Respective and Interactive Effects of O3 and CO2 and Drought Stress on Photosynthesis, Stomatal Conductance, Antioxidative Ability and Yield of Wheat Plants
Maysa M. Hatata1 , Reem H. Badar1 , Mohammad M. Ibrahim1,2 and Ibrahim A. Hassan1,3 *
1
Department of Botany,
Faculty of Science,
Alexandria University,
El Shatby,
21526
Alexandria
Egypt
2
Faculty of Science,
King Saud University,
Riyadh,
KSA
3
Centre of Excellence in Environmental Studies (CEES),
King Abdelaziz University, P.O.Box 80216,
Jeddah,
21589
KSA
DOI: http://dx.doi.org/10.12944/CWE.8.3.10
Effects of O3, Doubled CO2 Concentration and drought stress on wheat (Triticum aestivum L.) plants were studied in open-top chambers (OTC). Under doubled CO2 concentration, grain yield and biomass increased, the SOD activity, and carotenoid (Car) content also increased while relative conductivity yield parameters significantly decreased. But under Elevated O3 concentration, the SOD activity, Carotenooids decresaed. The final result was decreased grain yield and plant biomass. Interactive effects of doubled CO2 and O3 concentrations on soybean were mostly counteractive. However, the beneficial effects of concentration-doubled CO2 is more than compensate the negative effects imposed by doubled O3 and the latter in its turn partly counteracted the positive effects of the former.
Copy the following to cite this article:
Hatata M. M, Badar R. H, Ibrahim M. M, HASSAN I. A. Respective and Interactive Effects of O3 and CO2 and Drought Stress on Photosynthesis, Stomatal Conductance, Antioxidative Ability and Yield of Wheat Plants. Curr World Environ 2013;8(3) DOI:http://dx.doi.org/10.12944/CWE.8.3.10
Copy the following to cite this URL:
Hatata M. M, Badar R. H, Ibrahim M. M, HASSAN I. A. Respective and Interactive Effects of O3 and CO2 and Drought Stress on Photosynthesis, Stomatal Conductance, Antioxidative Ability and Yield of Wheat Plants. Curr World Environ 2013;8(3). Curr World Environ 2013;8(3). Available from: http://cwejournal.org?p=451/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-11-10 |
|---|---|
| Accepted: | 2013-12-02 |
Two aspects of global climate change that directly influence plant physiology, growth and productivity; increasing in concentrations of ambient ozone (O3) and carbon dioxide (CO2).1,2 Atmospheric CO2 is projected to continue rising to at least 550 ppb by 2050.3 The current annual average (O3) is predicted to continue increasing by 0.5–2% per year over the next century, mainly due to increases in precursor emissions from anthropogenic sources.4,5
Greenhouse effect is one of the important concern in present global change and the increase of concentrations of greenhouse gases is the main reason which resulted in the enhancement in the greenhouse effect. CO2 is the most important greenhouse and carbon source for plant photosynthesis. O3 in troposphere is essentially a pollutant6 and it restricts the growth of plant severely. The concentrations of CO2 and O3 have been increased continually and the responses of plant to them are regarded increasingly.
Ozone diffuses into the leaf apoplast via the stomata where it is rapidly converted into other reactive oxygen species (ROS) that signal a diverse metabolic response (Long and Naidu, 2002; Kangasjarvi et al., 2005; Hassan, 2006). Stress may promote the formation of harmful reactive oxygen species (ROS) which have the capacity to initiate chlorophyll bleaching, lipid peroxidation, protein oxidation, and injury to nucleic acids (Kangasjarvi et al., 2005). The effect of CO2 and O3 on plant growth and productivity has been determined separately for a large number of plant species, but very little work has focused on their interactive effect.2,6,7,8
The studies on combination effects of CO2 with temperature, moisture6,7 and effects of O3 with SO2 or NO28,9 on plant have been reported. Interactive effects of CO2 and O3 on winter wheat,10 potato,11,12 aspen and birch.13 However, the research on interactive effects of CO2, O3 and drought, are seldom.15 Although many studies addressed the effects of CO2, O3 and /or drought stress on plants in the developed world, no such study, to the best of knowledge, was conducted in developing world.
In this paper, respective and interactive effects of doubled CO2 and O3 concentration on wheat yield and biomass production, Photosynthesis, stomatal conductance, anti-oxidative ability and cell membrane lipid peroxidation of leaves in the context of free radical biology were studied in open-top chambers (OTC).
Though several previous studies report evocation of oxidative stress by water deficit stress in case of wheat4,9,26 at individual level, information on their comparative response to same degree of stress in terms of their stress sensitivity and functional variation is lacking. In order to fill this gap of knowledge, this study was undertaken to evaluate oxidative response to O3, CO2 and/or drought singly and in combination of wheat plants. The hypothesis behind the work was the sensitivity of plants to water stress might be associated with different abilities for their carbon fixation, and also these stresses may affect to the net assimilation through their impact on stomatal conductance as well as antioxidant enzymes.
Therefore the aims of the present study were to describe interaction of enhanced O3, elevated CO2 and water stress on growth, seed yield, photosynthetic activities, photosynthetic pigments, antioxidant enzymes such as glutathione (GR ), Peroxidase (POD), superoxide dismutase (SOD) and ascorbate Peroxidase (APX) as well as molecular biomarkers in durum wheat (Triticum aestivum L.) plants. Moreover, to study the importance of the accumulation of photosynthetic pigments which could change during exposure to multiple stresses, and this is the novelty of this experiment was a study of triple interaction of the mentioned factors.
Materials and Methods
Plant Materials, Growth Conditions and Experimental Design
Grains of wheat (Triticum aestivum L.) plants were sown in pots with 20x20cm2 filled with soil collected from top soil in the field. There were five plants/pot. They were transferred to four Open-Top Chambers (OTCS)19 when second foliage leaf appeared. The treatments were: (a) control (FA), (b) O3 without CO2 (O3), (c) FA with CO2 (CO2), (d) O3 and CO2 (O3 +CO2).
The experiment was split plot Latin square, one chamber was equipped with charcoal filter (FA) and the other was ventilated with FA + Target O3 (78 ppb/h) and the third was ventilated with FA +Target CO2 (450 ppm), while the fourth OTC was supplied with O3 (78 ppb) and CO2 (450 ppm).
There were 12 pots/chamber. Pots were distributed in a completely randomized block design (CRBD). Half of pots were irrigated to the field capacity while the other half was water-stressed to 0.5 MP.10,12 They were rotated within each chamber every week.
Biomass and Grain Yield
5 plants per pot were harvested for determination of grain and seed yield and yield attributes. The grains harvested were air-dried and the shoots, leaves and roots were dried at 80℃for 72h. Number of grains per ear was counted. Yield per ear, yield per plant, and 1000-grain weight were determined Stomatal conductance and Net photosyntheti measurements Net CO2assimilation rate (A) and stomatal conductance (gs) were measured using Infrared Gas Analyzer (IRGA,ciras-1PP System, Hitchin UK). Measurements were carried out on ten attached leaves per treatment on weekly basis Measurement of Photosynthetic Pigments, Photosynthetic pigments, chlorophyll a & b and carotenoids were extracted and from flag leaves and were determined by UV-spectrophotometer (LKB, UK.).35,36
Antioxidant Enzymes Assays
Extractions of antioxidant enzymes from the leaves of the four treatments were performed.21 Leaves were cut from each treatment and immersed in liquid nitrogen and kept in a deep freezer at 800C until the analyses were performed at University of Newcastle, UK.
Samples were weighed and ground at about 0C in25 m Tris–HCl buffer containing 3 mM MgCl2, then the homogenates were centrifuged at 20 000 for 15 min (Centrifuge 17 S/RS, Heraeus Sepatech). The supernatants were used for the enzyme assays and the results were expressed on protein basis.35
All assays were performed using a final volume of 1 mL, with at least duplicate assays undertaken on each sample. Moreover, the assays were end-point determinations.
SOD (EC 1.15.1.1) activity was monitored.21 The extraction mixture contained 50 mM phosphate buffer solution (pH 7.8), 13 mM L-methionine, 63 lM nitro blue tetrazolium and 2 lM riboflavin. The ability of the extract to inhibit the photochemical reduction of nitro blue tetrazolium was determined at 560 nm (Schimadzu UV-1201 spectrophotometer). The amount of the extract resulting in 50% inhibition of nitro blue tetrazolium reaction is defined as one unit of SOD activity.
Catalase (EC, 1.11.1.6) activity was assayed in enzyme extract reaction mixture containing 50 mM phosphate buffer (pH 7.4). The reaction was started by adding 10 mM H2O2, and the reduction in absorbance was determined at 240 nm.14,36
GPX (EC, 1.11.1.7) activity was determined by adding 50 mM phosphate buffer (pH 6.1), 1% H2O2 and 1% guaiacol to the extract, and the absorbance was determined at 470 nm.
APX (EC, 1.11.1.11) activity was determined according to Maehly & Chance (1954). The reaction mixture contained 50 mM potassium phosphate, 0.5 mM ascorbate, 0.1 mM ethylenedimethyl tartaric acid (EDTA) and 0.1 mM H2O2, and the absorbance was determined at 290 nm. Protein concentrations of leaf extracts were determined as described earlier.26,37
Data Analysis
Data were subjected to three-way analysis of variance (ANOVA), using O3, CO2 and drought treatments as factors, followed by a least significant difference test, and P values ≤ 0.05 were considered significant (using the STATGRAPHICS statistical package, Package 3, UK) based on plot means.
Results
Effects on Visible Injury Symptoms
Visible injury symptoms appeared on the upper surface of flag leaves as point brown spots and by the end of experiment.
Number of injured leaves were increased by 2-fold due to exposure to O3, while exposure to both CO2 and O3 increased it by 31% (Table 1). There was no significant effect (p > 0.05) of either CO2 or drought stress and their interaction on number of injured leaves and on degree of injury. Degree of injury increased by 6-fold when exposed to O3 and 4-fold due to exposure to both O3and CO2. Drought stress and CO2 protected plants against toxic effects of O3. Plants exposed to D had 39% less leaf injury while exposure to bothCO2 and D had 50% lower leaf injury than plants exposed to O3 alone (table 1).
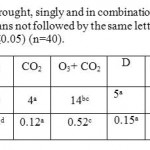 |
Table 1: Effect of CO2 and O3, drought, singly and in combination on foliar injury symptoms of Wheat leaves in year 2005. (Means not followed by the same letter in each row are significantly different from each other at P≤0.05) (n=40). Click here to View table |
Effect on Growth and Yield
O3 had greater effect on the numbers of ears per plant and the number of grains per ear than drought as these parameters was reduced by 20 and 26% respectively (Table 2). However, CO2 caused increased by 26% and 18% in these parameters respectively. Moreover, the percentage reduction in 1000 grain weight due to O3 (40%)was greater than that due to drought(29%),CO2 alone increased it by 23%,interaction between stresses was less than additive. O3 and Drought caused significant reductions in all yield parameters measured in this experiment (Reductions reached maximum of 63% in dry mass and 54% in 1000 grains weight).
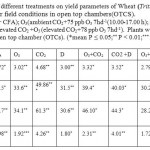 |
Table 2: Effects of different treatments on yield parameters of Wheat (Triticum aestivium L.) plants grown under field conditions in open top chambers(OTCS). FA (346 ppm CO2+ CFA); O3(ambient CO2+75 ppb O3 7hd-1(10.00-17.00 h); elevated CO2 (702 ppm1CO2 +CFA) elevated CO2 +O3 (elevated CO2+78 ppb O3 7hd-1). Plants were harvested 70 d after transfer to open top chamber (OTCs). (*mean P ≤ 0.05;** P < 0.01;*** P < 0.001). Click here to View table |
Effect on Stomatal Conductance and Photosynthetic Rates
It was clear that both O3 and drought had the greatest negative effects on stomatal conductance (gs) caused reduction by 41 and 50% respectively (over the entire period the experiment) (Fig.1). Moreover, interaction between O3 and drought was great reduction more than additive (58% reduction). On the other hand CO2 had a synergistic effect it caused increases to 12%, while it ameliorates toxic effects of O3 and drought when applied with other stresses. The interactive effects of CO2 and O3 are contradictory caused greater reduction in gs than CO2 and drought by 17 and 11%, respectively. Interactions between different treatments were less than additive (21% reduction). After 10 weeks O3 and drought exposure negative impacts were evident for Wheat plants revealed a 58% decline in stomatal conductance (gs).
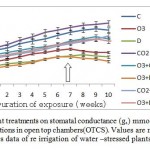 |
Figure 1: Effects 0f different treatments on stomatal conductance (gs) mmol m-2s-1 of Wheat plants grown under field conditions in open top chambers(OTCS). Values are means of 10 replicates ± 1SE. An arrow indicates data of re irrigation of water –stressed plants. Click here to View figure |
It was clear that O3 and drought had the greatest negative effect on net photosynthetic rates (A) (Fig.2). O3 and drought caused reduction in net photosynthetic rates (A) by 70% and 80%, respectively. On the other hand, CO2 increased net photosynthetic rates A by 6%. Interaction between O3 and CO2 decreased it by 8%, while O3 and drought decreased it to 12%. Exposure of Wheat plants to O3 and drought simultaneously had the greatest decline effect on the net photosynthetic rates A by 88%. Drought had more pronounced negative effects than those other parameters. CO2 mitigates toxic effects of drought and O3, it mitigated O3 and reduced its toxicity by to 23 % and drought to 19 %. Interactions between different treatments were less than additive.
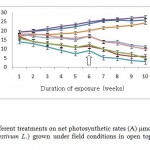 |
Figure 2: Effects of different treatments on net photosynthetic rates (A) µmol m-2 s-1 of Wheat plants (Triticum aestivum L.) grown under field conditions in open top chambers(OTCS). Legends as Figure 1. Click here to View figure |
Effects on antioxidant enzymes
A significant increase was observed in POD by 1.5-fold in Wheat plant treated with elevated CO2; in contrast, elevated CO2 had a reducing effect on GR and SOD by 11and 9 %, respectively. There was no significant (P≤0.05) effect of all treatments on APX.
O3 caused increases in activities of GR, POD by 18 and 36 % respectively, while SOD was decreased by 11%. Drought had more pronounced effect on these enzymes as it caused increases by 21% in POD while GR and SOD were decreased by 11 and 15%, respectively. The antioxidant enzymes; GR and SOD were significantly decreased in Wheat plants exposed to elevated CO2 + elevated O3 by 15 and 50%, respectively than in plants grown under normal conditions. CO2 followed the same pattern of O3. Interaction between O3, CO2 and drought and their multiple interactions were less than additive.
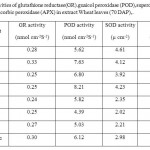 |
Table 3: Activities of glutathione reductase(GR) guaicol peroxidase (POD),superoxide dismutase (SOD) and Ascorbic peroxidase (APX) in extract Wheat leaves (70 DAP). Click here to View table |
Discussion
The results support the previous reports22,23,24,25 that biomass and yield of crop plant were significantly increased under high CO2 level but decreased under high O3 level. In our case, the increased yield and biomass under doubled CO2 were more than sufficient to eliminate the doubled O3-indced yield decrease as shown in the treatment of the combination of doubled CO2 and O3 concentration.
It is known that enriched CO2 increases photosynthesis1,12 by providing more carbon source. This is the basis for the increase in yield and biomass. The increased photosynthesis also provides more reducing power and more biosynthesis of chlorophyll and carotenoids as well as enhancement of antioxidants concemyraions. This would enhance the resistance of the plant to environment stresses, such as exposure to high O3. The enhancement of the antioxidative ability is especially important since O3 itself is a strong oxidant. Enriched CO2 also decrease stomata conductance of the leaves. This would reduce the flux of O3 into leaves though stomata. Take these two factors together, the effect of enriched CO2 in amelioration of the harmful effect of O3 is reasonable.
O3 as a strong oxidant, is highly injurious to the plant tissues. It inhibits photosynthesis,1,2,12 decrease yield and biomass production.35,36,38 It directly attacks the cell membranes inducing increase in lipid peroxidation and ion leakage. By inhibiting photosynthesis, the biosynthesis of the antioxidants or active oxygen scavengers may also be affected. The imbalance between the generation and scavenge of active oxygen would decrease the stress resistance of the plant.
It is interesting to note that when plants exposed to O3 was less than 20 days, they responded in a way that it induced higher SOD activity, higher chlorophyll and carotenoids content. This indicates that small dosage O3 or a short duration of exposure might not result in damage but induce acclimation response of the plant. But soon the accumulated dosage had increased to the level that intolerable to the plant and became injurious as indicated by the fast decrease in SOD activity, pigments content and increase in MDA accumulation and ion leakage.3,5,31 The decrease in the anti-oxidative ability and increased production induced by high O3 exposure is an indication of senescence. This is consistent to the fact that the plant exposed to doubled O3 shed their leaves 7-8 days earlier than others.
It was found in the present study that elevated CO2 confer some protection against O3, and it was clear that stress caused by CO2 predisposed leaves to injury caused by O3 and not vice versa and this explain how they interact to alleviate foliar injury.39
In the present study, increased GR concentration and POD activity in ambient air can be interpreted as response to oxidative stress imposed by O3 (Chernkova et al. 2000). However, the lack of significant changes in activities of SOD and AA in ambient air, which also observed in other O3 studies,40 differed from studies where activities of these enzymes increased in response to O3.41 The variability in the response of antioxidants to elevated O3 and CO2 among studies reflects differences in the magnitude of the perceived oxidative stress, the species-specific mechanisms involved in responses to changes in redox status, the plant capacity to cope with additional stress(s), experimental protocols and environmental conditions. Therefore, further studies are needed to further the understanding of the response of antioxidant metabolism to elevated O3, CO2 and drought singly and in combination.17,18,24,25,27,30
Conclusions
Ozone exposure reduced corn grain yield in response to 03 damage during the flowering process. Both O3 and CO2 had a major impact on physiological processes that are independent on PAR absorption. Therefore, radiation use efficiency (RUE) in response to gases treatments, in wheat, was significantly increased in response to CO2 enrichment and significantly reduced in response to O3-induced stress. While water use efficiency (WUE) was reduced in O3- stressed plants grown under water stress and elevated CO2. Similar results were observed for the control treatment and the high-O3/enriched-CO2 treatment, indicating that the damaging effect of 03 air pollution was counteracted by the beneficial effect of CO2 enrichment without any interactive effects between the two gases for the measured variables except for grain yield, during the first wheat experiment, where the CO2-enriched environment more than overcame the O3-induced stress.
Stomata closed under water stress treatment and decreased influx of O3 and CO3, which would affect growth, yield and physiology of both crops. Closure of stomata is beneficial as less O3 influx but reduction in CO2 influx would reduce photosynthetic rates of both crops and hence lowering yield
Acknowledgements
Part of this work was funded by a fellowship grant from UNESCO to RHB. Thanks to Late Prof Adel Aal (Alexandria University) for his suggestions and help at early stages of the work.
References
- Hassan, I.A. (2010). Interactive effects of O3 and CO2 on growth, physiology of potato (Solanum tuberosum L.). World J. Environ. & Sustainable Development, 7: 1–12.
- Rich, S. (1964). Ozone damage to plants. Annu. Rew. Phytopathology. 1964, 2:253-266.
- Solomon, S D. Qin, M. Manning, M. Marquis, K. Averyt, M. M. B. Tignor, H. L. Miller, Jr., and Z. Chen, Eds. (2007). Climate Change 2007: The Physical Science Basis. Cambridge University Press, 996 pp.
- Solomon, S., G. K. Plattner, R. Knutti, and Friedlingstein, P. (2009). Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA, 106, 1704–1709.
- Solomon, S. K. Rosenlof, R. Portmann, J. Daniel, S. Davis, T. Sanford, and G. K. Plattner, (2010). Contributions of stratospheric water vapor to decadal changes in the rate of global warming. Science, 327, 1219–1223
- Amunason, R. G. (1991). Seasonal changes in the pigments carbohydrates and growth of red spruce as affected by expose to ozone for two growth seasons. New Physiol. 1991, 118:127-137
- Bender, J. (1994). Response of cellular antioxidants to ozone in wheat flag leaves at different stages of plant development. Environ Pollut. 84:15-21
- Drake, B.G., Leadley, P.W. (1991). Canopy photosynthesis of crops and native plant communities exposed to long-term elevated CO2. Plant Cell Environ, 14:853-860
- Pooter, H. (1993). Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio, 104,77-97
- Idso, S.B., Allen, S.G., Anderson, M.G. (1989). Atmospheric CO2 enrichment enhances survival ofAzolla at high temperatures. Environ Exp Bot, , 29:337-341
- Polley, H.W., Hohnson, H.B., Mayeus, H.S. (1996). Carbon dioxide enrichment improves growth, water relations and survival of droughted honey mesquite (Prosopis glandulosa) seedlings. Tree Physiol, 1996, 16:817-823
- Olszyk, D.M., (1986). Effects of sulfur dioxide and ambient ozone on winter wheat and lettuce. J. of Environ, 15:363-369
- Osamu, I., Effects of NO2 and O3 alone or in combination on kidney bean plants (phaseolus vulgaris L.): Growth, partitioning of assimilates and root activities. J. Exper. Bot., 1985, 36(4): 652-662.
- Maehly A.C., Chance B. (1954) The assay of catalase and peroxidase. In: Methods of Biochemical analysis, pp. 357–424. Ed. D. Glick. New York: Interscience.
- Rudorff, B. F. T., Mulchi, C. L., Lee, E. H., et al., Effects of enhanced O3 and CO2 enrichment on plant characteristics in wheat and corn. Environ. Pollut., 1996,94:53-60
- Lawson, T., Craigon, J., Black, C.R. (2001). Effects of elevated carbon dioxide and ozone on the growth and yield of potatoes grown in open-top chambers. Environmental pollution, ,11(3): 479-491
- Noormets, A., McDonald, E.P., Dickson, R.E, Kruger, E.L., Sober A, Isebrands J.G and Karnosky D.F. The effect of elevated carbon dioxide and ozone on leaf- and branch- level photosynthesis and potential plant-level carbon gain in aspen. Trees, 001,15(5):262-270
- Lindroth, R. L., Kopper, B. J., Parsons, W. F. J., et al., Consequences of elevated carbon dioxide and ozone for foliar chemical composition and dynamics in trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera). Environmental Pollution, 2001, 115(3): 395-404
- Mulchi, C. L.,Slaughter, L., Saleem, M., et al., Growth and physiological characteristics of soybean in open-top chambers in response to ozone and increased atmospheric CO2. Agriculture Ecosystems and Environment. 1992, 38:107-108
- Wang C.Y, Gao S.H. (1994).The structure and performance of open top chamber (OTC). Advances in Environmental Science, 3(2): 19-31
- Li J.S., Wang H.C., Wang W.Y. et al., Effect of drought on the permeability and membrane lipid composition from maize leaves. Acta Phytophysiologia Sinica, 1983,9(3): 223-229.
- Lee E.H., Upadhyaya, A., Agrawal, M., Rowland R. A. (1997). Mechanism of ethylenediurea (EDU) induced O3 protection: re-examination of free radical scavenger systems in snap bean exposed to O3. Environmental and Experimental Botany, 38, 199 –209.
- Dhindsa, R.S., Matowe W. Drought tolerance in two Mosses: correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot., 1981,32:79-91
- Wang A.G., Luo G.H., Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiology Communications, 1990, (6): 55-57
- Haim D, Rabinowitch and Sklan D., Superoxide dismutase: a possible protective agent against sunscald in tomatoes (Lycopersicon esculentum Mill). Planta, 1980, (148): 162-167.
- Taie, W, Basahi, J. & Hassan.I.A (2013). Impact of ambient air on physiology, pollen tube growth and pollen germination in pepper (Capsicum annuum L.). Pakistan J. Botany, 45 (3): 2314 – 2322
- Zhang X.Z., Chen FHG., Wang R.F. et al., Experimental technique of plant physiology.Liaoning Technology Publishing Company, China. 1994, pp: 145-148
- Hao J.J, Liu Y.J., Experimental technique of plant physiology. Liaoning Technology Publishing Company, China. 2001, pp:71-74
- Uocking PJ, Mcyar C.P. Effect of CO2 environment and nitrogen stress on growth and partitioning of dry matter and nitrogen in wheat and maize. Aust J Plant Physci, 1991,18: 339
- Kch, K. E., Jones, H P., Avignc, W. T. (1986). Growth, dry matter partitioning and diurnal activities of RUBP carboxylase in cirtus seedlings maintained at two levels of CO2. Plant Physiol, ,67: 447
- Havelke V. D. Ackerson R. C. Boyle M. G. (1984). CO2-enrichment effect on soybean physiology I. Effects of long-term CO2 exposure. Crop Sci., 24:1146
- Yoshida. Z. (1973). Effects of CO2 enrichment at different stages of panicle development of yield of rice.Crop nutr, ,19:311 - 316
- Admos, R. M., A re-assessment of the economic effect of ozone on US agriculture. JAPCA, 1989, 39:960-968.
- McKee, I.F., Mulholland, B.J., Craigo, J., Black, C.R., and Long, S.P. (2000). Elevated concentrations of CO2 protects against and compensate for O3 damage to photosynthetic tissues of field-grown wheat. New Phytol., 146: 427 – 435.
- Noormets, A., McDonald, E.P., Dickson, R.E, Kruger, E.L., Sober A, Isebrands J.G anKarnosky D.F. (2011). The effect of elevated carbon dioxide and ozone on leaf- and branch- level photosynthesis and potential plant-level carbon gain in aspen. Trees, 15(5): 262-270.
- Ojanpera, K., Patsikka, E., and Ylaranta, T. (1997) Effects of low ozone exposure of spring wheat on net CO2 uptake, Rubisco, leaf senescence and grain filling. New Phytologist 138, 451–460.
- Hassan, I.A. (2006) Physiological and biochemical response of potato (Solanum tuberosum L. Cv. Kara) to O3 and antioxidant chemicals: possible roles of antioxidant enzymes. Annals of Applied Biology, 146: 134 – 142.
- Hassan, I.A., Basahi, J.M., Ismail, I.M. (2013). Gas exchange, chlorophyll fluorescence and antioxidants as bioindicators of airborne heavy metal pollution in Jeddah, Saudi Arabia. Current World Environment, 8(2) 203 – 213.
- Vorne, V., Ojanperä, K., De Temmerman, L., Bindi, M., Högy, P., Jones, M.B., Lawson, T. and Persson, K. (2002). Effects of elevated CO2 and O3 on what and corn quality in the European multiple-site experiment “CHIP- Project”. Eurp. J. Agron., 17: 369 – 381.
- Azevedo, R.A., Alas, R.M., Smith, R.J. and Lea, P.J. (1998). Response of antioxidant enzymes to transfer from elevated CO2 to air and O3 fumigation, in the leaves of wild-type and a catalse deficient mutant of barely. Physiol. Plant, 104: 280 – 292.
- Chernikova, T., Robinson, J.M., Lee, E.H. and Mulchi, C.L. (2000). O3 tolerance and antioxidant enzyme activity in soybean cultivars. Photosynthtica Research, 64: 15 – 26.







