Removal of chromium Cr(VI) from waste water using a waste filter cake from sugar industry
N. Partha1 * , K. Keruthiga1 and S. Renganathan1
1
Department of Chemical Engineering,
Alagappa College of Technology,
Anna University,
Chennai,
600 025
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.08
Sorption of Cr(VI) from an aqueous solution was studied using the filter cake obtained from the sugar industry at different pH (1 to 6) and initial chromium concentration (100 to 200 mg/L). From the result, it was observed that the percentage chromium removal was found to be decreased with increase in solution pH and the kinetic data fitted very well with the pseudo second order rate equation when compared to the pseudo first order rate equation. The equilibrium data was represented very well with the Freundlich isotherm model when compared to the Langmuir isotherm model.
Copy the following to cite this article:
Partha N, Keruthiga K, Renganathan S. Removal of chromium Cr(VI) from waste water using a waste filter cake from sugar industry. Curr World Environ 2008;3(2):257-260 DOI:http://dx.doi.org/10.12944/CWE.3.2.08
Copy the following to cite this URL:
Partha N, Keruthiga K, Renganathan S. Removal of chromium Cr(VI) from waste water using a waste filter cake from sugar industry. Curr World Environ 2008;3(2):257-260. Available from: http://www.cwejournal.org?p=130/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-07-30 |
|---|---|
| Accepted: | 2008-09-07 |
Introduction
Heavy metal pollution is a threat to human health, animals and plants and is mainly caused by industrialization and its consequences (Park, et al., 2007). Among the various heavy metals, pollution by chromium and its compounds is of considerable concern. This metal is highly reactive and has found widespread use in leather tanning, electroplating, metal finishing and chromate preparation processes (Parvathi and Nagendran, 2007). So the removal of chromium metal from wastewater is very important to create a pollution free environment.
Physical and chemical methods are used for the treatment of chromium metal effluents (Stafiej and Pyrzynska, 2007). The removal of chromium from wastewater through adsorption by activated carbon is an effective and expensive process (Mohan and Pittman, 2006). Therefore economic and eco-friendly technologies are focused (Volesky, 2007). Rather than the physical and chemical methods, sorption is used for the removal of chromium metal from effluents (Gode and Pehlivan, 2005). It is a popular technique used for chromium removal from wastewaters. Among the various sorbents, the removal of chromium from the effluents using filter cake is investigated recently. A survey of literature showed that little work has been done so far on chromium removal process using filter cake as adsorbent.
In the present study, filter cake from sugar industry was used for the removal of chromium (Cr(VI)) with the effect of initial chromium solution pH and chromium concentration in a batch mode. The equilibrium data was analysed using Langmuir and Freundlich adsorption isotherm model.
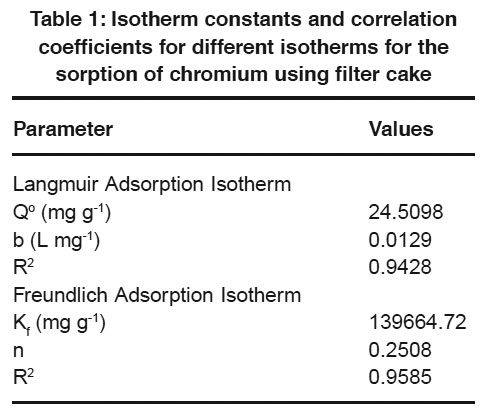 |
Table 1: Isotherm constants and correlation coefficients for different isotherms for the sorption of chromium using filter cake Click here to view table |
Material and Methods
Adsorbent
The filter cake obtained from sugar industry was collected from the Co- operative Sugars Limited, Mundiyambakam, Villupuram, India. It was treated with m-Chloro Benzoic acid with Chloroform at 60oC for 24 hrs to oxidize the adhering organic matter. Resulting material was then washed with distilled water and dried. The oxidized filter cake was powdered, ground, and sieved to the desired particle size. The experimental chromium (Cr(VI)) solutions were prepared from the stock solution.
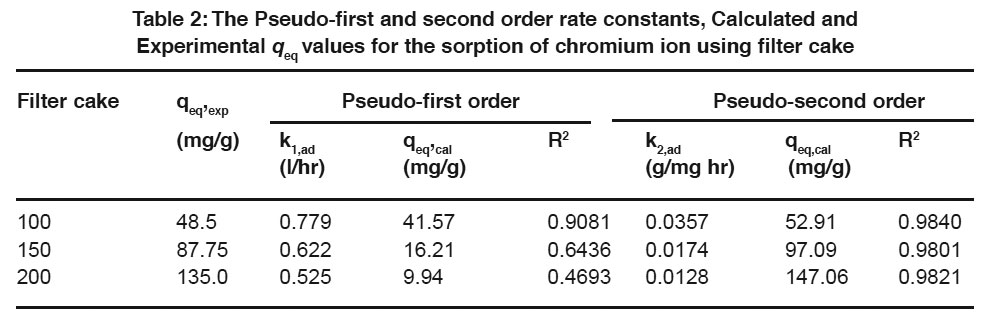 |
Table 2: The Pseudo-first and second order rate constants, Calculated and Experimental qeq values for the sorption of chromium ion using filter cake Click here to view table |
Batch Studies
Batch experiments were conducted to obtain the optimum operating conditions for the removal of chromium from an aqueous solution. Sorption experiments were carried out in a rotary shaker at 150 rpm at room temperature. Chromium solution was prepared to get a required chromium concentration at 30°C. Weighed amount of dried filter cake was introduced in to the chromium solution. Samples were withdrawn at different time interval and centrifuged at 10,000 rpm for 10 min and the absorbance of the supernatant was determined using UV-spectrophotometer.
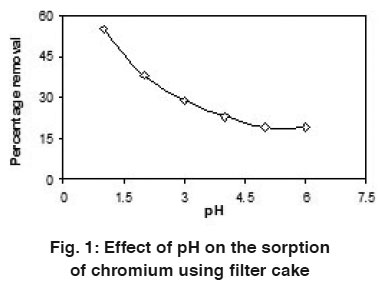 |
Figure 1: Effect of pH on the sorption of chromium using filter cake Click here to view figure |
Results and Discussion
Effect of Initial pH
The uptake capacity of filter cake was studied by varying the chromium (Cr(VI)) solution pH from 1 to 6 per 100 mL. The uptake capacity was found to be more at pH 1 when compared to all other pH studied in the present investigation. Fig.-1 shows the effect of solution pH on the uptake of chromium at 30°C. At lower pH values, the biomass will have a net positive charge. The reduction in adsorption capacity of chromium on sorbent with increasing pH can be attributed to change in surface characteristics and charge (Kouba and Zhuang, 1994). A negatively charged surface site on the sorbent does not favor the adsorption mechanisms with respect to pH (Hamadi, et al., 2001). So pH 1 was taken as optimum value for the corresponding experiments.
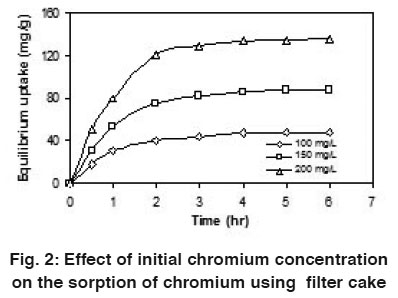 |
Figure 2: Effect of initial chromium concentration on the sorption of chromium using filter cake Click here to view figure |
Effect of Initial Chromium Concentration
With the influence of initial chromium concentration (from 100 to 200 mg/L), the uptake capacity was studied at 30°C. It was observed that the chromium uptake capacity was found to be increased linearly with contact time in the beginning, then non-linearly at slower rate and finally attained saturation called equilibrium time. Increase in initial chromium concentration provides an important driving force to overcome all mass transfer resistances of the chromium between the aqueous and solid phases, thus increases the uptake (Zhou and Banks, 1993).
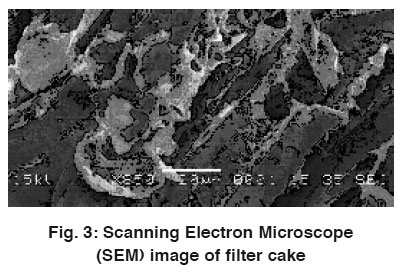 |
Figure 3: Scanning Electron Microscope (SEM) image of filter cake Click here to view figure |
Using coir pith, the maximum uptake capacity of chromium was previously reported as 76.3 mg/g by Namasivayam and Sureshkumar, (2008). However in this present investigation, the maximum uptake capacity was observed as 135.0 mg/g using filter cake at 200 mg/L initial chromium concentration. So this filter cake was found to be comparable with the other agricultural waste. Fig. 2 shows the plot of effect of initial chromium concentration on the uptake of chromium at 30°C.
Equilibrium Modeling
Langmuir and Freundlich adsorption isotherm models are generally used for the mathematical description of the sorption of chromium in aqueous waste streams. The expression for the Langmuir model is given as follows
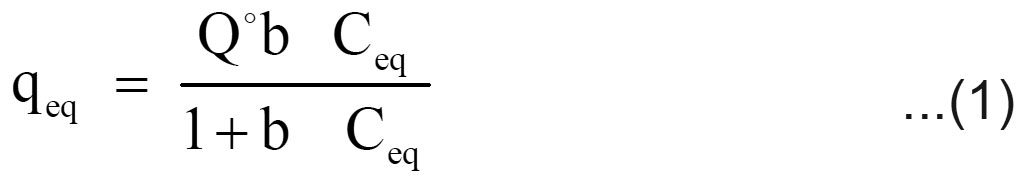
Where qeq (mg/g) and Ceq (mg/L) are the quantity of chromium adsorbed per unit weight of sorbent and unadsorbed chromium concentration in solution at equilibrium, respectively. Qo (mg/g) maximum quantity of chromium adsorbed per unit weight of sorbent to form a complete monolayer on the surface and b (L/mg) is a constant related to the affinity of the binding sites (Langmuir, 1918).
The Freundlich adsorption isotherm model is expressed by the following equation
where KF and n are the Freundlich constants of the system. KF and n indicate adsorption capacity and adsorption intensity, respectively (Freundlich, 1906).
The linearized Langmuir and Freundlich adsorption isotherms were obtained at 30°C for the sorption of chromium using filter cake. The calculated isotherm constants at 30oC are given in Table 1. In view of the values of linear regression coefficients in the Table 1, the Freundlich model
q K C 1/n
exhibitedq Fa slightlyeq better fit to the equilibrium data when compared to the Langmuir model in the studied concentration. The best fit of equilibrium model was determined based on the correlation coefficient R2.
Kinetic Modeling
The sorption mechanism and potential rate controlling steps have been investigated by using the pseudo first and pseudo second order kinetic models. The pseudo first order rate expression of Lagergren, (1898) is
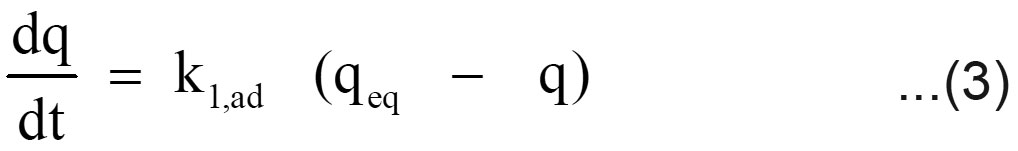
Where q is the amount of chromium adsorbed on the adsorbent at time t and k1, ad (min 1) is the rate constant for pseudo first order sorption. The integral form of equation (3) is
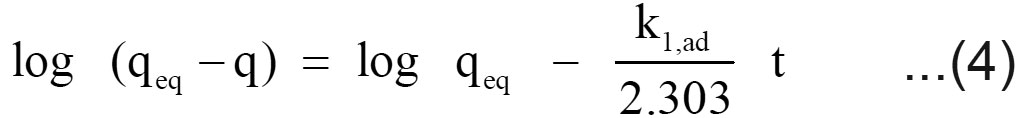
A linear fit of log (qeq – q) versus t shows the applicability of this kinetic model. From the slope and intercept, the pseudo-first-order rate constant (k1,ad) and qeq values were determined. The correlation coefficients of the pseudo first order kinetic model obtained for the sorption of chromium using filter cake was determined from the plot.
Expression for the pseudo second order kinetic model is
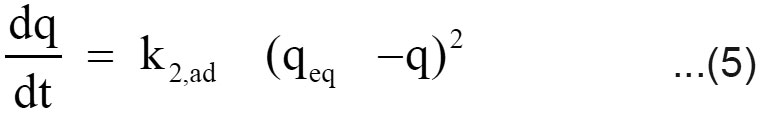
where k2,ad (g/mg min) is the rate constant of the pseudo second order kinetics (Ho and McKay, 1999). The integrated linear form of equation (5)
If the experimental data fits the plot of t/q versus t as linear relationship, the pseudo second order kinetic model is valid. Using equation (6), t/q was plotted against t for the removal of chromium using filter cake. The pseudo second order rate constant (k2,ad) and qeq values were determined from the slope and intercept of the plot.
The linearized form of the pseudo first and pseudo second order kinetic model at different initial chromium concentrations for the period of 360 minutes was analysed. The equilibrium adsorption capacity was found to be increased with an increase in initial chromium concentration (Table 2). The qeq calculated values were found to be closer to the qeq experimental values in the case of second order kinetics. From the analysis, it is observed that the kinetic data were found to be fitted very well with the Pseudo-second-order kinetic model (Hamadi, et al., 2001; Mohan, et al., 2005). Based on the above results; it was observed that the kinetic data fitted very well with the second order rate equation when compared to the first order rate equation.
SEM Imaging
Morphology of the biological specimens has been increasingly examined using Scanning Electron Microscopy (SEM). In the present study, the scanning electron micrograph was used to exemplify the surface morphology of the filter cake. As shown in the SEM micrograph, the rough and porous surface was found to be more in filter cake (Fig. 3). This surface property should be considered as a factor for providing an increase in the porous surface thereby increasing the uptake capacity of chromium.
Conclusion
Chromium removal experiment was conducted to study the effect of initial solution pHt and initial chromium1 concentration1. Filter cake was effectively used in the removal tof chromium using q k q 2 q batch study2,ad. Theqkinetic dataeq fitted very well with the pseudo second order rate equation when compared to the pseudo first order rate equation. Based on the above results, it was observed that the equilibrium data was found to be fitted very well with the Freundlich adsorption isotherm model with higher correlation coefficient when compared to Langmuir adsorption isotherms model.
References
- Freundlich, H.M.F.: Z. Phys., Chem., (1906) 57A, 385–470.
- Gode, F. and Pehlivan, E.: J. Hazard. Mater., (2005) 119, 175–182.
- Hamadi, N.K., Chen, X.D., Farid, M.M. and Lu, M.G.Q.: Chem. Eng. J., (2001) 83, 95–105.
- Ho, Y.S. and McKay, G.: Res. Conser. Recyc., (1999) 25, 171-193.
- Kouba, J.F. and Zhuang, P.: Fluid/Particle Sepn. J., (1994) 7, 87-90.
- Lagergren, S.: Kungliga Svenska Vetenskapsa. Handl. (1898) 24, 1-39.
- Langmuir, I.: J. Am. Chem. Soc., (1918) 40, 1361-1403.
- Mohan, D. and Pittman Jr, C.U.: J. Hazard. Mater., (2006) 137, 762–811.
- Mohan, D., Singh, K.P. and Singh, V.K.: Ind. Eng. Chem. Res., (2005) 44, 1027–1042.
- Park, D., Yun, Y.S., Ahn, C.K. and Park, J.M.: Chemosphere, (2007) 66, 939–946.
- Parvathi, K. and Nagendran, R.: Sep. Sci. Technol., (2007) 42, 625–638.
- Stafiej, A. and Pyrzynska, K.: Sep. Purif. Technol., (2007) 58, 49–52.
- Volesky, B.: Water Res., 4017–4029 (2007). 14. Zhou, J.L. and Banks, C.J. Chemosphere, (1993) 27, 607 620.







