A Simple Electrochemical Approach for Determination and Direct Monitoring of Drug Degradation in Water
M Tahir Soomro1 * , Iqbal M. I. Ismail1, 2 , Abdul Hameed1 and Mohammad Aslam1
1
Center of Excellence in Environmental Studies,
King Abdulaziz University P.O. Box 80216,
Jeddah,
21589
Saudi Arabia
2
Chemistry Department,
Faculty of Science,
King Abdulaziz University P.O. Box 80216,
Jeddah,
21589
Saudi Arabia
DOI: http://dx.doi.org/10.12944/CWE.8.3.05
Copy the following to cite this article:
Soomro M. T, Ismail I. M. I, Hameed A, Aslam M. A Simple Electrochemical Approach for Determination and Direct Monitoring of Drug Degradation in Water. Curr World Environ 2013;8(3) DOI:http://dx.doi.org/10.12944/CWE.8.3.05
Copy the following to cite this URL:
Soomro M. T, Ismail I. M. I, Hameed A, Aslam M. A Simple Electrochemical Approach for Determination and Direct Monitoring of Drug Degradation in Water. Curr World Environ 2013;8(3). Available from: http://www.cwejournal.org/?p=5073
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-07-23 |
|---|---|
| Accepted: | 2013-08-02 |
Most pharmaceuticals are persistent in wastewater especially the effluent from pharmaceutical industries and also from hospital wastes. Considerable attention has been already devoted for the removal of these pharmaceuticals from wastewater. Ibuprofen (IBP) and paracetamol (PCM) are two important pharmaceutical drugs which are regularly used to cure from fever, migraine, and diseases resulted from inflammatory disorder.1-4 Figure 1 shows the chemical structure of IBP and PCM.
It is reported that presence of IBP and PCM and their metabolites in wastewater have adverse effects on environment due to the ecotoxicity potential and their exposure could also be very harmful to human health.5, 6 Therefore their detection and removal from wastewater play vital role in wastewater treatment methodologies.
Several methods have been described in literature regarding detection of IBP and PCM using conventional or novel techniques. Such techniques include spectrophotometry, spectrofluorimetry, GC-MS, HPLC, electrophoresis and etc.1-4, 7-12 In addition electrochemical techniques are also widely use for determination of IBP and PCM in wastewater.12, 13 But unexpectedly only limited number of reports are found in literature illustrating the methods for removal of IBP and PCM from wastewater.5, 6
In the present work students are introduced to the electrochemical detection of IBP and PCM in water. Instrumentation for electrochemical measurements as compared with other available techniques is cost effective, simple and easy to handle. Sensitivity and fast analysis are additional advantages.
Cyclic voltammetry is one of the very versatile and most regularly used electrochemical technique in fundamental research. On the other hand square wave (SQW) voltammetry affords high sensitivity with fast analysis time and this is because of zero background charging current. An excellent material for supplementary reading is suggested to acquire necessary understanding of CV and SQW voltammetry.14-20 Both, CV and SQW voltammetry were employed to examine IBP and PCM in water.
The sunlight photocatalytic environmental remediation is regarded as the cheap and clean alternative of currently expensive technologies as it leads to the complete mineralization of pollutants essentially in water purification.21-23 Using cyclic voltammetry the sunlight photocatalytic degradation of pharmaceutical drugs were also studied. The method is novel and provide us opportunity to observe the complete mineralization of IBP and PCM present in wastewater. Using cyclic voltammetry the information of degradation product and mechanism of degradation, as a result of photocatalytic degradation, is also obtained
The work is purposefully designed to develop a easy protocol for determination and removal of pharmaceuticals drugs present in wastewater using electrochemistry.
 |
Figure 1: Structure of IBP and PCM Click here to View figure |
Experimental Reagents and Solutions
Ibuprofen (IBP) and paracetamol (PCM) tablets were purchased from local market in Jeddah, Kingdom of Saudi Arabia. Before cyclic voltammetric measurements IBP and PCM were purified by means of re-crystallization procedure. 5-10 g of IBP tablet was taken and grinded using pestle and mortar. A fine powder obtained was further added in 60-80 mL of acetone in 100 mL beaker. After that a continuous stirring was carried out using glass rod for approximately 10-20 minutes so that maximum amount of IBP dissolve in acetone. Then the mixture was filtered and acetone evaporated on hot plate at 40 ºC until thick liquid obtained. Finally the beaker was kept in vacuum oven for 2-3 hours to obtain purified crystals of IBP. For purification of PCM followed the same procedure.
Potassium chloride (BDH), potassium ferricyanide (HiMedia), sodium acetate (Sigma), and acetic acid (Sigma) were used as received from the supplier without further purification.
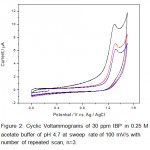 |
Figure 2: Cyclic Voltammograms of 30 ppm IBP in 0.25 M acetate buffer of pH 4.7 at sweep rate of 100 mV/s with number of repeated scan, n=3 Click here to View figure |
Milli-Q water was used throughout the study for making sample solutions. Stock solutions of 100 ppm of IBP and PCM were made in ethanol and used for further dilution using acetate buffer. All solutions were made in acetate buffer of pH 4.7 and concentration 0.25 M. All glassware were cleaned carefully, rinsed at least three times with double distilled water and oven dried at 100 ºC before use.
ZnNO3 (HiMedia), KOH (Loba Chemie), and ammonium metavanadate (NH4VO3, Loba Chemie) were purchased for synthesis of ZnO based photocatalyts. The ZnO based vanadium impregnated photocatalyst was synthesized by wet impregnation techniques using ammonium metavanadate as precursors for V6+ ions. The ZnO used in the preparation of impregnated catalyst was synthesized by hydrolyzing Zinc nitrate solution by KOH. The hydrated gel after filtration and drying was calcined at 500oC in muffle furnace. After impregnation the impregnated catalyst was again calcined for optimum surface geometry.
 |
Figure 3: Cyclic Voltammograms of 30 ppm PCM in 0.25 M acetate buffer of pH 4.7 at scan rate of 100 mV/s with number of repeated scan, n=3. An inset graph showing cyclic voltammogram of PCM scanned between 0 V to + 1.5 V vs. Ag/AgCl. Click here to View figure |
Apparatus and Procedure
A VSP multi-channel potentiostat (Biologic Science Instrument, USA) provided with Ec-lab Software was used for carrying out all voltammetric measurements. A Glassy carbon (GC) working electrode with a diameter of 3 mm, a platinum (Pt) counter electrode, and a Ag/AgCl reference electrode were purchased (Biologic Science Instrument) and used as received. The electrochemical cell of maximum capacity of 10-20 mL equipped with gas bubbler and gas outlet assembly was used. All voltammetric measurements was accomplished using usual procedure such as by immersing working, counter and reference electrodes in an electrochemical cell. All experiments were performed at ambient temperature. The 30 ppm sample solutions of IBP and PCM were tested for their identification. Variable scan rate from 10-500 mV/s was employed using cyclic voltammetry. The square wave voltammetric parameters are as follows: pulse height 25 mV, pulse width 50 ms, and step height 10 mV. All cyclic voltammograms presented were recorded against Ag/AgCl reference electrode and all potential values are in volts vs. Ag/AgCl reference electrode.
The working electrode was pretreated (activated) by performing 10 cycles in 0.1 M H2SO4 between 0 V to 2 V at 100 mV/s. The working electrode surface was cleaned first using diamond paste and then with alumina paste by manual procedure. After that the polished electrode was rinsed with double distilled water and immersed in 5% HNO3 solution for 5 second then rinsed again with double distilled water and air dried before use. No deterioration of working electrode was detected during voltammetric measurements. The background current was also measured in absence of pharmaceutical drugs in acetate buffer.
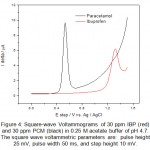 |
Figure 4: Square-wave Voltammograms of 30 ppm IBP (red) and 30 ppm PCM (black) in 0.25 M acetate buffer of pH 4.7. The square wave voltammetric parameters are: pulse height 25 mV, pulse width 50 ms, and step height 10 mV. Click here to View figure |
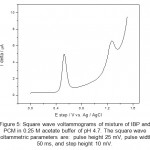 |
Figure 5: Square wave voltammograms of mixture of IBP and PCM in 0.25 M acetate buffer of pH 4.7. The square wave voltammetric parameters are: pulse height 25 mV, pulse width 50 ms, and step height 10 mV. Click here to View figure |
Sunlight Photocatalytic Degradation
To study the photocatalytic degradation 50 ppm solution of IBP in simple Milli-Q water was used. 100 mg vanadium impregnated ZnO powder was added in 100 mL solution of IBP and stirred for 30 minutes to get the homogenous dispersion. After that the solution was exposed to sunlight. For cyclic voltammmetric measurements 10 mL of IBP was taken at different period of time and diluted using acetate buffer and Milli-Q water. The photocatalytic degradation of PCM was also studied followed the above mentioned procedure.
Results and Discussion
Voltammeric Response at Bare Glassy Carbon Electrode
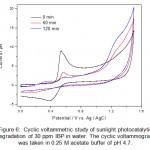 |
Figure 6: Cyclic voltammetric study of sunlight photocatalytic degradation of 30 ppm IBP in water. The cyclic voltammogram was taken in 0.25 M acetate buffer of pH 4.7. Click here to View figure |
The absence of reduction peak on the reverse scan indicating that the formation of highly reactive intermediate product upon oxidation which reacted either with solvent or dimerized quickly into a dimer. The electrochemical oxidation of IBP fits into an EC reaction that is electron transfer reaction followed by homogeneous chemical reaction.
A - e = B (1)
B → Product (2)
The characteristic cyclic voltammogram obtained for PCM is represented in figure 3. To obtain a well defined peak the potential was scanned between 0 V to +1 V. An inset graph in figure 3 also recorded for the potential range of 0 V to +1.5 V just to confirm that there is no other peak for PCM. The cyclic voltammogram of PCM is interpreted as quasi reversible process, with ∆E ≥ 100 mV/n, coupled with a slow chemical reaction i.e., an EC reaction.
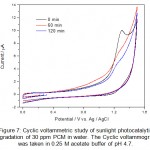 |
Figure 7: Cyclic voltammetric study of sunlight photocatalytic degradation of 30 ppm PCM in water. The Cyclic voltammogram was taken in 0.25 M acetate buffer of pH 4.7. Click here to View figure |
Electrochemically well defined response depends on many factors and some of them are electrode material, supporting electrolyte, and solvent decomposition potentials (i.e., potential window). Because water oxidize itself when potential applied further than +1.23 V and due to this water decomposition background current the working range (potential) is restricted to +1.5 V when using glassy carbon as working electrode. So beyond +1.5 V the oxidation or reduction peak, if any, is obscured.
From the cyclic voltammograms of IBP and PCM one can also deduce that the oxidation of PCM is easier than IBP. The potential difference in peak position is found to be 800 mV which allows easy identification of IBP and PCM in same solution. Overlapped square wave (SQW) voltammograms over one another are shown in figure 4 obtained for IBP and PCM. Square wave voltammogram for a mixture of IBP and PCM was also recorded and is shown in figure 5. As expected the peaks of IBP and PCM are clearly resolved and identified without any problem and letting us analysis of mixture from wastewater. To obtain a well define square wave voltammogram optimization studies were also done. The optimal values of square wave voltammetric parameters were obtained and are as follows: pulse height 25 mV, pulse width 50 ms, and step height 10 mV. All square wave voltammograms were recorded using such parameters.
Voltammetric Study of Sunlight Photocatalytic Degradation in water
Sunlight photocatalytic degradation offers a new alternative against persistent organic pollutants present in wastewater. During the CV analysis of the degradation products in the photocatalytic degradation studies it was observed that CV is a powerful alternative of other analytical techniques such as UV-Visible spectroscopy and HPLC for monitoring the degradation products. The sunlight photocatalytic degradation of IBP and PCM are shown in figure 6 and figure 7. The studies were carried out in different time intervals. The complete degradation and/or mineralization of IBP and PCM were observed in about one hour. No side products or metabolites were detected in cyclic voltammetric investigations. The complete mineralization of pharmaceutical drugs into inorganic ions also evidenced from the increase of the background current in cyclic voltammograms of IBP and PCM.
Conclusion
The protocol demonstrated above is successfully applied for the determination of IBP and PCM in water using cyclic voltammetry and SQW voltammetry. Sunlight photocatalytic degradation study of IBP and PCM was also carried out using cyclic voltammetry. Cyclic voltammetry is very useful for identification of pharmaceutical drugs. On the other hand for quantitation square wave voltammetry is more superior than CV and can be easily used for that purpose. The developed protocol given students an understanding about electrochemical instrumentation used for pharmaceutical drug determination in wastewater. In addition, students also learned about novel method for removal of pharmaceutical drug from wastewater. As a result students received experience in both electrochemistry and method development.
Acknowledgment
For carrying out the work the Ministry of Higher Education (MOHE) and King Abdulaziz University (DSR) are highly appreciated for their technical and financial support.
References
- Miner D. J., Rice J. R., Riggin R. M. and Kissinger P. T., Analytical Chemistry, 53, 2258 (1981).
- Motoc S., Manea F., Pop A., Pode R. and Burtica G., Advanced Science, Engineering and Medicine, 3, 7 (2011).
- Hamoudová R. and Pospíšilová M., Journal of Pharmaceutical and Biomedical Analysis, 41, 1463 (2006).
- Wangfuengkanagul N. and Chailapakul O., Journal of Pharmaceutical and Biomedical Analysis, 28, 841 (2002).
- Escher B. I., Baumgartner R., Koller M., Treyer K., Lienert J. and McArdell C. S., Water Research, 45, 75 (2011).
- Ort C., Lawrence M. G., Reungoat J., Eaglesham G., Carter S. and Keller J., Water Research, 44, 605 (2010).
- Kachoosangi R. T., Wildgoose G. G. and Compton R. G., Analytica Chimica Acta, 618, 54 (2008).
- Ghoneim E. M. and El-Desoky H. S., Bioelectrochemistry, 79, 241 (2010).
- Whelan M. R., Ford J. L. and Powell M. W., J Pharm Biomed Anal, 30, 1355 (2002).
- Khoshayand M. R., Abdollahi H., Shariatpanahi M., Saadatfard A. and Mohammadi A., Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 70, 491 (2008).
- Ternes T. A., Trends in Analytical Chemistry, 20, 419 (2001).
- Manea F., Motoc S., Pop A., Remes A. and Schoonman J., Nanoscale Research Letters, 7, 331 (2012).
- Stefan-van Staden R.-I., Mashile T., Mathabathe K. C. and van Staden J. F., Instrumentation Science & Technology, 37, 197 (2009).
- Chen A. and Shah B., Analytical Methods, 5, 2158 (2013).
- Bard A. and Faulkner L., Electrochemical methods: fundamentals and applications, Wiley (2001).
- Heinze J., Angewandte Chemie International Edition in English, 23, 831 (1984).
- Kissinger P. T. and Heineman W. R., Journal of Chemical Education, 60, 702 (1983).
- Mabbott G. A., Journal of Chemical Education, 60, 697 (1983).
- Krause M. S. and Ramaley L., Analytical Chemistry, 41, 1365 (1969).
- Wang J., Bollo S., Lopez Paz J. L., Sahlin E. and Mukherjee B., Analytical Chemistry, 71, 1910 (1999).
- Hameed A., Montini T., Gombac V. and Fornasiero P., Photochemical & Photobiological Sciences, 8, 677 (2009).
- Fatin S., Lim H., Tan W. and Huang N., Int. J. Electrochem. Sci, 7, 9074 (2012).
- Reddy S., Kumara Swamy B. E., Vasan H. N. and Jayadevappa H., Analytical Methods, 4, 2778 (2012).







