Gas Exchange, Chlorophyll Fluorescence and Antioxidants as Bioindicators of Airborne Heavy Metal Pollution in Jeddah, Saudi Arabia
I.A Hassan1,2 * , J.M. Basahi2,3 and I.M. Ismail2,4
1
Faculty of Science,
Alexandria University,
Alexandria,
21526
El Shatby
Egypt
2
Centre of Excellency in Environmental Studies,
King Abdulaziz University, P.O. Box 80216,
Jeddah,
21589
KSA
3
Faculty of Environment, Meteorology and Arid Land Agriculture,
King Abdulaziz University,
Jeddah,
21589
KSA
4
Department of Chemistry,
Faculty of Science,
King Abdulaziz University, P.O. Box 80203,
Jeddah,
21589
KSA
DOI: http://dx.doi.org/10.12944/CWE.8.2.05
Copy the following to cite this article:
Hassan I. A, Basahi J. M, Ismail I. M. Gas Exchange, Chlorophyll Fluorescence and Antioxidants as Bioindicators of Airborne Heavy Metal Pollution in Jeddah, Saudi Arabia. Curr World Environ 2013;8(2) DOI:http://dx.doi.org/10.12944/CWE.8.2.05
Copy the following to cite this URL:
Hassan I. A, Basahi J. M, Ismail I. M. Gas Exchange, Chlorophyll Fluorescence and Antioxidants as Bioindicators of Airborne Heavy Metal Pollution in Jeddah, Saudi Arabia. Curr World Environ 2013;8(2). Available from: http://www.cwejournal.org/?p=4818
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-07-09 |
|---|---|
| Accepted: | 2013-08-05 |
The rapid increasing population in urban areas led to anthropogenic activities and fossil fuel combustion. Emissions from road traffic that uses fossil fuel, industry, agriculture, sewage sludge, and waste incineration are the chief sources of air pollution.1,2 Air pollutants especially heavy metals are hazardous and toxic to human beings depending on their concentrations in the food stuff.3,4 Presence of airborne heavy metals in vegetable crops above the permissible limit may lead to severe health hazards to the people consuming it.5 So the estimation of their levels in contaminated food is very important for the safety of human health.3,6
Increasing industrialization, urbanization and vehicular traffic in Jeddah city could increase levels of heavy metals in air and soil2 which lead to a high pollution pressure on the biota and eventually, would pose a threat to food safety and human health.7,8
Metal pollutants found as superficial contaminants on leaves thereby, they are especially useful as biological indicators to assess air pollution indicator for metal pollution.9-17 Because of the different characteristics of foliar uptake, accumulation and translocation of atmospheric heavy metals by leaves, plant leaves are used as bioindicators and/or biomonitors of heavy metal pollution in the terrestrial environment.18–20 Although it was reported that mosses and lichens are good monitors of heavy metal pollution, higher plants can be used as biomonitors in areas that do not have these species.21–23
Photosynthesis (PN) is inhibited by air pollution and other environmental stresses.24–28 Ouzounidou et al.,29 found reductions in rate of photosynthesis stomatal conductance (gs), the maximum quantum yield of primary photochemistry, variable fluorescence (Fv) and chlorophyll concentration in Ni-stressed wheat. Recently, a marked toxicity of heavy metal pollution to photosynthetic apparatus in maize plants was reported.30 They found a decline of fluorescence induction kinetics as well as of chlorophyll and carotenoid concentrations in Ni-stressed plants of maize. However, the main mechanism primarily affecting photosynthesis in response to heavy metals is not clear.17 Heavy metals have detrimental effects on the enzymatic capacity and gs of the photosynthetic apparatus.31
In Saudi Arabia, air pollution due to the heavy metals arises from road traffic that uses fossil fuel, industry, agriculture, sewage sludge, and waste incineration as well as from the dust storms.32-34 However, Studies regarding the contamination of heavy metals in the vegetable crops are scanty. Therefore, it is important to study the heavy metals contamination in plants m that could presumably be used as a biological indicator of heavy metal pollution so as to decide if it is safe or not for human consumption.2,34
Airborne heavy metals are hazardous and toxic to human beings depending on their concentrations in the food stuff.4 During the past few decades, there has been an increase in the use of levels of higher plant as biomonitors of heavy metal pollution in the arid and semi-arid environments such as Saudi Arabia.9,32-36
The aim of present study was aimed at evaluating lettuce (Lactuca sativa L. cv Romaine) leaves as a biomonitor of airborne heavy metals in order to assess whether the vegetable crops were safe for human consumption.
Material and Methods
Plant material, growth conditions and experimental design Seeds of Lettuce (Lactuca sativa L. cv Romaine) plants were washed with distilled water to remove excess pesticides or herbicides and to break dormancy. Experimental design and growth conditions were discussed elsewhere.2 Gas exchange and fluorescence measurements The photosynthetic gas-exchange measurements were done by a portable photosynthesis system LI 6000 (Li- Cor, USA). The pots were located in a climatic box, where plants were adapted for 1 h at a photon flux density (PFD) of 450 mmol m-2 s-1 (PAR). The leaf gas-exchange was determined under the following conditions: PFD of 900 mmol m-2 s-1, leaf temperature of 31.50C, ambient CO2 concentration of ca 400 mmol mol-1 and relative air humidity of about 65%. For each measurement, the first top fully developed leaves from the main stems of six plants were used on weekly basis.16
Chlorophyll fluorescence was measured by a Fluorescence Monitoring System (FMS, Hansatech Instruments, U.K.). Measurements were made in ambient [CO2] (Ca, 450 mmol mol-1) on individual leaves enclosed into a leaf cuvette under a rate of 0.44 L min-1 air flow, relative humidity within the cuvette at 50-55%, a leaf temperature of 400C and 900 mmol m-2 s-1 of light intensity.31 The maximum quantum yield of PSII in dark adapted leaves was estimated by the ratio between variable and maximal fluorescence, Fv/Fm = (Fm - F0)/Fm. The efficiency of water-splitting apparatus was estimated by ratio between basal and variable fluorescence, F0/Fv.37 Oxygen concentration was lowered to 1.5% when testing leaf gas exchange under non-photorespiratory conditions17
Gas exchange parameters and chlorophyll fluorescence yield were measured simultaneously.
Pigment Concentration
Chlorophylls (a & b) were extracted in 85% acetone and measured on a UV-1800 Spectrophotometer (SHIMADZU) and their concentrations were calculated.21 Leaves of the same age as those in the gas-exchange analyses were used.
Antioxidant enzymes
Tissue samples of 5 young and 5 expanded leaves were homogenized and dialyzed.38 The dialyzed samples were used for enzymatic and protein content determinations. Activities of CAT, POX, and SOD were determined.39 One unit of CAT and POX is defined as the number of mmoles of H2O2 consumed per minute, and one unit of SOD as the enzyme content which gives 50% inhibition of cytochrome c reduction.
Lipid peroxidation
Lipid peroxidation of lettuce leaves (n = 10) was determined by measuring malondialdehyde (MDA) production.40 Tissues samples were homogenized in 0.1% trichloro acetic acid, centrifuged (20,000g, 15min) and the supernatants were collected. To 1 ml aliquots of supernatant, 4 ml of a solution of 20% trichloroacetic acid and 0.5% thiobarbituric acid was added; the mixture was heated (95 1C; 30min),quickly cooled, and then centrifuged (10,000g,10min). Supernatants were used to determine MDA content at 532 nm.41
Elemental analysis
The elemental analysis was performed by inductively coupled plasma optical emission spectrometry (ICP-OES) using IRIS Intrepid II XSP instrument.2 Six point calibration procedure was applied with multi-element calibration solution (Merck ICP multi-element standard solution IV).42
Statistical Analysis
Data were subjected to one way ANOVA, using the SATATGRAPHICS statistical software package. Least Significant Difference (LSD) Test was applied to assess the significant differences among the mean values of different attributes. The values are means of ten replications. Data were log transformed prior to analysis to ensure normality and equality of variance. The relationships between sites and different parameters were assessed using correlation analysis. There were 6 replicates
Results
Toxicity symptoms and plant growth Lettuce plants developed visible injury symptoms, especially in older leaves collected from industrial and urban areas which exhibited chlorotic and brown necrotic lesions (Fig. 1). Furthermore, accentuated necrosis and leaf fall were observed in the oldest plant leaves collected from the same areas during the third week of exposure
.
 |
Figure 1: Small chlorotic stippling on the old leaves of the plant. (a) Control plants, (b) plants collected from urban and industrial areas. Arrows indicate Chlorotic and necrotic lesions on leaves Click here to View figure |
The growth of lettuce shoots was significantly reduced in industrial, urban and residential areas (p <0.05) when compared to the control. Length of shoot was decreased by 51, 41, 25, 30 and 18% in plants collected from industrial, urban, suburban, residential and rural sites, respectively (Fig 2).
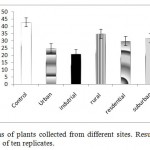 |
Figure 2: Shoot lengths of plants collected from different sites. Results are expressed as mean +/- 1 SE of ten replicates. Click here to View figure |
Figure 3 shows that soils collected from industrial and suburban sites have the highest concentrations of heavy metals.
 |
Figure 3: Percentage of different heavy metals collected from different areas. Click here to View figure |
Gas exchange, Chlorophyll Fluorescence and Pigments
Net Photosynthetic rates (PN) were decreased by 53, 51, 37, 36 and 14% in plants collected from industrial, urban, suburban, residential and rural sites, respectively (Table 1). Stomatal conductance (gs) was also decreased by 46, 49, 29, 33 and 12% in plants collected from the same sites, respectively (Table 1).
Table 1 also shows that the maximum quantum yield of PSII (Fv/Fm) was decreased significantly (P ≤ 0.05) at industrial and urban sites by 13 and 10%, respectively, while the reductions were insignificant (P > 0.05) in other sites (Table 1). Leaves collected from different sites had a higher basal fluorescence (F0) level (p ≤ 0.01), and a significant decrease in both maximal fluorescence induction (Fm) and variable fluorescence (Fv) value (p ≤ 0.05) when compared to control.
The superficial observations were consistent with the chlorophyll contents and the fluorescence parameters (Table 1). Chl a , b and Chl a/b ratio were decreased by 70, 59 and 27% in plants collected from industrial area, and by 64, 57 and 16 in plants collected from Urban areas, respectively. These parameters were decreased at other sites but at relatively lower extents (Table 1).
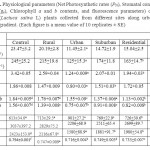 |
Table 1: Physiological parameters (Net Photosynthetic rates (PN), Stomatal conductance (gs), Chlorophyll a and b contents, and fluorescence parameters) of lettuce (Lactuca sativa L) plants collected from different sites along urbanization gradient. (Each figure is a mean value of 10 replicates ± SE) Click here to View table |
Antioxidant enzymes and Lipid peroxidation
SOD was increased by 38, 40, 18, 32 and 31% in leaves collected from industrial, urban, suburban, residential and rural areas, respectively (Table 2). On the other hand, CAT activities were reduced by 41, 38, 18, 22 and 14% in the same site respectively (Table 2). Moreover, POX was reduced by 30, 28, 11 in leaves collected from industrial, urban, suburban sites, respectively, while there was no significant (P > 0.05) effect on leaves collected from residential or urban areas (Tab.2).
Lipid peroxidation as measured by MDA content in lettuce leaves increased significantly (p ≤ 0.05) in plants collected from industrial, urban, suburban and residential areas by 52, 30, 21 and 23%, respectively (Table 2). Rural area had no significant (P > 0.05) effect on MDA (Table 2).
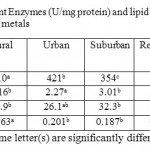 |
Table 2: Response of Antioxidant Enzymes (U/mg protein) and lipid peroxidation (nmol/g fresh wt.) to accumulation of heavy metals Click here to View table |
A least-squares linear regression analysis was obtained for all sites and different physiological and biochemical markers (Table 3). The results show that the correlation coefficients (r) were significant at p<0.001 for gas exchange measurements (PN, gs. Fv/Fm), Chl contents, SOD, CAT, POX and MDA (Table 3).
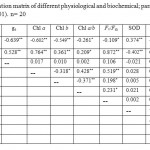 |
Table 3: Correlation matrix of different physiological and biochemical; parameters measures (*p< 0.01, **p< 0.001). n= 20 Click here to View table |
Discussion
Urban atmospheres, particularly those of megacities tend to have higher concentrations of heavy metals and other pollutants than rural (agricultural) ones, reflecting varying contents of contaminants from industrial and vehicular emissions as well as ash and soot coal fires.5,7,14,43,44 Nevertheless, here are limited studies on environmental pollution by heavy metals in Saudi Arabia.
The reduction in growth recorded in the present study is in agreement with the results reported in literature about effects of heavy meal pollution on growth and yield of lettuce (L. sativa L.), bean (phaseolus vulgaris L.) and Lupinus albus L. plants40,42-48
Chlorophyll content is often measured in plants in order to assess the impact of environmental stress, as changes in pigment content are linked to visual symptoms of plant illness and photosynthetic productivity.46–49 Researchers have reported decreased chlorophyll in several different plant species under the impact of heavy metals.21 Heavy metals inhibit metabolic processes by inhibiting the action of enzymes, and this may be the most important cause of inhibition.21,47,50,51 The percentage reduction in Chl. Contents reported in our study is higher than those recorded in other urban areas in Turkey.21,51 and Nigeria.48 This higher percentage of reduction in Chl content of lettuce in the present study is an indicator of disturbances of the pigment synthesis mechanism and inhibition of degradation due to heavy metal effects. Such reductions in Chl content would lead to reduction in photosynthetic rates and eventually growth. Both chlorophyll and A showed a strong negatively correlation with urban and industrial sites, which are characterized by high heavy metal contents in their soils.
The chlorophyll ratio, which is used as a stress indicator, decreased significantly with increasing metal concentrations. Such alteration indicates a change in the PSII/PSI ratio in stressed leaves.47
Plants have evolved a complex antioxidant system to mitigate oxidative stress caused by heavy metals and by other biotic and abiotic stresses. These antioxidants play an important role in the cellular defense strategy. Metals are known to cause molecular damage to plant cells either directly or indirectly through the burst of Reactive Oxygen Species (ROS), which can react with fatty acids leading to the peroxidation of lipids, destroying biological membranes.40
Antioxidants like POX, SOD and CAT are ubiquitous and they play an important role in detoxification of toxic metal ions.47,53 They play a crucial role in plant growth and development. Moreover, they are a potential indicator for metal toxicity.21,51,54
Our results demonstrated that SOD increased linearly with urbanization and contents of heavy metals in soils. Excess of heavy metals can persuade oxidative stress in plants, which can escort formation of ROS. Antioxidant enzymes may alter the H2O2 to the H2O in the plant cells and counteract the toxicity effect of H2O2.54-55 Hence to shield cells against oxidative stress, antioxidant enzymes augmented proportionally, which is also consistent with our results.
On the other hand, activities of CAT and POX were decreased linearly with increasing concentrations of heavy metals. Both increases and decreases were detected in POX and CAT.21,51,54 Exposure to high concentrations of heavy metals resulted in a decreased antioxidant capacity.56–57 In our study, CAT and POX were inhibited with extended exposure to heavy metals at different sites, in exposed leaves. This is in a agreement with other studies bean, (Phaseolus vulgaris L.)58-59 pea, (Pisum sativum L.),60 and in lettuce (Lactuca sativa L.) plants.40
MDA is a cytotoxic product of lipid peroxidation and its formation is routinely used as a general indicator of the extent of lipid peroxidation resulting from oxidative stress.40,61 The elevated MDA content obtained in lettuce leaves in the present study suggests that heavy metals, induced oxidative damage in lettuce as evidenced by increased lipid peroxidation through either indirect production of ROS or through inhibition of oxidative stress enzymes.40 Furthermore, MDA content was increased in leaves of a mangrove plant (Bruguiera gymnorrhiza) when exposed to multiple metals.62 Therefore lipid peroxidation is recommended as a biomarker of heavy metal stress for pollution monitoring purposes.
In general, airborne heavy metal pollution induced senescence in lettuce in the present study, as measured in general as photosynthetic efficiency reduction, decrease in the overall antioxidant capacities of lettuce plants and a MDA production. These alterations were accompanied by an inhibition in the classical endpoint, shoot growth, at the end of exposure. These biomarkers could be used in integrative approaches with classical endpoints in ecotoxicological tests; especially this study was conducted real field conditions. Therefore they could form the basis for monitoring and be predictive of early effects of this pollutant before they give rise to significant changes in natural community structures.
Conclusion
Laboratory and field studies have provided encouraging insights into the capacity of lettuce plants to act as biomonitors of air pollution through the use of biomarkers. However, a better understanding of the overall process of metal-induced senescence, describing the cascade of their effects in plants is needed for a selection of relevant biomarkers of heavy metal stress. Lettuce plants proved to be suitable as usage in environmental studies as a bioindicator.
Acknowledgements
Authors are indebted to Scientific Deanship at King Abdulaziz University for continuous support. This work is supported by a grant (4/H/1433). E would like to Thank Prof N. Saied (Braunschweig, Germany) for her technical support and Ms Samah Shata for her keen assistance.
References
- Celik, A., Kartal, A., Akdogan, A., Kaska, Y. Determination of heavy metal pollution in Denizli (Turkey) by using Robinio Pseudoacacia L. Environ. Int. 31 (1), 105, 2005.
- Hassan, I.A., Bashai, J.M. Assessing roadside conditions and vehicular emissions using lettuce plants grown nearrRoadside in Jeddah, Saudi Arabia. Polish Journal of Environ.Studies, 22 (2).387 – 393, 2013.
- Senesi, G.S., Baldassarre, G., Senesi, N., Radina, B. Trace Element inputs into Soils by Anthropogenic Activities And Implications For Human Health. Chemosphere 39 (2), 343, 1999
- Järup, L. Hazards Of Heavy Metal Contamination. Brit. Med. Bull. 68 (1), 167, 2003.
- Hassan, I.A., Gewifal, I.M. Heavy Metals In Egyptian Soils: Uptake By Vegetable Crops. Egyptian J. Botany, 38, 119, 1999.
- Aldjain, I.M, Al-Whaibi, M. H. Al-Showiman, S S. Siddiqui, M.H. Determination Of Heavy Metals In The Fruit Of Date Palm Growing At Different Locations Of Riyadh. Saudi Journal Of Biological Sciences, 18, 175, 2011.
- Ali, E. A. Damage To Plants Due To Industrial Pollution And Their Use As Bioindicators In Egypt. Environ. Pollut., 81 (3), 251, 1993.
- Zheng, N. Wang, Q.C. Zheng, D.M. Health Risk Of Hg, Pb, Cd, Zn And Cu To The Inhabitants Around Huludazn Plant In China Via Consumption Of Vegetables. Sci. Total Environ. 381, 81, 2007.
- Al-Shayeb, S.M., Al-Rajhi, M.A., Seaward, M.R.D. The Date Palm (Phoenix Dactylifera L.) As A Biomonitor Of Lead And Other Elements In Arid Environments. Sci. Total Environ. 168 (1), 1, 1995.
- Khairiah, J., Zalifah, M.K., Yin, Y.H., Aminha, A. The Uptake Of Heavy Metals By Fruit Type Vegetable Grown In Selected Agricultural Areas. Pak. J. Biol. Sci. 7 (2), 1438, 2004.
- Chojnacka, K., Chojnacki, A., Gorecka, H., Gorecki, H. Bioavailability Of Heavy Metals From Polluted Soils To Plants. Sci. Total Environ. 337, 175, 2005.
- Hashmi, D.R., Siddiqui, I., Shaikh, G.H. Accumulation Of Heavy Metals In Tarry Deposit On Leaves At Various Locations Of Karachi. Chem. Soc. Pak. 28, 125, 2006.
- Kardel, F., Wuyts, K., Babanezhad, M., Vitharana, U.W.A., Wuytack, T., Potters, G., Samson, R. Assessing Urban Habitat Quality Based On Specific Leaf Area And Stomatal Characteristics Of Plantago Lanceolata L. Environmental Pollution 158, 788, 2010
- Abou El Saadat, E. M., Hassan, M. R. Hassan, I. A., Weheda, B. M. Heavy Metal Content In Leaves Of Ficus Retusa Grown In Contaminated And Uncontaminated Sites In Northern Egypt And Mitigation Of Their Toxic Effects By Washing Treatments. Universal J. Of Environ. Research And Tech. 1(4), 408, 2011.
- Simon, E., Braun, M. Vidic, A., Bogyó, D. Fábián, I. Tóthmérész, B. Air Pollution Assessment Based On Elemental Concentration Of Leaves Tissue And Foliage Dust Along An Urbanization Gradient In Vienna. Environmental Pollution 159, 1229, 2011.
- Vassilev, A. Tsonevb, T. Yordanovb, I. Physiological Response Of Barley Plants (Hordeum Vulgare) To Cadmium Contamination In Soil During Ontogenesis. Environ. Pollut. 103, 287, 1998.
- Velikova, V., Tsonev T., Loreto F., Centritto M. Changes In Photosynthesis, Mesophyll Conductance To Co2, And Isoprenoid Emissions In Populus Nigra Plants Exposed To Excess Nickel. Environmental Pollution 159, 1058,2011.
- Aksoy, A., Ozturk, M. A. Nerium Oleander L. As A Biomonitor Of Lead And Other Heavy Metal Pollution In Mediterranean Environments. The Science Of The Total Environment, 205, 145, 1997.
- Tomasevic, M., Vukmirovic, Z., Rajsic, S., Tasic, M., & Stevanovis, B. Characterization Of Trace Metal Particles Deposited On Some Deciduous Tree Leaves In An Urban Area. Chemosphere, 61, 753, 2005.
- Achakzai, A. K., Bazai Z. A., Kayani, S. A. Accumulation Of Heavy Metals By Lettuce (Lactuca Sativa L.) Irrigated With Different Levels Of Wastewater Of Quetta City. Pak. J. Bot. 43(6), 2953, 2011
- DoÄŸanlar, B.Z., Atmaca, M. Influence Of Airborne Pollution On Cd, Zn, Pb, Cu, And Al Accumulation And Physiological Parameters Of Plant Leaves In Antakya (Turkey). Water Air Soil Pollut. 214,509, 2011. Doi 10.1007/S11270-010-0442-9.
- Elbagermi, M. A., Edwards H. G. M., & Alajtal, A. I. Monitoring Of Heavy Metal Content In Fruits And Vegetables Collected From Production And Market Sites In The Misurata Area Of Libya. Isrn Analytical Chemistry Volume 2012, Article Id 8, 5, 2012. Doi:10.5402/2012/827645
- Clijsters, H., Van Assche, F. Inhibition Of Photosynthesis By Heavy Metals. Photosynthesis Research 7, 31, 1985.
- Ahmed, H., Häder, D.P. Rapid Ecotoxicological Bioassay Of Nickel And Cadmium Using Motility And Photosynthetic Parameters Of Euglena Gracilis. Environmental And Experimental Botany 69, 68, 2010.
- Kammerbauer, J., Dick, T. Monitoring Of Urban Traffic Emissions Using Some Physiological Indicators In Ricinus Communis L. Plants. Arch. Environ. Contam. Toxic. 39, 161, 2000.
- Niinemets, Ü. Mild Versus Severe Stress And Bvocs: Thresholds, Priming And Consequences. Trends In Plant Science 15, 145,2010.
- Hassan, I.A. Interactive Effects Of Salinity And Ozone Pollution On Photosynthesis, Stomatal Conductance, Growth, And Assimilate Partitioning Of Wheat (Triticum Asetivum L.) Photosynthetica, 42 (1): 111, 2004.
- Ouzounidou, G., Moustakas, M., Symeonidis, L., Karataglis, S. Response Of Wheat Seedlings To Ni Stress: Effects Of Supplemental Calcium. Arch. Of Environ. Contam. & Toxicol. 50, 346, 2006.
- Drãzkiewicz, M., Baszyňski, T. Interference Of Nickel With The Photosynthetic Apparatus Of Zea Mays. Ecotoxi & Environ. Safety. Doi:10.1016/ J.Ecoenv. 2010. 02.001.
- Seregin, I.V., Kozhevnikova, A.D. Physiological Role Of Nickel And Its Toxic Effects On Higher Plants. Russian J. Plant Physio. 53, 257, 2006.
- Aganchich, B., Wahbi, S., Loreto, F., Centritto, M. Partial Root Zone Drying: Regulation Of Photosynthetic Limitations And Antioxidant Enzymatic Activities In Young Olive (Olea Europaea) Saplings. Tree Physiology 29, 685, 2009.
- Bounessah, M., Al-Shayeb, S. M. Assessment Of Plant Leaves As Biomonitors For Atmospheric Pollution By Lead In Arid Environment: A Comparative Study. Asian J. Of Chemistry, 17(2), 1928, 2005.
- Nrdc, 2005. Toxic Metals In New Orleans Air. Available At: (Retrieved On 12/08/2010).
- Aldjain, I. M. A, Al-Whaibi, M. H. B. Al-Showiman, S.S. C, Siddiqui M. H. Determination Of Heavy Metals In The Fruit Of Date Palm Growing At Different Locations Of Riyadh. Saudi Journal Of Biological Sciences 18, 175, 2011. Doi:10.1016/J.Sjbs.2010.12.001
- Al-Whaibi, M.H. Biology Of Date Palm, Second Ed. Scientific Publications, King Saud University Press, Riyadh, Saudi Arabia (In Arabic). 2008
- Bounessah, M., Al-Shayeb, S.M., Al-Ghefaili, K.M., Abdulfatah, B. Assessment Of Lead Levels In Dust And Date Palm (Phoenix Dactylifera L.) In 6–10 Year-Old School Children Environment In Riyadh City, Saudi Arabia. Asian J. Chem. 13 (4), 1435, 2001.
- Kriedemann, P.F., Graham, R.D., Wiskich, J.T. Photosynthetic Dysfunction And In Vivo Chlorophyll A Fluorescence From Manganese- Deficient Wheat Leaves. Australian Journal Of Agricultural Research 36, 157, 1985.
- Santos, C.L.V., Campos, A., Azevedo, H., Caldeira, G. In Situ And In Vitro Senescence Induced By Kcl Stress: Nutritional Imbalance, Lipid Peroxidation And Antioxidant Metabolism. J. Exp. Bot. 52, 351, 2001.
- Hassan, I.A. Physiological And Biochemical Response Of Potato (Solanum Tuberosum L. Cv. Kara) To O3 And Antioxidant Chemicals: Possible Roles Of Antioxidant Enzymes. Ann Appl. Biol. 148, 197, 2006. Doi:10.1111/J.1744-7348.2006.00058.X
- Monteiro, M. S. Santos C. Soares A. M. V. M. Mann R. M. Assessment Of Biomarkers Of Cadmium Stress In Lettuce. Ecotoxicology And Environmental Safety 72, 811, 2009. Doi:10.1016/J.Ecoenv.2008.08.002
- Hassan, I.A., Tewfik, I. Co2 Photoassimilation, Chlorophyll Fluorescence, Lipid Peroxidation And Yield In Cotton (Gossypium Hirsutum L. Cv Giza 65) In Response To O3. World Rev. Sci. Techno. & Sustain. Develop. 3(1), 70, 2006.
- Suruchi, F., Pankaj K. Assessment Of Heavy Metal Contamination In Different Vegetables Grown In And Around Urban Areas. Research J. Of Environmental Toxicology, 5, 162. 2011, Doi: 10.3923/Rjet.2011.162.179
- Nasaralla, M.M, Ali, E.A., Lead Accumulation In Edible Portions Of Crops Grown Near Egyptian Traffic Roads. Agric. Ecosys. & Environ. 13, 73, 1985.
- Rovinsky,F.Y., Burtseav, L.V. Chucheva, T.B. Heavy Metal In The Vegetation As Indicators For The Environmental Pollution In The Area Of The Former Ussr. In: ‘Plants As Biomonitors: Indicators For Heavy Metals In The Terrstrian Environments”, B. Markert (Ed.), 507 – 1993.
- Costa, G., Morel, J. L. Water Relations, Gas-Exchange And Amino-Acid Content In Cd-Treated Lettuce. Plant Physiol. Biochem.32, 561, 1994.
- Costa, G., Spitz, E. Influence Of Cadmium On Soluble Carbohydrates, Free Amino Acids, Protein Content Of In Vitro Cultured Lupinus Albus. Plant Sci. 128, 131, 1997.
- Zengin F. K., Munzuroglu O. Effects Of Some Heavy Metals On Content Of Chlorophyll, Proline And Some Antioxidant Chemicals In Bean (Phaseolus Vulgaris L.) Seedlings. Acta Biologica Cracoviensia Series Botanica 47(2), 157, 2005.
- Adu, A.A. Aderinola, O.J. Kusemiju, V. Heavy Metals Concentration In Garden Lettuce (Lactuca Sativa L.) Grown Along Badagry Expressway, Lagos, Nigeria. Transnational J. Of Science & Technology 2(7), 115, 2012.
- Parekh D, Puranl´K R. M., Srl´Vastava H.S. Inhibition Of Chlorophyll Biosynthesis By Cadmium In Greening Maize Leaf Segments. Biochemie Physiologie Der Pflanzen 186, 239, 1990.
- Oncel, I., Keles Y, Ustun A. S. Interactive Effects Of Temperature And Heavy Metal Stress On The Growth And Some Biochemical Compounds In Wheat Seedlings. Environmental Pollution 107, 315, 2000.
- Baycu, G., Tolunay, D., Ozden, H., Gunebakan, S. Ecophysiological And Seasonal Variations In Cd, Pb, Zn And Ni Concentrations In The Leaves Of Urban Deciduous Trees In Istanbul. Environmental Pollution, 142, 545, 2006.
- Singh S, And Sinha S. Accumulation Of Metals And Its Effects In Brassica Juncea (L.) Czern. (Cv. Rohini) Grown On Various Amendments Of Tannery Waste. Ecotoxicology And Environment Safety 62,118, 2005.
- Sharma, S. Sharma, P. Shankari P., Gupta, V. Morphological And Biochemical Response Of Cicer Arietinum L. Var. Pusa-256 Towards An Excess Of Zinc Concentration. Life Science Journal, 7 (1), 95, 2010
- Achakzai, A.K., Bazai, Z.A., Kayani, S.A. Accumulation Of Heavy Metals By Lettuce (Lactuca Sativa L.) Irrigated With Different Levels Of Wastewater Of Quetta City. Pak. J. Bot. 43(6), 2953, 2011.
- Fodor, F. Physiological Responses Of Vascular Plants To Heavy Metals. In: Prasad, M.N.V., Strzalka, K. (Eds.), Physiology And Biochemistry Of Metal Toxicity And Tolerance In Plants. Kluwer Academic Publishers, Dordrecht, Netherlands, Pp. 149–2002.
- Sanita´Di Toppi, L.,Gabbrielli, R. Response To Cadmium In Higher Plants. Environ.Exp.Bot.41, 105, 1999.
- Chaoui, A., Mazhoudi, S.,Ghorbal, M.H., El Ferjani, E.,1997.Cadmium And Zinc Induction Of Lipid Peroxidation And Effects On Antioxidant Enzyme Activities In Bean (Phaseolus Vulgaris L.). Plantsci.127,139, 1997.
- Chaoui, A., Mazhoudi, S., Ghorbal,M.H.,El Ferjani,E. Cadmium And Zinc Induction Of Lipid Peroxidation And Effects On Antioxidant Enzyme Activities In Bean (Phaseolus Vulgaris L.). Plantsci.127,139, 1997.
- Chaoui, A., El Ferjani, E. Effects Of Cadmium And Copper On Antioxidant Capacities, Lignifications And Auxin Degradation In Leaves Of Pea (Pisum Sativum L.) Seedlings. C.R.Biol.328, 523, 2005.
- Hassan, I.A., Twefik, I. Co2 Photoassimilation, Chlorophyll Fluorescence, Lipid Peroxidation And Yield In Cotton (Gossypium Hirsitum L. Cv Giza 65) In Response To O3. World Rev. Sci., Technol., Sustain. Develop., 3(1), 70, 2006. Doi: 10.1204/Wrstsd.2006.008764
- Zhang, F.-Q.,Wang,Y.-S.,Lou,Z.-P.,Dong,J.-D. Effect Of Heavy Metal Stress On Antioxidative Enzymes And Lipid Peroxidation In Leaves And Roots Of Two Mangrove Plant Seedlings (Kandelia Candel And Bruguiera Gymnorrhiza). Chemosphere 67,44, 2007.







