate substitutes for animal bioassays. The establishment of the fact that microcystins is the reason for the acute liver damage has encouraged studies using hepatocytes (liver cells).42 The toxicity is determined by leakage of a key hepatic enzyme, lactate dehydrogenase (LDH) from hepatocytes.43 Typically, isolated rat hepatocytes are incubated with pure toxin or bloom extracts for a specified time and then the practicality of the cells is measured using the (3, 4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) test. The bioassay provided the first authenticated report for comparison of toxicities with the change in structure of microcystin and showed that MC-LR is most toxic and MC-RR is at least 100 times less toxic as compared to MC-LR.39
Bioassay Using Plants and Plant Extracts
Secondary metabolites including microcystins produced by cyanobacteria and microalgae have been shown to possess algicidal or herbicidal properties. Bioassay using Anacystis, Phormidium, Plectonema and Chlorella has been used to investigate algicidal effects posed by Oscillatoria. Although, scanty or no literature exist for the cost effectivity and sensitive plant-based bioassay for the detection of cyanotoxins in drinking water. Pan et al., (2002) examined the effect of a microcystin-LR extract on the growth of Lepidium sativum over 6 days. Exposure to 10μg L-1 microcystin-LR concentration give rise to a substantial reduction in root and leaf lengths as well as fresh weights of seedlings when linked to the controls.37 Glutathione S-transferase and glutathione peroxidase activities were also significantly high in plants examined. The study shows that of Lepidium sativum bioassay can be use against microcystins, though the effect of microcystins other than MC-LR and other cyanobacterial toxins have not been included in the study. The use of this bioassay needs vast exploration. Cylindrospermopsin was shown to have negative effects on the germination of pollen from tobacco (Nicotiana tabacumcv Samsun NN). Pollen germination was inhibited by cylindrospermopsin between 5 and 1000 μg ml−1. The inhibition of tobacco pollen germination may be open for utilization as a bioassay for cylindrospermopsin, though the method needs a pre-concentration step for the checking the samples from aquatic environment.44
Enzyme Bioassay
Microcystins and nodularins are reported to inhibit protein phosphates (PP) 1 and 2A. In this way, the protein phosphatase inhibition assay has proved to be a subtle screening method for microcystins and nodularins. Microcystins bind equally well with PP1 and 2A. Earlier versions of PP1 and 2A bioassay were based on thequantitation of 32P-phosphate released from a radio labelled substrate. This bioassay was sensitive to subnano gram levels of microcystin and nodularin. The technique has also been used successfully for quantitation of microcystins in environmental samples such as drinking water formerly and afterward water treatment. Being sensitive enough, this method was not used widely because of the use of radioactive substrate, which necessitates specialized laboratory equipment and regulations.45
Determination of Cyanotoxins using Analytical Methods
The most reliable methods of detecting cyanotoxins in aquatic environment is by the usage of high enactment liquid chromatography (HPLC), this method permits the characterization of the toxins into toxins kinds and variants.46 The limitation of the HPLC method in cyanotoxin detection is the fact that it is expensive and difficult to access.47 The concentration of cyanotoxin in the tissues of animals is usually determine using the high-performance liquid chromatographic (HPLC, LC) technique attached with UV, photodiode array (PDA) and/or mass spectrometer (MS) detectors was applied.48 These process permits further detailed identification according to retention time but possess further limitations which include prolonged examination time resulting from purification, concentration and scarcity of commercially cyanotoxins standards.49 This method is not the best method for the quick determination of cyanotoxin with low concentration and continuous sample investigation. The use of HPLC-MS/MS analysis with direct aqueous injection can be used for the fast detection of the different form of cyanotoxins in aquatic environment because the method those not involve purification of sample before analysis.50,51 Studies on the solid-phase extraction (SPE) –liquid chromatography (LC) – mass spectrometry (MS) technique was used to distillate and detect nine cyanotoxins concurrently, the studies was effective in the monitoring of cyanotoxin in aquatic environment.52
The matrix assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) technique permits the rapid recognition of particular microcystin variants. The technique consumes lesser time owing to omission of purification progressions and needs lesser amounts (microgram vs. milligram) of the sample if compare with HPLC or bioassays.38 Gas chromatographic (GC) technique is founded on oxidation of microcystins, which splits the Adda ((2S, 3S, 8S, 9S)-3-amino-9-methoxy-2, 6, 8-trimethyl-10-pheny1deca-4, 6-dienoic acid) side chain to produce 3-methoxy-2-methyl-4-phenyl butyric acid(MMPB), which is then detected by using either GC,GC/MS or by HPLC/fluorescence.53 Studies by Kaya and Sano (1998) found detection limit of this method to be about 0.4µg of total microcystin concentration expressed in microcystin-LR, but uncovering limit hinge on the concentration water. Capillary electrophoresis (CE) is employed in the segregation and quantification of the biological mixtures including cyanotoxins, though, the sensitivity of these method is low if compared with HPLC.54 Vasas et al., (2004) had shown the application of CE for the analysis of complex matrices. The study suggested that the method ought to be combined with other analytical methods like micellar electro kinetic chromatography. Microcystins can also be detected using using thin layer chromatography (TLC) in way that similar to PDA detection in HPLC based on their characteristic of UV spectra.46 With appropriate detection systems; UV spectra of the detached components can be recorded. Different TLC techniques for the segregation of microcystins have been reported by.55 TLC can give quantifiable outcomes of toxin, but this method ought to be regarded as a screening technique pending the addition of extra development. The toxin gene in a aquatic environment have been quantify using the Quantitative real-time PCR 56. 57 established a quick and precise method used simultaneously as qualitative and molecular technique which can be used in the detection of numerous species inside the detrimental algae bloom community. The method was designed for 14 species-specific probes and 4 sets of specific primers. Multiple-simultaneous detection was attained with a bead array method using a flow cytometer and color-coded microspheres, which are conjugated to the developed probes. Following a parallel double PCR amplification, which engaged universal primers in a single reaction and a set of species-specific primers in multiplex detection, was implemented in a cost-effective and highly specific analysis. This multi-format requisited less than 4 h to complete sample collection and up to 100 dissimilar species can be identified simultaneously in a single sample.55
Conclusion
The methods of quantifying cyanotoxins in aquatic environment including bio-analytical methods, molecular methods and bioassay methods were compared with the efficiency of the use of analytical methods in the quantification of cyanotoxins from aquatic environment. Although, analytical methods are highly effective in the determination of cyanotoxins in aquatic environment these methods require high level laboratory skills and expertise whereas bio-analytical methods, molecular methods and bioassay method are highly sensitive, easily accessible and effective in the quantification of cyanotoxins in aquatic environment. These emerging techniques are important tools that can be used to prevent the toxicity associated with algae blooms because they have the capacity to detect the presences of micro-toxins in aquatic environment before algae blooms occur. These study also show that biological method of cyanotoxins detection in aquatic environment have limitations that make analytical methods the best option for the detection of cyanotoxins in aquatic environment despite the fact that high skills are needed to actualized the aim, it is thereby recommended that more research should be conducted to eradicated the challenges associated with the use of biological method for the detection of cyanotoxins in aquatic environment.
Acknowledgement
The first and second author are thankful to the department of Biological sciences, Bayero university Kano.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
References
- Grattan LM, Holobaugh S, Morris JG, Jr. Harmful Algal Blooms and Public Health. Harmful Algae. 2016;57(B):2-8.
- Ugya AY, Imam TS, Li A, Ma J, Hua X. Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: a mini review. Chemistry and Ecology. 2019:1-20.
- Omar WMW. Perspectives on the use of algae as biological indicators for monitoring and protecting aquatic environments, with special reference to malaysian freshwater ecosystems. Trop Life Sci Res. 2010;21(2):51-67.
- Buratti FM, Manganelli M, Vichi S, et al. Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Archives of toxicology. 2017;91(3):1049-1130.
- Ugya AY. The Efficiency of Lemna minor L. in the Phytoremediation of Romi Stream: A Case Study of Kaduna Refinery and Petrochemical Company Polluted Stream. Journal of Applied Biology and Biotechnology. 2015;3(1):011-014.
- Ugya AY, Imam TS. Temporal Heavy metals Variation in Vegetables Sampled at Kasuwan Mata, Kaduna ,etropolis, Nigeria. Malaysia Journal of sciences. 2017;36(6):63-73.
- Funari E, Testai E. Human health risk assessment related to cyanotoxins exposure. Critical reviews in toxicology. 2008;38(2):97-125.
- Main CR, Salvitti LR, Whereat EB, Coyne KJ. Community-Level and Species-Specific Associations between Phytoplankton and Particle-Associated Vibrio Species in Delaware's Inland Bays. Appl Environ Microbiol. 2015;81(17):5703-5713.
- de la Cruz A, Logsdon R, Lye D, Guglielmi S, Rice A, Kannan MS. Harmful Algae Bloom Occurrence in Urban Ponds: Relationship of Toxin Levels with Cell Density and Species Composition. J Earth Environ Sci. 2017;25:704-726.
- Ugya AY, Imam TS, Ma J. Mini-review on the Efficiency of Aqutic Macrophytes as Mosquito Larvicide. Journal of Applied Botany and Food Quality. 2019;92:320-326.
- Hoagland P, Anderson DM, Kaoru Y, White AW. The economic effects of harmful algal blooms in the United States: Estimates, assessment issues, and information needs. Estuaries. 2002;25(4):819-837.
- Ugya AY, Imam TS, Hua X, Ma J. Efficacy of Eicchornia crassipes, Pistia stratiotes and Nymphaea lotus in the Biosorption of Nickel from Refinery Wastewater. Applied Ecology and Environmental Research. 2019;17(6).
- Stewart I, Seawright AA, Shaw GR. Cyanobacterial poisoning in livestock, wild mammals and birds – an overview. In: Hudnell HK, ed. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. New York, NY: Springer New York; 2008:613-637.
- Ugya AY, Hua X, Agamuthu P, Ma J. MOLECULAR APPROACH TO UNCOVER THE FUNCTION OF BACTERIA IN PETROCHEMICAL REFINING WASTEWATER: A MINI REVIEW. Applied Ecology and Environmental Research. 2019;17(2):3645-3665.
- Rapala J, Sivonen K, Luukkainen R, Niemelä SI. Anatoxin-a concentration inAnabaena andAphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxicAnabaena-strains — a laboratory study. Journal of Applied Phycology. 1993;5(6):581-591.
- van der Merwe D. Chapter 31 - Cyanobacterial (Blue-Green Algae) Toxins. In: Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents (Second Edition). Boston: Academic Press; 2015:421-429.
- Yunes JS. Chapter 22 - Cyanobacterial Toxins. In: Mishra AK, Tiwari DN, Rai AN, eds. Cyanobacteria. Academic Press; 2019:443-458.
- Kumar J, Singh D, Tyagi MB, Kumar A. Chapter 16 - Cyanobacteria: Applications in Biotechnology. In: Mishra AK, Tiwari DN, Rai AN, eds. Cyanobacteria. Academic Press; 2019:327-346.
- Ugya AY, Hua X, Ma J. BIOSORPTION OF Cr3+ AND Pb2+ FROM TANNERY WASTEWATER USING COMBINED FRUIT WASTE. Applied Ecology and Environmental Research. 2019;17(2):1773-1787.
- Bláha L, Babica P, Maršálek B. Toxins produced in cyanobacterial water blooms - toxicity and risks. Interdiscip Toxicol. 2009;2(2):36-41.
- Carmichael WW, Azevedo SM, An JS, et al. Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins. Environ Health Perspect. 2001;109(7):663-668.
- Zanchett G, Oliveira-Filho EC. Cyanobacteria and cyanotoxins: from impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins (Basel). 2013;5(10):1896-1917.
- Haque F, Banayan S, Yee J, Chiang YW. Extraction and applications of cyanotoxins and other cyanobacterial secondary metabolites. Chemosphere. 2017;183:164-175.
- Svircev Z, Lalic D, Bojadzija Savic G, et al. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of toxicology. 2019;93(9):2429-2481.
- Greer B, Maul R, Campbell K, Elliott CT. Detection of freshwater cyanotoxins and measurement of masked microcystins in tilapia from Southeast Asian aquaculture farms. Analytical and bioanalytical chemistry. 2017;409(16):4057-4069.
- Moreira C, Ramos V, Azevedo J, Vasconcelos V. Methods to detect cyanobacteria and their toxins in the environment. Appl Microbiol Biotechnol. 2014;98.
- Gaget V, Lau M, Sendall B, Froscio S, Humpage AR. Cyanotoxins: Which detection technique for an optimum risk assessment? Water research. 2017;118:227-238.
- Trifiro G, Barbaro E, Gambaro A, et al. Quantitative determination by screening ELISA and HPLC-MS/MS of microcystins LR, LY, LA, YR, RR, LF, LW, and nodularin in the water of Occhito lake and crops. Analytical and bioanalytical chemistry. 2016;408(27):7699-7708.
- Lawrence J, Niedzwiadek B, Ménard C, et al. Comparison of Liquid Chromatography/Mass Spectrometry, ELISA, and Phosphatase Assay for the Determination of Microcystins in Blue-Green Algae Products. Journal of AOAC International. 2001;84:1035-1044.
- Hiskia A, Spoof L, Kaloudis T, Meriluoto J. Determination of Cyanotoxins by Highâ€Performance Liquid Chromatography with Photodiode Array. In:2017:203-211.
- Moreira C, Matos A, Mendes R, Antunes A. Plant Cyanotoxins: Molecular Methods and Current Applications. In: Carlini CR, Ligabue-Braun R, eds. Plant Toxins. Dordrecht: Springer Netherlands; 2017:339-360.
- Kaushik R, Balasubramanian R. Methods and Approaches Used for Detection of Cyanotoxins in Environmental Samples: A Review. Critical Reviews in Environmental Science and Technology. 2013;43(13):1349-1383.
- Medlin LK, Orozco J. Molecular Techniques for the Detection of Organisms in Aquatic Environments, with Emphasis on Harmful Algal Bloom Species. Sensors (Basel). 2017;17(5):1184.
- Ferrão-Filho AdS, Kozlowsky-Suzuki B. Cyanotoxins: bioaccumulation and effects on aquatic animals. Mar Drugs. 2011;9(12):2729-2772.
- Du X, Liu H, Yuan L, et al. The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review. Toxins (Basel). 2019;11(9).
- Agrawal M, K. Protocols on Algal and Cyanobacterial Research. Narosa Publishers, New Delhi. 2010:281-288.
- Pan H, Song L, Liu Y, Börner T. Detection of hepatotoxic Microcystis strains by PCR with intact cells from both culture and environmental samples. Archives of Microbiology. 2002;178(6):421-427.
- Welker M, Fastner J, Erhard M, Döhren H. Applications of MALDI-TOF MS analysis in cyanotoxin research. Environmental toxicology. 2002;17:367-374.
- Brunson M, W, Greg Lutz C, Durborow R, M. Algal Blooms in Commercial Fish Production Ponds. Southern Regional Aquaculture Centre(SRAC) Publication 1994;466.
- Li R, Carmichael WW, Brittain S, et al. Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria). Toxicon : official journal of the International Society on Toxinology. 2001;39(7):973-980.
- Hurley W, Wolterstorff C, MacDonald R, Schultz D. Paralytic shellfish poisoning: a case series. West J Emerg Med. 2014;15(4):378-381.
- Abdoli N, Heidari R, Azarmi Y, Eghbal MA. Mechanisms of the statins cytotoxicity in freshly isolated rat hepatocytes. Journal of biochemical and molecular toxicology. 2013;27(6):287-294.
- Srilakshmi VS, Vijayan P, Raj PV, Dhanaraj SA, Chandrashekhar HR. Hepatoprotective properties of Caesalpinia sappan Linn. heartwood on carbon tetrachloride induced toxicity. Indian journal of experimental biology. 2010;48(9):905-910.
- Ibelings BW, Backer LC, Kardinaal WEA, Chorus I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae. 2015;49:63-74.
- Bitton G, Koopman B. Bacterial and enzymatic bioassays for toxicity testing in the environment. Reviews of environmental contamination and toxicology. 1992;125:1-22.
- Poon KF, Lam MH, Lam PK, Wong BS. Determination of microcystins in cyanobacterial blooms by solid-phase microextraction-high-performance liquid chromatography. Environmental toxicology and chemistry. 2001;20(8):1648-1655.
- Tsutsumi T, Nagata S, Hasegawa A, Ueno Y. Immunoaffinity column as clean-up tool for determination of trace amounts of microcystins in tap water. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2000;38(7):593-597.
- Kubickova B, Babica P, Hilscherová K, Šindlerová L. Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environmental Sciences Europe. 2019;31(1):31.
- Triantis T, Tsimeli K, Kaloudis T, Thanassoulias N, Lytras E, Hiskia A. Development of an integrated laboratory system for the monitoring of cyanotoxins in surface and drinking waters. Toxicon : official journal of the International Society on Toxinology. 2010;55(5):979-989.
- Hedman CJ, Krick WR, Karner Perkins DA, Harrahy EA, Sonzogni WC. New measurements of cyanobacterial toxins in natural waters using high performance liquid chromatography coupled to tandem mass spectrometry. Journal of environmental quality. 2008;37(5):1817-1824.
- Ma J, Ugya YA, Isiyaku Au, Hua X, Imam TS. Evaluation of Pistia stratiotes fractions as effective larvicide against Anopheles mosquitoes. Artificial Cells, Nanomedicine, and Biotechnology. 2019;47(1):945-950.
- Yen HK, Lin TF, Liao PC. Simultaneous detection of nine cyanotoxins in drinking water using dual solid-phase extraction and liquid chromatography-mass spectrometry. Toxicon : official journal of the International Society on Toxinology. 2011;58(2):209-218.
- Kaya K, Sano T. A photodetoxification mechanism of the cyanobacterial hepatotoxin microcystin-LR by ultraviolet irradiation. Chemical research in toxicology. 1998;11(3):159-163.
- Vasas G, Gaspar A, Pager C, et al. Analysis of cyanobacterial toxins (anatoxin-a, cylindrospermopsin, microcystin-LR) by capillary electrophoresis. Electrophoresis. 2004;25(1):108-115.
- Pelander A, Ojanperä I, Lahti K, Niinivaara K, Vuori E. Visual detection of cyanobacterial hepatotoxins by thin-layer chromatography and application to water analysis. Water research. 2000;34:2643-2652.
- Koskenniemi K, Lyra C, Rajaniemi-Wacklin P, Jokela J, Sivonen K. Quantitative real-time PCR detection of toxic Nodularia cyanobacteria in the Baltic Sea. Appl Environ Microbiol. 2007;73(7):2173-2179.
- Scorzetti G, Brand LE, Hitchcock GL, Rein KS, Sinigalliano CD, Fell JW. Multiple simultaneous detection of Harmful Algal Blooms (HABs) through a high throughput bead array technology, with potential use in phytoplankton community analysis. Harmful Algae. 2009;8(2):196-211.






