Environmental Implications of pH in a Pervious Concrete Pavement on Highway BR-319, Amazonas, Brazil
Evailton Arantes de Oliveira 1
*
 , Maria do P. S. Lamêgo Oliveira1
, Maria do P. S. Lamêgo Oliveira1
 , Arlene M. Lamêgo da S. Campos3
, Arlene M. Lamêgo da S. Campos3
 , Murilo Ferreira dos Santos 2
, Murilo Ferreira dos Santos 2
 , Jessica Adriane Afonso Souza 2
, Jessica Adriane Afonso Souza 2
 , Maria João Correia de Simas Guerreiro4
, Maria João Correia de Simas Guerreiro4
 and Maria Alzira Pimenta Dinis 4
and Maria Alzira Pimenta Dinis 4

Corresponding author Email: 35986@ufp.edu.pt
DOI: http://dx.doi.org/10.12944/CWE.13.2.03
Copy the following to cite this article:
Oliveira E. A. D, Santos M. F. D , Sou za J. A. A , Campos A. M. L. D. S, Oliveira M. D. P. S. L, Guerreiro M.J.C.D.S, Dinis M.A.P. Environmental Implications of pH in a Pervious Concrete Pavement on Highway BR-319, Amazonas, Brazil.Curr World Environ 2018;13(2). DOI:http://dx.doi.org/10.12944/CWE.13.2.03
Copy the following to cite this URL:
Oliveira E. A. D, Santos M. F. D , Sou za J. A. A , Campos A. M. L. D. S, Oliveira M. D. P. S. L, Guerreiro M.J.C.D.S, Dinis M.A.P. Environmental Implications of pH in a Pervious Concrete Pavement on Highway BR-319, Amazonas, Brazil. Curr World Environ 2018;13(2). Available from: http://www.cwejournal.org/article/1088/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2018-05-25 |
|---|---|
| Accepted: | 2018-08-23 |
| Reviewed by: | 
 Dr. Avinash Prabhu (India)
Dr. Avinash Prabhu (India)
|
| Second Review by: |

 Dr. Simona Azzali (Singapore)
Dr. Simona Azzali (Singapore)
|
| Final Approval by: | Dr. Umesh Chandra Kulshrestha |
Introduction
Concrete has cement as a binder, therefore, it is subject to two phenomena: calcination and carbonation.1 The cement manufacturing process consumes energy and generates carbon dioxide (CO2) due to calcination, whereas carbonation of cement, a natural process of calcium carbonate formation in the concrete hydration process, uptakes CO2 from the environment, an interesting environmental factor that may contribute to reduction of this greenhouse gas.2,3 In fact, cement carbonation in construction may reduce CO2 in the environment, with a positive impact on the greenhouse effect. Nonetheless, carbonation may contribute to concrete pathology by steel corrosion in reinforced concrete4. The importance of investigating the environmental implications of pH variation in drainage water from porous concrete is related to cement carbonation.5 Upon the hydrolysis reaction, an increase in the solubility of calcium carbonate, incorporates carbon dioxide, leading to an acidity in the water, once the system is in equilibrium. Sustainability requirements in today’s constructions compel to investigate materials whose waste can be recycled.3 This research work focused on the effects of carbonation in porous concrete, which may include recycling of concrete by using it as an aggregate contributing to pavements6 construction sustainability. The main objective of this study was to investigate evidence of carbonation in specimens of pervious concrete, under laboratory and field conditions of pressure and temperature, and to verify the integrity of specimens of pervious concrete subject to different types of water: distilled, alkaline ionized water, carbonated water and tap water.
Materials and Methods
This study was developed in Manaus, in the Amazon region in Brazil, and involved laboratory and field experiments.
Pervious Concrete Specimens
Pervious concrete specimens were formed based on the Brazilian standard NBR 57387, that defines the procedures to mold and cure concrete specimens. Based on studies of traits, preparation methods, standard quality control as well as permeability concrete optimization methods8, ten cylindrical specimens of pervious concrete were formed, with a cement to coarse aggregate 1:4.4 ratio and a water/cement factor of 0.3 to compression (Table 1).
Table 1: Pervious Concrete Specimens Characteristics
|
Material |
Ratio |
|
cement : coarse aggregate ratio |
1:4.4 |
|
water / cement factor |
0.3 |
|
aggregate (4.8 a 9.5 mm) (kg/m3) |
1660 |
|
cement (kg/m3) |
374 |
The specimens were molded into cylindrical shapes of 10 cm in diameter by 20 cm. The compaction consisted on applying 15 strokes with a shank, by layer of equal thickness. Vibration, curing and demolding were performed according to the guidelines of Brazilian standard NBR 5738, considering that the characteristics of pervious concrete differ from common concrete.9
The infiltration rate was evaluated using distilled (DIS), ionized (ION), tap (TAP) and carbonated (CAR) water (Figure 1). Water adsorption was calculated by weight difference after the samples were left to air dry for 24 hours and after water (DIS, ION, TAP, and CAR) had infiltrated through the specimen.
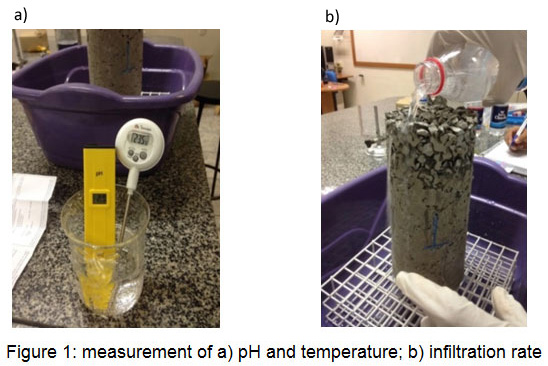 |
Figure 1: measurement of a) pH and temperature; b) infiltration rate |
The pH variation during carbonation and calcination10: the chemical reaction in cement manufacture, where calcium carbonate dissociates into carbon dioxide and calcium oxide, requires a lot of thermal energy and releases its carbon dioxide into the atmosphere. Carbonation (equations 1 and 2) is the inverse reaction of calcination (Equation 3), where carbon dioxide is slowly absorbed by the concrete during its lifetime (Equations 1 to 3).
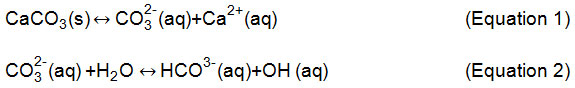
The hydrolysis reaction consumes CO32- (aq) and increases the solubility of calcium carbonate. Therefore, the incorporation of carbon dioxide creates an acidity in water, which can be detected by pH measurement, after the equilibrium displayed in Equation 3:
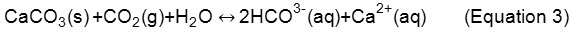
Thus, the indication of pH change can be used as a method to verify if carbonation in the specimens of pervious concrete is occuring.13 Evaluation of pH difference was performed in each sample (Figure 1), with a time interval of 24 hours, in the following order: DIS, ION, TAP, CAR. The samples were left to air dry for 24 hours, after which weighing with a precision scale of ± 0.2 g would take place.
Results
Pervious concrete specimen average infiltration rate, pH e temperature before/after percolation are presented in Table 2.
Table 2: Experimental Data
|
Sample |
Weight |
Infiltration rate |
pH before/after percolation |
temperature before/after percolation (ºC) |
||||||
|
(g) |
(mm/hr) |
DIS |
ION |
TAP |
CAR |
DIS |
ION |
TAP |
CAR |
|
|
1 |
3,354.30 |
1.14 |
7.4/ 7.1 |
9.6/ 8.7 |
8.5/ 5.1 |
5.4/ 3.2 |
22.7/ 21.9 |
23.5/21.9 |
27.0/ 24.2 |
24.3/ 21.8 |
|
2 |
3,236.90 |
1.27 |
8.2/ 7.1 |
9.8/ 8.7 |
8.6/ 5.1 |
5.1/ 3.2 |
22.9/ 22.1 |
23.2/ 21.5 |
25.9/ 22.8 |
24.3/ 21.3 |
|
3 |
3,308.20 |
1.31 |
7.6/ 7.1 |
9.6/ 8.7 |
7.8/ 5.1 |
4.6/ 3.2 |
22.5/ 21.3 |
22.9/21.2 |
27.1/ 23.4 |
23.5/ 21.1 |
|
4 |
3,304.80 |
1.23 |
7.6/ 7.1 |
9.6/ 8.7 |
8.5/ 5.1 |
4.7/ 3.2 |
22.5/ 21.6 |
22.4/ 20.9 |
27.0/ 22.9 |
23.1/ 21.1 |
|
5 |
3,285.80 |
1.27 |
7.5/ 7.1 |
9.5/ 8.7 |
8.3/ 5.1 |
4.6/ 3.2 |
22.3/21.1 |
22.3/ 21.1 |
25.3/ 22.3 |
22.7/ 20.7 |
|
6 |
3,148.50 |
1.22 |
8.0/ 7.1 |
9.6/ 8.7 |
8.8/ 5.1 |
4.6/ 3.2 |
22.2/ 21.8 |
22.6/ 21.5 |
25.3/ 22.3 |
22.7/ 20.6 |
|
7 |
3,257.00 |
1.55 |
7.7/ 7.1 |
9.4/ 8.7 |
8.3/ 5.1 |
4.5/ 3.2 |
21.8/ 20.6 |
22.6/ 22.3 |
26.1/ 23.4 |
22.5/ 21.4 |
|
8 |
3,405.90 |
1.26 |
7.4/ 7.1 |
9.3/ 8.7 |
8.2/ 5.1 |
4.0/ 3.2 |
21.3/ 20.6 |
22.7/ 22.7 |
25.8/ 23.6 |
22.6/ 22.0 |
|
9 |
3,164.30 |
1.31 |
8.2/ 7.1 |
9.2/ 8.7 |
9.0/ 5.1 |
4.3/ 3.2 |
21.5/ 21.2 |
21.1/ 20.0 |
25.3/23.9 |
22.4/ 22.0 |
|
10 |
3,269.90 |
1.26 |
8.8/ 7.1 |
9.4/ 8.7 |
8.5/ 5.1 |
4.3/ 3.2 |
21.6/ 21.5 |
21.1/19.9 |
25.0/ 24.3 |
22.8/ 21.7 |
Discussion
Infiltration Rate
As shown in Figure 3, the infiltration rate showed little variation between specimens for the same water type. The highest rates (smaller filtration time) were always under distilled water, suggesting that minerals may affect infiltration rates. Infiltration rate is within the expected parameters.15
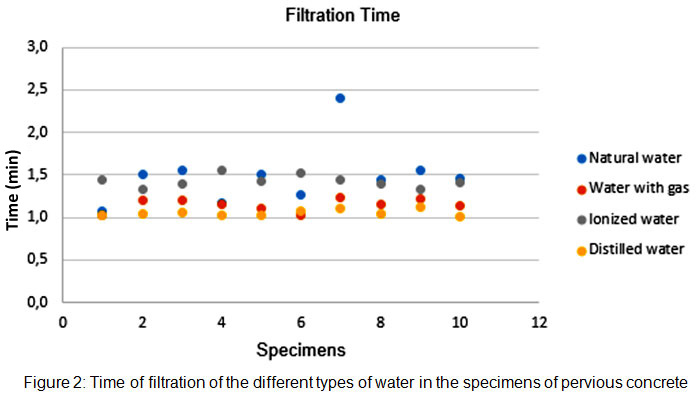 |
Figure 2: Time of filtration of the different types of water in the specimens of pervious concrete Click here to view figure |
Nonetheless, the Kruskal-Wallis ANOVA test, using filtration time of tap water as a grouping variable, demonstrates that samples are homogeneous, with a p-value=0.429 for CAR, p-value of 1.000 for ION, and p-value of 0.317 for DIS. Table 3 shows the descriptive statistics of filtration times for the four water types.
Table 3. Filtration Time Descriptive Statistics
|
|
Water type |
|||
|
|
Tap |
Carbonated |
Ionized |
Distilled |
|
mean |
1.5 |
1.1 |
1.4 |
1.1 |
|
standard deviation |
0.361 |
0.079 |
0.070 |
0.038 |
|
Variance |
0.130 |
0.006 |
0.005 |
0.001 |
Water Adsorption
Water adsorption in the specimens varied from 1.2% to 1.6%, with distilled and tap water showing less adsorption than carbonated or ionized water (Table 3), based on the non-parametric Kruskal-Wallis test with seven degrees of freedom, using weight difference of water as a grouping variable. Results showed a p-value= 0.339 for CAR, p-value= 0.357 for ION, and p-value=0.339 for DIS, implying that samples were homogeneous.
Table 4. Adsorption on Pervious Concrete Specimens
|
Types of water |
Adsorption (%) pervious concrete specimens |
|
Distilled water (DIS) |
1.2 |
|
Ionized water (ION) |
1.6 |
|
Tap water (TAP) |
1.2 |
|
Carbonated water (CAR) |
1.5 |
Figure 2 shows difference in weight before and after the infiltration process, suggesting that water adsorption is higher under ionized water, followed by carbonated water, distilled and tap water, suggesting that water with higher carbon concentration tends to adsorb more to concrete. Differences between samples are attributed to differences in porosity due to concrete molding.
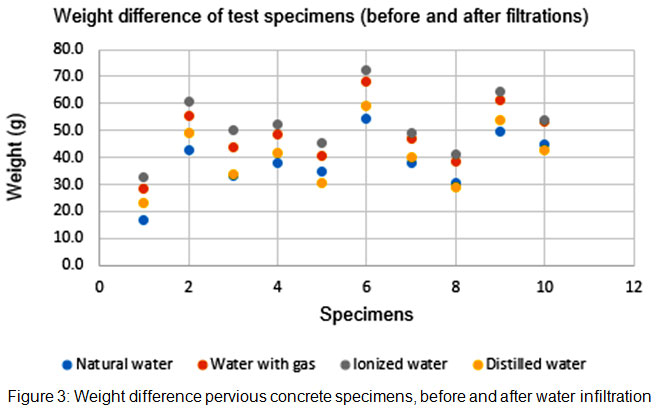 |
Figure 3: Weight difference pervious concrete specimens, before and after water infiltration Click here to View figure |
As shown in Figure 4, all water samples filtered through the pervious concrete specimens changed their pH. Tap water and carbonated water showed higher pH differences, whereas ionized and distilled water showed smaller differences. These results suggest that pervious concrete adsorbs CO2 from carbonated water, changing its pH.
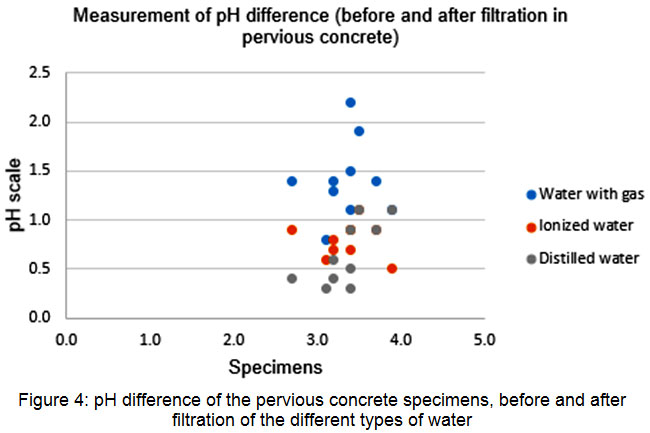 |
Figure 4: pH difference of the pervious concrete specimens, before and after filtration of the different types of water Click here to View figure |
The Kruskal-Wallis test, using the difference data of pH of tap water as a grouping variable, resulted in a p-value of 0.860 for CAR, p-value of 0.471 for ION, and p-value of 0.481 for DIS, implying that samples are homogeneous (p > 0.05).
Descriptive statistics (Table 5), the natural water presented the mean with the greatest difference of values between pH before and pH after filtration, while ionized water and distilled water showed the lowest mean with the difference between pH before and after pH filtration. It is assumed that natural water needs less filtration time to undergo greater pH changes, i.e., water with gas, ionized water and distilled water need to remain contained in the pores of the pervious concrete to undergo greater pH changes.
Table 5: pH Descriptive Statistics
|
|
Water type |
|||
|
|
Tap |
Carbonated |
Ionized |
Distilled |
|
Mean |
3.4 |
1.4 |
0.8 |
0.7 |
|
Standard deviation |
0.331 |
0.401 |
0.176 |
0.321 |
|
Variance |
0.109 |
0.161 |
0.031 |
0.103 |
Having pervious concrete large and interconnected pores in addition to the typical micropores of cement paste11, this structure allows ambient air and water to more easily reach the inner spaces. When water flows through pervious concrete, pH of water changes due to the calcium hydroxide and calcium carbonate in the cement slurry12, with lower pH values, indicating more calcium carbonate or a greater amount of carbonation. The pH change indicates that water encountered calcium hydroxide, with a pH increase. Nonetheless, in this experiment there was no time for formation of calcium carbonate, therefore, it was not possible to detect the carbonation by the reduction of pH in the samples, in these laboratory experiments.
Conclusions
The results obtained in the laboratory and in the field showed that:
low absorption of tap and distilled water by pervious concrete specimens, is an advantage if it is intended to be used as road paving material, increasing stability under rainfall conditions;
filtration time, associated to infiltration rate, showed little variation under different water types, suggesting that pervious concrete is use is not constrained by carbon concentration in water;
pH increase in water samples after infiltration through pervious concrete specimens, in the laboratory and in the field experiments suggest contact of rainwater with the calcium hydroxide in the cement paste, but carbonation and CO2 sequestration could not be detected probably because there was not enough time for calcium carbonate formation.
These preliminary studies suggest that carbonation was not detected by the methodology used, so it would be interesting to perform other tests, such as X-ray diffraction to identify the chemical components in the samples being tested that serve as evidence of carbonation in the cement.
Acknowledgments
The authors are grateful to University Fernando Pessoa (Porto - Portugal) for the guidance provided, Uninorte University - Laureat International for permission to use the Chemistry Laboratory and Concrete Laboratory of the Coordination of the Civil Engineering Course, Manaus, Amazon, Brazil, and the National Department of Infrastructure and Transport – DNIT, Brazil, for the support of the scientific research taking place. This research had no external funding sources.
References
- Haselbach l., Thomle J. An Alternative Mechanism for Accelerated Carbon Sequestration in Concrete. Sustainable Cities and Society, 2014;12:25.
CrossRef - Thomle J., Haselbach L. The Declining pH of Waters Exfiltrated Through Pervious Concrete. Sustainable Cities and Society, 2011;12:25.
- Pade C., Guimarães M. The CO2 Uptake of Concrete in 100 Years Perspective. Cement and Concrete Research, 2007;37:1348-1356.Hõltz F. C. Use of Permeable Concrete in Urban Drainage: Technical Feasibility and Environmental Impact Analysis. Master's Dissertation UFRS, School of Engineering, Porto Alegre, Brazil, 2011.
- Ho L. S., Nakarai K., Ogwa Y., Sasaki T., Morioka M. Effect of Internal Water Content on Carbonation Progress in Cement-treated Sand and Effect of Carbonation on Compressive Strength. Cement and Concrete Composites, 2018; 85:9.
CrossRef - Branch J. L., Epps R., Kosson D. S. The Impact of Carbonation on Bulk and ITZ Porosity in Microconcrete Materials with Fly ash Replacement. Advances in Materials Science & Engineering, 2018; 103:170.
- El-Hassan H., Kianmehr P. Sustainability Assessment and Physical Characterization of Pervious Concrete Pavement made with GGBS. MATEC Web of Conferences, 2017; 120:07001.
CrossRef - Associação Brasileira de Normas Técnicas. NBR 5738: Molding and Cure of Cylindrical and Prismatic Concrete Samples. (In Portuguese), Rio de Janeiro, 1994.
- Rangelov M., Nassiri S., Chen Z., Russell M., Uhlmeyer J. Quality Evaluation Tests for Pervious Concrete Pavements Placement. International Journal of Pavement Research and Technology, 2017;10(3):245.
CrossRef - Batezini R., Balbo J.T. Study on the Hydraulic Conductivity by Constant and Falling head Methods for Pervious Concrete. IBRACON Structure and Materials Journal, 2015, 8(3), 248-259.
- Arrigoni A., Pelosato R., Melià P., Ruggieri G., Sabbadini S., Dotelli G. Life Cycle Assessment of Natural Building Materials: The Role of Carbonation, Mixture Components and Transport in the Environmental Impacts of Hempcrete blocks. Journal of Cleaner Production, 2017;149:1051.
CrossRef - Georget F., Prévost J. H., Huet B. Impact of the Microstructure Model on Coupled Simulation of Drying and Accelerated Carbonation. Cement and Concrete Research, 2018;104:1.
CrossRef - Hunnicutt W., Struble L., Mondal P. Effect of Synthesis Procedure on Carbonation of Calcium-silicate-hydrate. Journal of the American Ceramic Society, 2017;100: 3736.
CrossRef






