Assesment of ground water quality of Pali district (Rajasthan)
Jaipal Garg1 * and Gita Seth1
DOI: http://dx.doi.org/10.12944/CWE.1.2.07
An assessment of physico-chemical characteristics of ground water samples from the bore wells and dug wells of different 22 locations of Pali district in Rajasthan . Totally 18 parameters were analysed .In many locations maximum parameters within the permissible limit and about 59% samples sites showed higher concentration of fluoride content than the permissible limit and 36 % of the water samples showed higher range of total dissolved solids than the permissible limit. The hydro-chemical facts of ground water of this area were found to be dominated by sodium bicarbonate and sodium chloride.
Copy the following to cite this article:
Garg J, Seth G. Assesment of ground water quality of Pali district (Rajasthan). Curr World Environ 2006;1(1):139-144 DOI:http://dx.doi.org/10.12944/CWE.1.2.07
Copy the following to cite this URL:
Garg J, Seth G. Assesment of ground water quality of Pali district (Rajasthan). Curr World Environ 2006;1(1):139-144. Available from: http://www.cwejournal.org/?p=1018
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2006-07-12 |
|---|---|
| Accepted: | 2006-09-21 |
Introduction
Water, next to air is a vital natural resource responsible for the existence and development of life on the earth, even though our country is one of the wettest country of the world and has substantial fresh water resource, there is a chronic shortage of safe water specially in some of major towns where urbanization has taken place. The shortage varies from mild to acute depending upon the geographical, topographical, climate, hydrogeological and other factors. The area chosen for the present study is Pali district in Rajasthan. The study area, situated in south-west of Rajasthan and lies between 24° 45' and 26° 27' N Latitude and 72° 48' and 74° 24' E Longitude, is one of the most important industrial district of Rajasthan due to presence of large number of small scale industries and also use of fertilizers and pesticides. In the Pali town 800 hard processing textile units are discharging their effluent in to Bandi river which has created alarming situation in ground water quality deterioration of the area .Their are some chemical steel, paper and dye industries are also working in this area. The hydro-geological formation encountered in the area is younger alluvium which comprises of unconsolidated sand, gravel, silt and clay along the main river and channels of various streams in the area. These deposits are discontinuous and have limited thickness. Sand is mostly brown sand, the superficial in colour, fine to medium, well rounded mainly of quartz with some ferromegnesium minerals and feldspar and well sorted by wind action .It is disintegrated product of older rocks present in surrounding area.Pali district lies in arid zone of Western Rajasthan having very scanty rains and very low for ground water reserves. It covers the villages Bassi, Nimaj, Sojat, Kirwa, Perwa, Radawas, Sumerpur, Jaitran, Khudala, Birani etc.
The forest reserves in the area are very few due to scanty rainfall and scarce ground water reserves and comes under the subsidiary edaplic type of dry tropical forest. Khejra, Babul, Gular, Kair and Jamum are the main species found along the nullahas. The district lies within the drainage basin to Luni river and its tributaries namely Jawai, Mithri, Bandi, Lilri, Guhia, Radi and Raipur Luni. These streams are empheral in nature and flow only in direct response to precipitation.
Vijayram et al. (1989) found the concentration of total hardness, calcium, chloride, magnesium and sulphate beyond permissible limit in ground water of sembattu, Tiruchirappalli due to presence of tanneries. Shankar and Muthukrishaman (1994) observed the concentration of total dissolved solids, total hardness, chloride and calcium, were higher then the permissible limit in the ground water of Madras city due to rapid industrialization.
Prasad and Ramchandra (1997) studied the ground water of industrial zone in Jeedimetala (AP) and found the concentration of total dissolved solids, Fluoride, Nitrate greater than the permissible limit. Bhat and Ganesh Hegde (IJEH 1997) in their paper on ground water quality in Uttar Kannada district of Karnatatka describes physico-chemical quality of water of that area. The study indicates that nearly 50% of the water range is moderately hard to very hard types. Tiwari5 (IJEH, 2001) “Hydro geochemistry of under ground water in and around Hattpur city (M.P.)” describes physico-chemical characteristics of collected water samples.The study of ground water quality involves a description of the occurrence of various constituent in ground water and relation of these constituents to water use. Kulshresta (2002) studied about physico-chemical characteristics of ground water and effluents in Sanganer of Jaipur district in Rajasthan.
Studies7 on defluoridation capacity of different parts of Tamarindus indica was done in our laboratory and it was observed that fruit powder has maximum capacity of defluoridation at room temperature.Geochemical study of fluoride in ground water of Rajasthan and study of Physico-chemical characteristics of ground water of adjoining area of NH-8 in Jaipur district ,Rajasthan was done in our laboratory8-9 and it was observed that many places have more fluoride value than the permissible level. In continuation of our work on physico-chemical study on ground water of Rajasthan, we report here the study on the area of Pali district in Rajasthan.
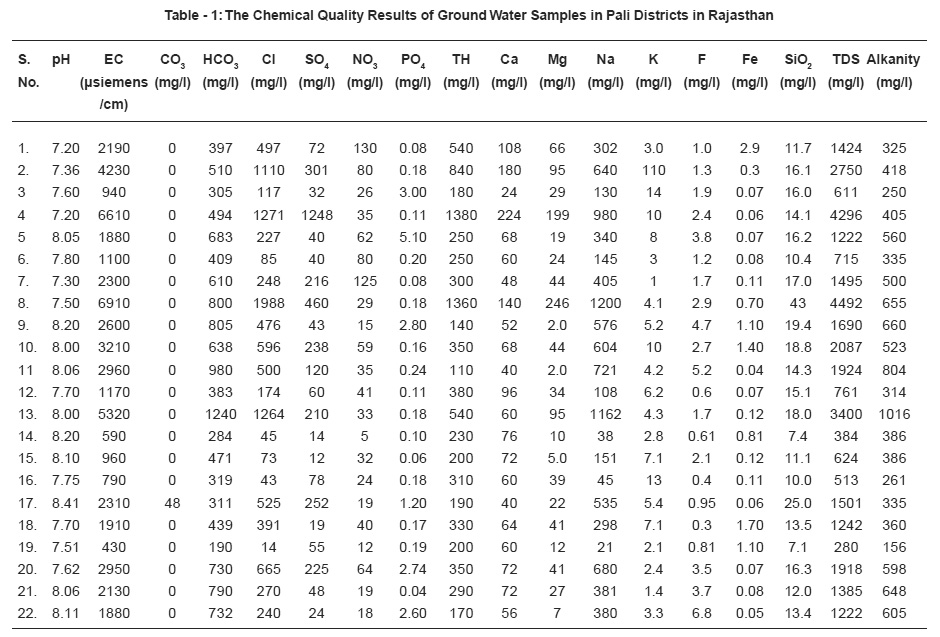 |
Table - 1: The Chemical Quality Results of Ground Water Samples in Pali Districts in Rajasthan Click here to view table |
Material and Methods
The water samples collected include both irrigation and domestic wells and they are located in various Geological formations, in a polythene bottles from the study area which covers whole Pali district for determination of Carbonate (CO3), Bicarbonate(HCO3), Chloride(Cl), Sulphate (SO4), Nitrate(NO3), Phosphate(PO4) and dissolved Silica(Si-SiO2), Total Hardness(TH), Calcium(Ca), Magnesium(Mg), Sodium(Na), Potasium(K), Fluoride (F) and for determination of iron, sample were collected in different bottles treated with dilute HCl (1:1).
pH, EC of the water samples was measured in the field using field lab kit. Ca, Mg, TH, Cl, CO3, HCO3, were analysed by volumetric titration, Na and K were measured by flame photometer, Sulphate(SO4), Nitrate(NO3), Phosphate(PO4)and dissolved Silica (Si-SiO2) were analysed by Ultraviolet-Vissible Spectrophoto-meter-108 Systronic make. The methods used are as per described10 in APHA 1992.
Results and Discussion
The chemical quality results of ground water samples are reported in Table 1 and distribution of major quality parameters shown in Table -2.
pH
pH measurement is one of the most important frequently used test in water chemistry. The pH of study area was minimum 7.2 at Jaitpura and maximum 8.41 at Kriwa during the tenure of work. All the values are usually on alkaline side and under the acceptable limit of water quality standard (6.3 -9.2) of India and therefore use of water for various purposes is acceptable.
Fluoride
During the tenure of work Sumerpur sample showed minimum value 0.30 mg/L and Birani sample maximum value 6.8. Acute determination of fluoride ion has increased in importance with the growth of practice of fluoridation of water supplied as a public health measure. Fluoride concentration of approximately 1.0 mg/L in drinking water effectively reduces dental strength without harmful effect on health. It is observed that 59% ground water sanker have higher value then the permissible limit. Beyond permissible limit fluoride content is harmful. Dentals bone fluorosis was observed in the people consuming this water.
Chloride ion
Chloride concentration is not only an index of eutrophication but also the indication of pollution caused by septic tank effluents, animals and potash fertilizers. Chloride level over 1000 mg/L in drinking water indicates a risk of corrosion in transport pipes, if the level excess 250 mg/L there is a risk of change in the taste of the water. A high level of chloride kills plants and animals. During the tenure of the work, Chandarwar sample showed minimum value 14 mg/L and maximum 1988 mg/L at Bassi. Permissible limit of chloride concentration for ground water is 250 mg/L and maximum permissible limit11 1000 mg/L, therefore treatment of water is suggested before use for various purposes.
Nitrate
Maximum permissible limit of nitrate is 100 mg/L as given be BIS (Bureo of Indian standards). Minimum value of nitrate observed 5 mg/L at Sandera and Maximum value of nitrate observed 130 mg/L at Sojat. In the present studies it was observed that most of the value of samples have nitrate with in the permissible limit, only three samples were found to have more value then the permissible level.
Iron
This is fourth abundant element of earth occurs cosmopolitically in ground water of study area, it varies from (0.04-2.9 mg/L). Deficiency of iron causes decrease in hemoglobin and cytochromes, while excess causes damage to tissues by iron accumulation. Water sample of Sojat has maximum value of iron 29 mg/L, which is beyond permissible limit and renders the water unsuitable for a number of domestic purposes.
Silica
In the present study Silica varies from (7.4-25 mg/L) and there is no guide line for this constituent as it does not effect human and animal system.
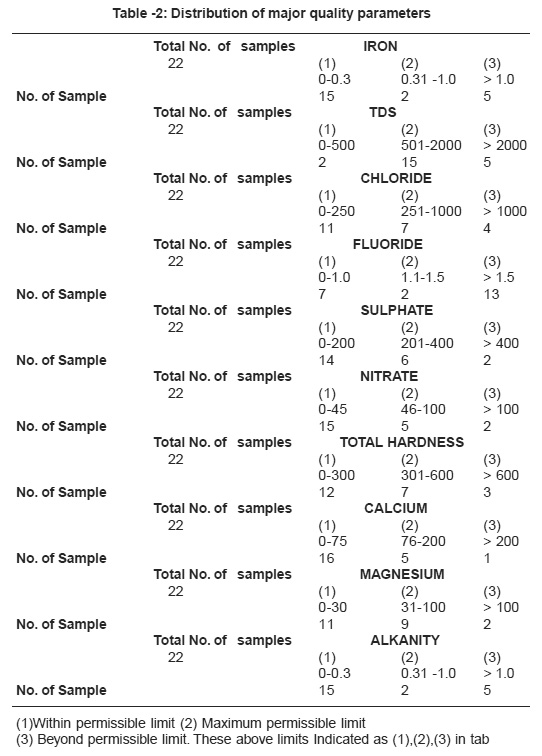 |
Table -2: Distribution of major quality parameters Click here to view table |
Total Hardness
Hardness of water as calcium carbonate is an important measure of pollution. Increase in value pertains to excess of salts of calcium, magnesium and iron. Water hardness can cause other problems in homes such as increased soap consumption with preventing of soap and detergents from lathering by giving rise to an insoluble curdy precipitation. Source of calcium in igneous rock and minerals like silicates, pyroxenes etc. and magnesium in ground water from igneous rock primarily drives from ferromagnesian minerals, like olivine, pyroxines etc. In sedimentary rock, Magnesium occur as magnetite and other carbonate and some time mix calcium carbonate. Ca and Mg along with their carbonate, sulphate and chloride make the water hard, temporary and permanent both. These ions in few samples are observed to have pretty high amount therefore this water attributes to both temporary and permanent hardness. The total hardness as CaCO3 ranges from (110-1380 mgl). The major ion contributing it are Ca and Mg. Maximum permissible limit of hardness is 600 mg/L as given by BIS. The maximum value of hardness is observed 1380 mg/ L at Jaitpura. In the present study it was observed that most of the samples have value of hardness within the permissible limit, only few water sample were found to have more value then the permissible level in the study area.
Alkanity
The alkanity is mainly due to bicarbonate ions formed by bacterial decomposition of organic maters as well as mineral ion exchange which varies from (156-1016 mg/L) in study area. 22.2% water samples have more value of Alkanity than the permissible limit in the study area. The maximum value of Alkanity was observed 1016 mg/L at Pritevipura.
Salinity
The conductivity values range (430-6910 micromhos/cm).The values of conductivity is higher at many places due to high values of Na and Cl ion in the water samples. The maximum value of conductivity 6910 micro mhos/cm was observed at Bassi. The salinity of water increases with increasing concentration of TDS, chloride, sulphate, etc.
Total Dissolved Solids
Higher values of TDS are attributed to the presence of colloidal or finely divided suspended matter which does not readily settle. High levels of TDS in drinking water may cause objectionable taste, have laxative and cause foaming.Excessive amounts may be unsuitable for aquatic life and poor for crop irrigation. Drinking water with an extremely low level of dissolved salts is not very potable. The value of total hardness indicate the level of salt content in water. According to ICMR norms for drinking water the general acceptability concentration limit of total hardness is 500 mg/L and maximum permissible limit is 1500 mg/L. For Agricultural utility the optimum range should below 3000 mg/L. In the present study the value of total hardness is observed in the range from 384-4492 mg/L. 36% water samples have more value of total hardness in the study area.
Sodium
The value of sodium varies 21-1200 mg/L. The maximum value of sodium was observed 1200 mg/L at Bassi site and minimum value of sodium was observed 21 mg/L at Chandarwar of Pali district.
Sulphate
Sulphate content more than 200 mg/L is objectionable for many domestic purposes. Sulphate is contributed to ground water from fertilizers. Sulphate was observed from(12-1248 mg/L) in the present study area. The maximum value of sulphate was observed 1248 mg/L at Jaitpura and minimum value of sulphate was observed 12 mg/L at perwa of Pali district.
Phosphate
The value of phosphate varies from 0.04-3.00mg/L in the present study area. The maximum value of phosphate was observed 3 mg/L at Gunjoj of Pali district.
Conclusions
The analysis of water samples reveals that the water in few samples is suitable for both domestic and irrigation purposes. The study clearly indicates that most of the parameter under taken to assess the water quality, are in the permissible limits, except - TDS,F and Alkanity which were observed in 36%, 59%, 22.2% samples respectively beyond permissible limit for drinking purposes. These higher concentrations of ions indicates the ground water of the study area as saline and alkaline. The water of the wells were not found suitable for potable due to occurrence of high concentration of TDS, Cl, Fe, F Alkanity. It is observed that high fluoride value is harmful for potable purposes as it may cause mottling of teeth and be hazardous for health. Suitable defluoridization techniques like blending with low fluoride water, ion exchange and chemical treatment etc. should be adopted to keep fluoride concentration of water within permissible limits. Excessive use of fertilizers both phosphatic and potassic should be avoided. It is recommended that strict vigilance and constant monitoring are needed to maintain water quality
References
-
K Vijayram., L Jamesh., P Geraldine., Varader Jan TS., Pollution studies of ground water in sembattu, Tiruchirappalli, Indian J. Env. Prot., (1989) 9(10), 746.
-
V Shankar Bhavani., N Muthu Vrishnana; Ground water study in Madras City, (Tamilnadu); J Env. Prot. (1994) 14(3), 176.
-
B.V.Prasad; P.Ramesh Chandra; “Ground water quality in an Industrial zone “Poll.Res. (1997) 16(2), 105.
-
D.M. Bhat; Ganesh R. Hegde; Ground water quality in Uttar Kannada district of Karnataka, IJEH (1997) 39, 61.
-
D.R Tiwari., “Hydrogeo Chemistry of under ground water in and around Chhatarpur city, district Chhatarpur, IJEH (2001) 43, 176.
-
S.Kulshresta., “Physico-chemical character-istics of ground water and effluents in Sanganer town of Jaipur city 2002. National Envi. Poll. Tech. (2002) 1453.
-
A.Kumar; M.K.Samota; Sanjay Gupta., Gita Seth; “Studies on defluoridation capacity of Tamarindus indica” Orient. J. Chem., (2005) 21(2), 291-294.
-
A.Kumar., Mahesh. K Samota., Gita Seth; “Geochemical study of Fluoride in ground water of Rajasthan” Chemistry an Indian Journal, (2005) 2(6): 191-193.
-
Jaipal Garg; Akhalesh Kumar; Kavita. Batheja, , Gita Seth; “Physico-chemical characteristics of ground water of adjoining area of NH-8 in Jaipur district ,Rajasthan” Chemistry an Indian Journal, (2006) 3(8).
-
Standard method for examination of water and waste water American Public Health Association Washington DC, 1134 18th ed. (1992).
-
Guidelines for drinking water quality, 1, recommendations, WHO Geneva (1993) (172-181) 53-79.







