Lead: A toxic substance, its effect and removal
K.N. Singh1 * , G.S. Ojha2 and S.N. Singh1
1
Department of Chemistry,
S.G.R. (P.G.) College Dobhi,
Jaunpur,
222 149
India
2
Department of Chemistry,
R.S.K.D. (P.G.) College,
Jaunpur,
222 001
India
DOI: http://dx.doi.org/10.12944/CWE.1.2.21
Lead is a relatively abundant metal in nature, occurring in lead minerals. The major source of lead is the combustion of leaded petrol. The most lead intake by a big city dweller is from diet 200-300 µg per day, air and water adding a further 10-15 µg per day, each of this total intake, approximately 200 µg of lead is exerted while 25 µg is stored in the bones every day. The present work deals the effect of lead on human health and its removal by Dithiazone and Atomic-Absorption Method (Chelation Extraction).
Copy the following to cite this article:
Singh K.N, Ojha G.S, Singh S.N. Lead: A toxic substance, its effect and removal. Curr World Environ 2006;1(2):209-212 DOI:http://dx.doi.org/10.12944/CWE.1.2.21
Copy the following to cite this URL:
Singh K.N, Ojha G.S, Singh S.N. Lead: A toxic substance, its effect and removal. Curr World Environ 2006;1(2):209-212. Available from: http://www.cwejournal.org/?p=1045
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-10-18 |
|---|---|
| Accepted: | 2006-11-30 |
Introduction
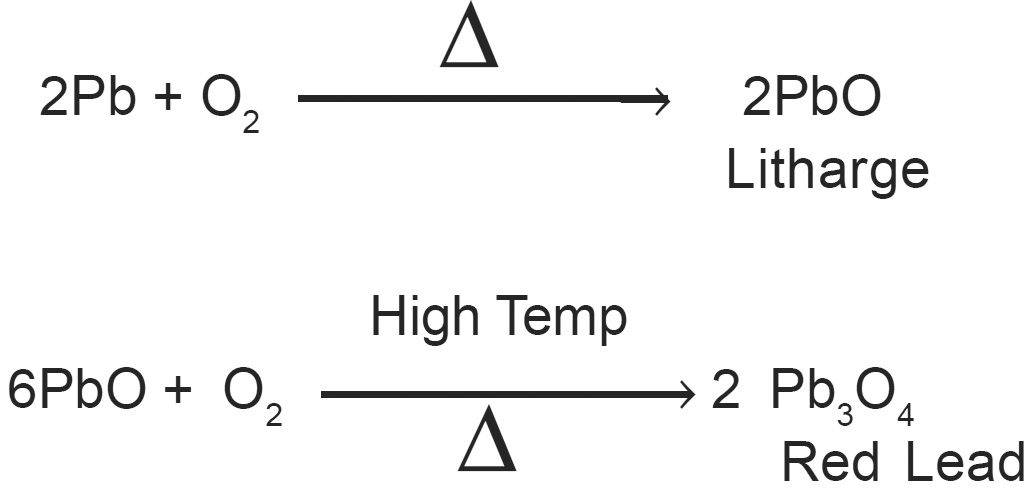
Lead (Plumbum) Pb-82, a bluish-grey metal, mp 3270 C. It is so soft and can be cut easily with a knife. When heated with air or oxygen it forms litharge and red lead.
With water it forms slightly soluble lead hydroxide which is poisonous in nature.
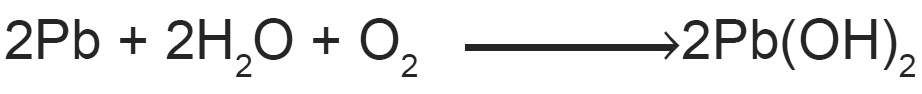
Several brands of shampoos contain lead sulphide to give hair a dark hue. Worley etal (1968) reported 88% PbS in mascara, face powder, pastes and liquids also contain 65% lead, paints, welding stainless steal, glass, fabric cottons, food colours, PVC plastics and cigarette smoking is a major source of lead poisoning.
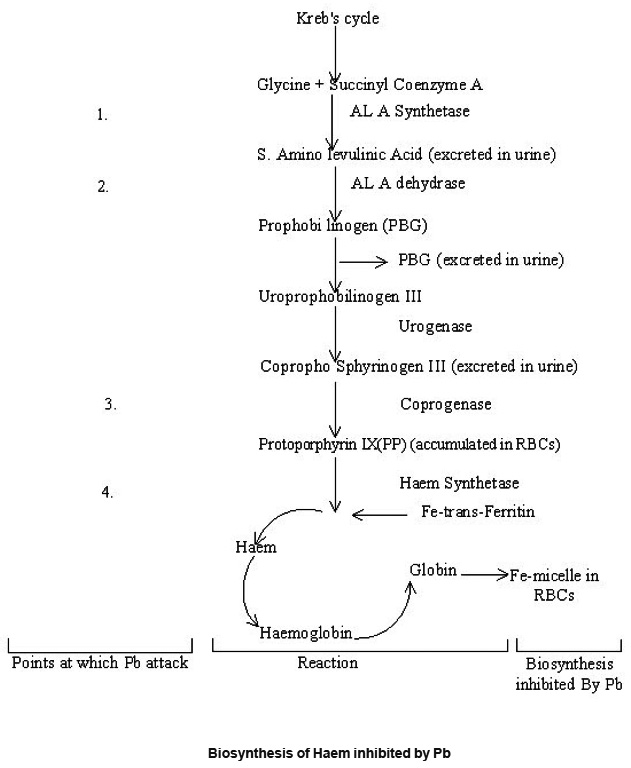
Lead effects on Central Nervous System (CNS) primarily causes neural degradation, microcytic anemia due to inhibition of haem synthesis and shortened life span of circulating RBCs is caused by lead. It also alters the morphology of kidney-glomerular fibrosis (Neurological, haematological and nephrological problems are associated mainly). Its toxicity depends on its route of administration and the chemical compound with which it is bound. Lead can also disturb the structure-functioning relationship of DNA molecule. It can combine with specific biochemical residues like sulphydryl, carboxyl and imidazole group. The major biochemical effects of Pb in its interference with haem synthesis, which lead to haematological damage. Kreb’s cycle clearly expresses the inhibition of biosynthesis of haem by lead as given below-
Biosynthesis of Haem inhibited by Pb
It also affects the IQ (Intelligence Quotient) of children. It is transported by the blood and stored in teeth, bones and soft tissues including brain.
Experimental
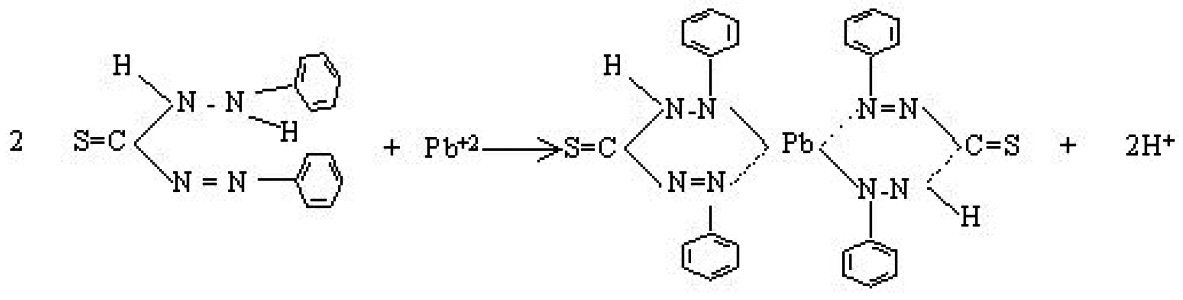
Lead in made to react with dithiazone at a pH of about 11.5 to form lead dithiazonate which is soluble in chloroform. In the presence of alkaline cyanide solution, the free green coloured dithiazone is not extracted by chloroform. The interference of Bismuth and Tin is eliminated by preliminary treatment of the sample with dithiazone at a pH about 2.3. This method is applicable to test the raw water, potable water, polluted water and waste water.
Reagents:
- Lead free distilled water
- 0.2N-NH3 Solution → Diluted 3.5 ml ammonia solution to 100 ml with water.
- Dithiazone stock solution 0.1% → Dissolved 0.1 g dithiazone in 100 ml Chloroform.
- Dithiazone working solution ’! 12 ml of stock dithiazone solution is taken in 100 ml water in separating funnel, added 20 ml 0.2 N NH3 solution and shaken well. Allowed the two phases to separate and rejected the lower chloroform layer. Filtered the aqueous layer through a wetted filter paper (To remove droplets of chloroform) into a 50 ml bottle.
- Sodium hexa metaphosphate solution 10% → Dissolved 10 gs sodium hexa metaphosphate in 100 ml distilled water, removed traces of lead by extraction with stock dithiazone solution after adjusting the pH to 9 with concentrated NH3 solution. Acidified the aqueous layer in the separating funnel using HCl and then extracted with chloroform to remove free dithiazone until the chloroform layer becomes colourless. Rejected the chloroform layer, adjusted the pH of aqueous solution to 9.5 by adding ammonia solution.
- Hydroxylamine hydrochloride solution 1% → Dissolved 1 g of NH2OH.HCl in 100 ml distilled water.
- Alkaline cyanide solution prepared a mixture of 340 ml. NH3 (S.G. 0.88) + 680 ml water → Dissolved 1.5 gs sodium sulphate and sodium sulphite in the mixture, added 30 ml 1% potassium cyanide solution and shaken well kept in the tightly stoppered bottle.
- Lead stock solution → Dissolved 15.99 gs lead nitrate in small amount of water, added 10 ml conc. HNO3 and made upto 1000 ml. 1 ml = 1.0 mg Pb
- Lead working solution → Diluted 5 ml of lead stock solution in 500 ml distilled water. (Freshly prepared when required). 1ml = 10 mg Pb.
Procedure
Standards → Placed 50 ml of lead free distilled water into a series of short stemmed separating funnels and took 1 ml, 1.5 ml, 2 ml, 2.5 ml 3.0 ml and 4 ml of lead working solution into them. Took 50 ml sample, 50 ml blanks and 50 ml of different standards in different bottles. Added 1.0 ml sodium hexa metaphosphate solution, 1 ml hydroxylamine solution and mixed well. After this 30 ml alkaline cyanide solution, 0.5 ml dithiazone working solution and 10 ml chloroform are added with them.
Now all the funnels are shaken vigorously and allowed the layers to separate, dried the stem of the funnel with filter paper strips and removed the chloroform layer in the optical cell.
Measured the optical density using spectrophotometer at 510 nm.
Results and Discussion
Lead is serious cumulative body poison. Natural water seldom contains more than 20 µg / L. Although values as high as 400 µg/ L have been separated in different types of layers to water.
Lead in a water supply comes from industrial, mine, and smelter discharges from the dissolution of old lead plumbing. Tap waters that are soft, acidic and not suitably heated may contain lead resulting from an attack on lead service pipes. It is toxic and therefore, a stringent limit has been specified for lead in potable water. The limiting concentration of lead in drinking water prescribed by WHO is 0.1 mg / L. Several countries use 0.1 mg/ L as a standard. Symptoms of lead poisoning is numerous. The severely affected areas include the nervous system, renal system, digestive and muscular systems. It is reported that the traces of lead in metal-plating baths will affect the smoothness and brightness of deposits. Inorganic lead salts in irrigation water may be toxic to plants. In the testing of water for various uses, their action on lead is of great importance. Natural water that are highly acidic, or containing large amount of free CO2 and are low in calcium and magnesium bicarbonates is capable to dissolve significant amount of lead. Organic matter present in acidic water also increases the plumbo-solvency. So, for characterisation of the water, to determine the plumbo-solvency parameter is essential.
On the basis of above facts lead is to be specially tested when pollution/plumbo-solvency is suspected in water which give us ideas whether the lead content of potable water and waste water is within the acceptable limit or not.
Acknowledgements
The authors are thankful to the Principal; Dr. P.K. Singh S.G.R.(P.G.) College Dobhi, Jaunpur for providing necessary facilities for performing this type work.
References
- Sandel, E.B., “Colorimetric Determination of Traces of Metals” 3rd edn. Inter Science Publishers, New York. (1950).
- Abott. D.C. and Harris, J.R., Analyst (1962) 87, 387.
- Her Majesty’s stationary office, “Analysis of Raw, Potable and Waste Water”. HMSO, London, (1972).
- Indian Standard IS No. 7022 Part (I) (1973) and IS No. 7022, Part (II) (1974). “Glossary of Terms Relating to Water Sewage and Industrial Effluents”.
- Cleseri, L.S, Greenberg etal, “Standard Methods for the Examination of Water and Waste Water” APHA, 17th edn. Washington D.C. U.S.A. (1989).
- Singh, A.K., Singh, D.P. and Singh, V.N. Environ Technol. Letters, (1988) 9 1753.
- Chaturvedi, A.K., Pathak, K.C. and Singh, V.N., Applied Clay Science (1988) 3, 337.







