Geographical Peculiarities of Pinus sibirica Du Tour Natural Regeneration as Related to its Seed Crops
Nikolai V. Tantsyrev3
 , Seyed Omid Reza Shobairi12
*
, Seyed Omid Reza Shobairi12
*
 , Vladimir A. Usoltsev34
, Vladimir A. Usoltsev34
 , Sun Lingxiao12
, Sun Lingxiao12
 , Zhang Haiyan12
, Zhang Haiyan12
 , Li Chunlan12
, Li Chunlan12
 , He Jing12
, He Jing12
 , Samira Hemmati Roudbari5
, Samira Hemmati Roudbari5
 , Behnam Asghari Beirami6
, Behnam Asghari Beirami6
 and Qirghizbek Ayombekov1
and Qirghizbek Ayombekov1

1
Xinjiang Institute of Ecology and Geography,
Chinese Academy of Sciences,
Urumuqi,
China
2
University of Chinese Academy of Sciences,
Beijing,
Chaina
3
Ural State Forest Engineering University,
Faculty of Forestry,
Sibirskiy Trakt,
Yekaterinburg,
Russia
4
Institute Botanical Garden of Ural Branch of RAS,
Marta str.,,
Yekaterinburg,
Russia
5
Department of Soil Science,
Faculty of Agriculture,
University of Zanjan,
Zanjan,
Iran
6
Department of Photogrammetry and Remote sensing,
Faculty of Geodesy and Geomatics,
K. N. Toosi University of Technology,
Teharn,
Iran
Corresponding author Email: omidshobeyri214@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.1.10
Copy the following to cite this article:
Tantsyrev N. V, Shobairi S. O. R, Usoltsev V. A, Lingxiao S, Haiyan Z, Chunlan L, Jing H, Roudbari S. H, Beirami B. A, Ayombekov Q. Geographical Peculiarities of Pinus sibirica Du Tour Natural Regeneration as Related to its Seed Crops. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.10
Copy the following to cite this URL:
Tantsyrev N. V, Shobairi S. O. R, Usoltsev V. A, Lingxiao S, Haiyan Z, Chunlan L, Jing H, Roudbari S. H, Beirami B. A, Ayombekov Q. Geographical Peculiarities of Pinus sibirica Du Tour Natural Regeneration as Related to its Seed Crops. Curr World Environ 2024;19(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2024-01-03 |
|---|---|
| Accepted: | 2024-03-08 |
| Reviewed by: | 
 Izolda Matchutadze
Izolda Matchutadze
|
| Second Review by: |

 Rishikesh Singh
Rishikesh Singh
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Identifying patterns in the dynamics of woody plant populations during their renewal stage, which profoundly influences the subsequent development and structure of biogeocenosis, is a significant challenge in forest ecology. Alongside seed production, the distribution of seeds from tree species that contribute to forest formation is a crucial stage to consider. The functioning of forest ecosystems is not governed by rigid laws but rather exhibits stochastic patterns rooted in the theory of probability 1. Currently, the study of plants is primarily focused on accumulating information, preceding the stage of generalization.
The regeneration of five-needle pines of the Cembrae subdivision, which have wingless seeds and rely on mutualism with Nucifraga birds, remain an unresolved problem despite numerous studies. Nutcrackers play a crucial role in facilitating the dispersal and renewal of these plants by creating soil food reserves, spreading seeds over long distances 2,3,4,5. However, most studies on this topic focus only on specific aspects of the "seeds-nutcracker-seedlings" chain. Some studies discuss the yield dynamics and seed productivity of forest stands 6,7,8,9,10, while others examine seed dispersal and caching by nutcrackers 2,5,11,12,13,14,15,16. Although these studies provide a general forecast of the influence of these factors on subsequent renewal, they often lack a quantitative assessment and fail to capture the dynamics of the whole process.
The Siberian stone pine (Pinus sibirica Du Tour), a typical representative of the five-needle pines, is widely distributed in Russia. Studies on its natural regeneration mainly focus on quantifying undergrowth based on environmental conditions 5,9,17,18,18,20. Some articles suggest the selective introduction of seeds by nutcrackers 17,18,19,20, as these birds deliberately cache seeds in specific microhabitats with preferred environmental conditions 21. This behaviour highlights a fundamental difference between Pinus sibirica and all anemochorous and some zoochorous plants, whose seeds accidentally fall on suitable soil surface for germination. Therefore, studying the natural regeneration of Pinus sibirica and closely related species in Cembrae sp. requires a synecological interdisciplinary approach that incorporates principles from ornithology and zoopsychology 22, going beyond the ordinary silvicultural methods.
Currently, the ecology of Nucifraga birds remains relatively studied well 11,23,24,25 and there is a growing understanding of their cognitive abilities 2,12,15,26,27,28. It has been observed that each nutcracker maintains individual caches 16,28, which are carefully arranged to prevent competition and theft by others 28,29,30. These caches nutcrackers find using exceptional visual memory and landmarks unaffected by snow cover, serve as a vital food source for nutcrackers and their offspring throughout the autumn-winter-spring period. Unutilized caches germinate and give rise to dense clusters of seedlings known as "nests". However, there is a lack of quantitative data on the population dynamics of nutcrackers in relation to the seed production of five-needle pines 16,24,31,32,33. Furthermore, the investigation of the nutcracker's role and the influence of seed production on the renewal dynamics of these pine species is still limited 15,31,34,35,36. Since these studies have been conducted using different methods, at various times and in diverse regions, combining their results is often challenging. Nonetheless, they collectively contribute to a general understanding of the intricate processes and complex relationships within the "seeds-nutcrackers-seedlings" system.
The objective of our research was to explore the relation of the quantity of one-year-old Pinus sibirica seedlings to productivity of the cones in three regions where they grow. We aim to provide an explanation for the observed correlations, focusing on the role of the thin-billed nutcracker (Nucifraga caryocatactes macrorhynchos Brehm C. L.). Our findings are based on extensive, long-term studies conducted in the Northern Urals.
Material and methods
A comparative investigation was conducted to study the patterns of seed crop dynamics and renewal of Siberian stone pine in different mountain forest regions. The research locations included Southern Yakutia (Aldan Highlands 59º40´ N., 125º24´ E.) in 2012, Northern Urals ("Denezhkin Kamen’" mountain region 60º25´ N., 59º32´ E.) in 2013, and South-Eastern Baikal region (Khamar-Daban mountain range 51º32´ N., 103º32´ E.) in 2017 (Fig. 1). Throughout these regions, Siberian stone pine trees are distributed across various forest stands either individually, in groups, or as dominant species. The sampled areas were chosen within 160-180-year-old forests of Siberian stone pine in the Aldan Highlands and the "Denezhkin Kamen’" mountain (Northern Urals), located at an elevation of 500-550 meters above sea level. In addition, subalpine Siberian stone pine woodlands in the Khamar-Daban ridge were chosen at an altitude of 1700 meters above sea level (Fig. 1).
 | Figure 1: Allocation of plots studied: a – in the Aldan Highland; b – in the Northern Urals; c – in the Khamar-Daban mountain range. 1 – the area of growing Pinus sibirica Du Tour.
|
We examined the patterns of yield dynamics in Siberian stone pine forests and the regeneration dynamics of Pinus sibirica seedlings across multiple regions over similar time periods. Specifically, the study covered the Aldan Highlands from 2000 to 2012, the Northern Urals from 1997 to 2012, and the Khamar-Daban mountain range from 2002 to 2015. To ensure the integrity of the data, sample plots were selected that had not experienced any significant disturbances in the past three to four decades, such as fires, extensive windfall, or logging, which could impact the regeneration of Siberian stone pine 37. Another criterion was the absence of dense thickets (with no more than 0.5–1.0 thousand specimens per ha (thous. spec./ha)) of shrubs and woody plants under 2 meters in height (total projective cover not exceeding 12%), as these could impede the nutcracker's access to the soil substrate. Environmental conditions, abundance, vitality, and age of the Pinus sidirica undergrowth (young trees) were assessed at 30–40 accounting sections measuring 5x5 meters, systematically and evenly distributed within the respective areas. The accounting sections were placed every 25 meters within the respective forest areas and at the same elevation, forming two or three parallel lines. The quantification of Siberian stone pine renewal focused on two parameters: the number of "nests" (dense clusters of seedlings grown up from seed caches) and the number of undergrowth or seedlings within them. The age of the undergrowth was determined to a one-year accuracy by calculating the annual vertical increments of the stem. The dynamics of initial seedling generations were reconstructed on the basis of the undergrowth age and the calculated coefficients (presented in Table 1) of the survival curves (shown in Fig. 2) previously proposed for the environmental conditions specific to the study regions [38].
Table 1: Empirical coefficients of survival curves of the Pinus sibirica undergrowth for study areas
Age of the undergrowth, years old | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
Aldan Highland | 1.9 | 2.4 | 2.8 | 3.2 | 3.6 | 3.9 | 4.3 | 4.6 | 4.9 | 5.2 | 5.4 | 5.7 | 5.9 | 6.2 | 6.4 |
Northern Urals | 1.8 | 2.4 | 2.9 | 3.4 | 3.8 | 4.3 | 4.7 | 5.1 | 5.5 | 5.9 | 6.3 | 6.7 | 7.0 | 7.4 | 7.7 |
Khamar-Daban mountain range | 1.6 | 2.1 | 2.7 | 3.2 | 3.6 | 4.1 | 4.5 | 4.9 | 5.4 | 5.7 | 6.1 | 6.5 | 6.9 | 7.3 | 7.6 |
The approach used to develop survival curves for Siberian stone pine seedlings in suitable environmental conditions is based on the findings from counting their numbers in 100–200 "nests" that have sprouted from seed caches within the moss cover and are 1–16 years old. Annual seedlings per "nest" can range from 1-2 to 25-30 [3; 11; 17; 38]. Our own data indicates that under the canopy of parent stands in the Aldan Highlands and the Northern Urals, the average number of annual seedlings per "nest" is approximately 5.4±0.4 and 6.7±0.6, respectively. In the subalpine parent woodlands of the Khamar-Daban ridge, the average number is 7.2±0.6 seedlings. The subsequent survival of Siberian stone pine seedlings follows a hyperbolic function (Fig. 2). In all the regions examined, the most significant decline (73%) in the number of seedlings within the "nests" occurs during the first five years of their life, reducing to 1.4–1.9 individuals. Afterward, the decline in the number of individuals within the "nests" is more gradual. By the age of 10, approximately 1.2–1.6 individuals (21–23% of the initial count) remain within the "nests," and by the 15th year, only around 14–16% of the initial count, corresponding to a single young tree.
 | Figure 2: Empirical survival curves of the Pinus sibirica undergrowth in its “nests” [38] from the initial number of one-year-old seedlings (%): a – in the Aldan Highland; b – in the Northern Urals; c – in the Khamar-Daban mountain range.
|
The annual fluctuations in the crop yield of Siberian stone pine cones, which typically contain 60–80 seeds, is determined by the presence of fallen mature cone scars on the annual shoots of the branch (c/s) in specific years [39]. This assessment is conducted by examining 5–8 branches from the upper portion of 15–20 tree crowns within the designated sample areas. From 0 to 5 mature cones can be found per shoot in some years. These visible scars on the shoots, indicating cone presence, can be observed for a period of 15–17, and sometimes up to 20 years. The traces left by aborted cones (unripe cones that have fallen) of the same year differ from mature cones in terms of size, resin amount, and degree of overgrowth.
In the Northern Urals, visual observations of nutcracker activity were conducted exclusively for the period from 1997 to 2012. These observations took place annually in August–September along fixed accounting routes measuring 7 kilometers in length. The observation area spanned a 50-meter wide strip on both sides of the route, covering the 30–40-day period when nutcrackers were actively harvesting seeds 31. The occurrence of nutcrackers was measured by recording their number per hour (birds/hour) during the observation period.
The Microsoft Office Excel program was used for the standard statistical processing of the materials received and for the presentation of graphs. We used linear regression equations. On the graphs of the dynamics of the regeneration, nutcracker occurrence and cone yields the calculated standard errors of the mean values have been inserted.
Results
In the Aldan Highlands, on a sample plot within a berry-green-moss forest type, there is a stand with an average height of 23 meters (with a sum of cross-sectional areas of 29.5 square meters per hectare). This stand consists of Pinus sibirica trees (50%), Picea obovata Ledeb (20%), Pinus sylvestris L (10%), Larix gmelinii Rupr (10%), and Betula pendula Roth (10%). In general, over the period from 2001 to 2012, number of Siberian stone pine undergrowth appeared on the moss substrate with a projective cover of 89.2% dominated by Pleurozium schreberi Brid, was 4.0 thousand specimens per hectare (thous. spec./ha) in 3.2 thousand "nests" per hectare. In the Northern Urals, under the canopy of a forest stand (consisting of Pinus sibirica 60%, Pinus sylvestris 20%, Larix sukaczevii Djil 10%, Betula pendula 10%) with a height of 22 meters (with a sum of cross-sectional areas of 32.5 square meters per hectare) in a geographically similar berry-green-moss forest type, the total number of Siberian stone pine undergrowth that appeared between 1998 and 2012 on the Pleurozium schreberi cover (96%) was 2.9 thous. spec./ha in 2.2 thousand "nests" per hectare. In the subalpine woodlands of the Khamar-Daban ridge, characterized by a height of 16 meters (with a sum of cross-sectional areas of 6.2 square meters per hectare) and predominantly consisting of Pinus sibirica (90%) and Picea obovata (10%), the total number of Siberian stone pine undergrowth from 2003 to 2015 was 3.5 thous. spec./ha in 1.8 thousand "nests" per hectare.
In the low-mountain Siberian stone pine forests on the Aldan Highlands and the Northern Urals, an average of 2.2–2.4 fallen cone scars per annual shoot (c/s) can be taken as an indication of a high relative crop. In the subalpine woodlands of the Khamar-Daban ridge, this indicator ranges from 1.8–2.2 c/s. Over a similar period, the average relative crop of cones in the Aldan Highlands (1.9 c/s) is approximately 1.5 times higher compared to the Urals (1.4 c/s) and Khamar-Daban (1.4 c/s). This difference in cone yields possibly explains the more abundant annual renewal of Siberian stone pine under the canopy of the parent stand in the Aldan Highlands, with an average of 1.3 thousand seedlings per hectare. In similar conditions of the geographically replacing forest type in the Northern Urals, the average is 1.0 thousand seedlings per hectare. Consequently, the total accumulation of undergrowth over the period under review is almost 1.5 times greater in the Aldan Highlands. In the subalpine woodlands of the Khamar-Daban ridge, the smaller number of undergrowth "nests" (1.8 thousand per hectare) may be attributed to the dense thickets of Bergenia crassifolia (L) Fritsch and Rhododendron aureum Georgi, which have a projective cover of 76.3% and hinder the nutcracker's access to the soil substrate for seed caching. The preferred substrate for nutcrackers, Pleurozium schreberi moss cover, only constitutes 25.6% of the total projective cover. Under the canopy of the forest stands in the Aldan Highlands and the Urals, openwork thickets of forest grasses and Vaccinium uliginosum L., Vaccinium vitis idea L., Vaccinium murtillus L., which reach heights of 20–30 cm and have a total projective cover of 35–40%, do not impede the nutcracker's access.
The analysis of Siberian stone pine yields in the three regions revealed diverse dynamics. The Aldan Highlands exhibited lower chronological variability (Cv = 21.6%) compared to the Urals (Cv = 46.5%) and Khamar-Daban (Cv = 30.9%). In the Aldan Highlands, there were minimal fluctuations in cone crops between 2000 and 2011, as depicted in Fig. 3a. The period from 2000 to 2004 showed consistently increased yields, followed by a relatively stable period of high yields from 2005 to 2009 and in 2011. However, 2010 was an exception with lower productivity (1.1 c/s). During the period from 1997 to 2011 in the Northern Urals, there were two years with high cone crops (1999 and 2004) separated by four years with low to medium crops, as shown in Fig. 3b. This cyclic pattern of alternating high and low crops every 4-6 years, which was previously observed in this region 8,32,40, was expected to continue with another high crop in the following years. However, contrary to expectations, there was a decline in cone crops in 2011, resulting in an absolute crop failure (0 c/s). The yields of subalpine woodlands in the Khamar-Daban ridge exhibited noticeable fluctuating dynamics from 2003 to 2014. This pattern involved a repetition of relatively high and increased yields after one year, alternating with low and medium yields (Fig. 3c).
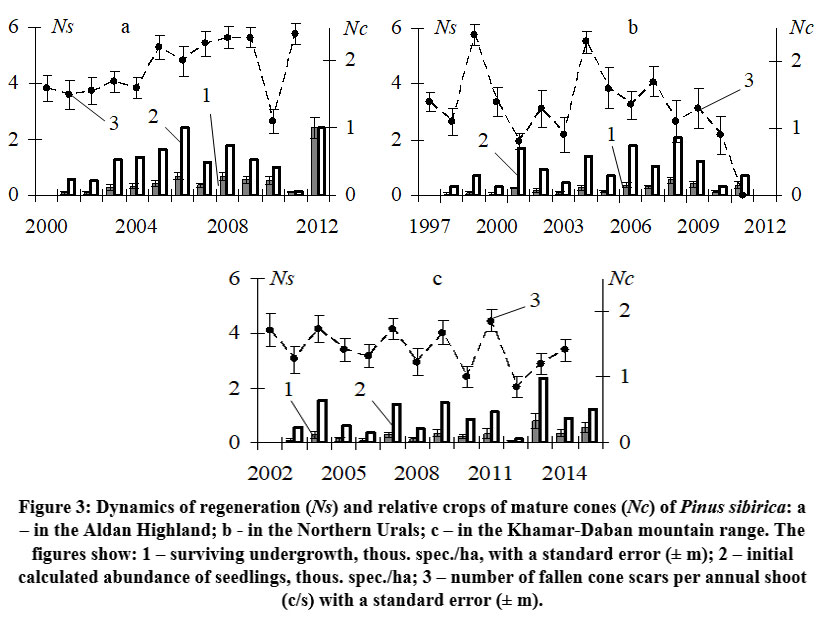 | Figure 3: Dynamics of regeneration (Ns) and relative crops of mature cones (Nc) of Pinus sibirica: a – in the Aldan Highland; b - in the Northern Urals; c – in the Khamar-Daban mountain range.
|
The dynamics of seedling generations in the studied regions are not consistent and can exhibit substantial fluctuations (Cv = 50.0–98.2%), even in the Aldan Highlands where cone yields are relatively stable (Fig. 3a). However, even minor fluctuations of cone yields have an impact on the subsequent emergence of seedlings under the canopy of the parent stand There is a relatively close positive relationship (R2 = 0.46) between the number of one-year-old seedlings during the period 2001–2012 and the previous year's cone crops (Fig. 4a). Unlike the Aldan Highlands, both the Northern Urals (Fig. 3b) and the subalpine woodlands of the Khamar-Daban ridge (Fig. 3c) exhibit a decrease in seedling numbers immediately the year after high cone yields, and vice versa, an abundant appearance of seedlings after a relatively weak crop. This negative relationship between one-year-old seedling numbers and the previous year's cone yield is evident (Fig. 4c, e). The fluctuations in the dynamics of seedling generations somewhat mirror the fluctuations in cone yields, but with a two-year delay. In these regions, a higher abundance of seedlings is observed primarily two years after high cone yields, as indicated by the positive close relationships (R2 = 0.49 in the Northern Urals and R2 = 0.48 in Khamar-Daban) between seedling numbers and the crop of the year before last (Fig. 4d, f).
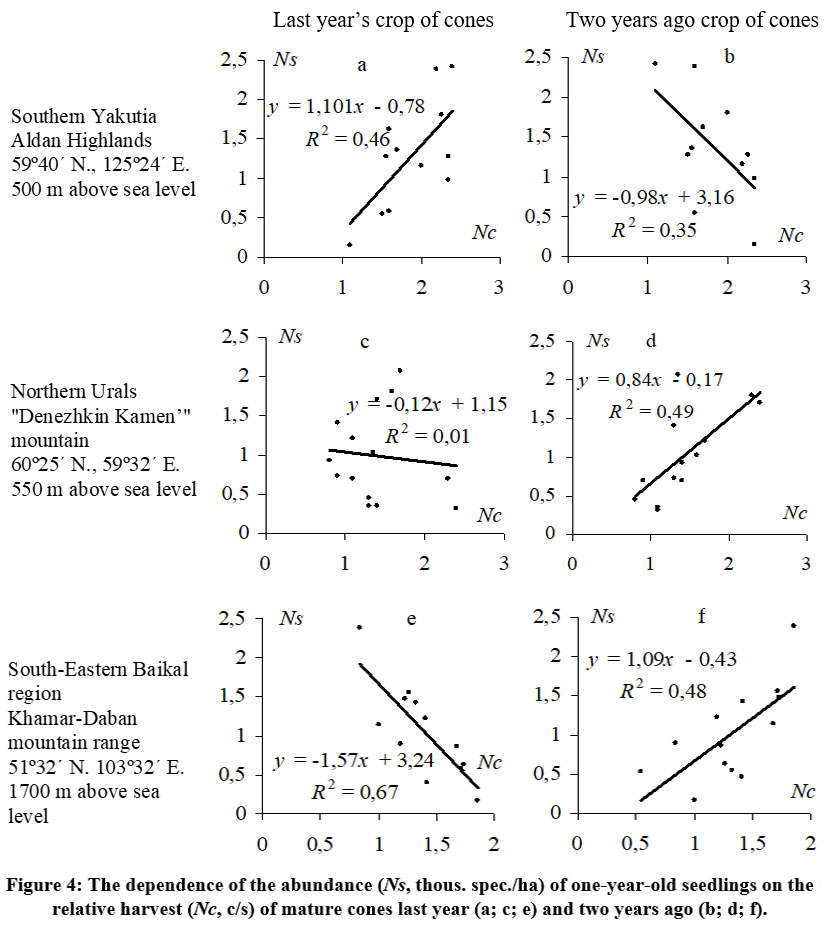 | Figure 4: The dependence of the abundance (Ns, thous. spec./ha) of one-year-old seedlings on the relative harvest (Nc, c/s) of mature cones last year (a; c; e) and two years ago (b; d; f).
|
Discussion
A correlational visual relationship indicates the existence of a pattern without providing an explanation or revealing the underlying mechanism of factor interaction. Thus it differs from a functional real relationship 40,41. Correlation merely identifies parallel variations in characteristics, which could be influenced by certain third factors 42.
The observed direct positive correlation between the abundance of one-year-old seedlings of Pinus sibirica and the level of last year’s relative cone crop in the Aldan Highlands' Siberian stone pine forest (Fig. 4a) appears to be natural and easily understandable. Similar relationships have been noted in anemochore forest-forming species and in Pinus sibirica in certain conditions of Western Siberia 36 and the mountain forest-tundra ecotone in the Northern Urals [43]. Previous studies on the natural regeneration of Siberian stone pine and other wingless-seeded five-needle pines in various regions of their habitat have also reported a similar dependence of seedling numbers on the seed crop from the year before last 31,35,44,45,46, as we have found in the Northern Urals (Fig. 4d) and Khamar-Daban (Fig. 4f).
The presence of a soil seed bank containing dormant Siberian stone pine seeds that germinate after two winters is often considered the primary reason for the observed relationships between the generation of one-year-old seedlings and the crop from two years prior 47. However, our study in the Aldan Highlands contradicts this conclusion. Furthermore, in the Northern Urals, no seedlings were found in the following year (2012) after a completely lean year (0 c/s) in 2011. In any scenario, there is a consistent pattern involving the emergence of seedlings from the seeds of the previous year's crop. The observed relationship between their abundance and the harvest from two years prior is actually influenced by an unaccounted factor. In the context of our study, one potential intermediary factor between seed yields and Siberian stone pine regeneration is the activity of nutcrackers.
The annual assessment of the relative nutcrackers occurrence (birds/hour) in the Northern Urals at the time of the seed caches season in August-September (1997-2012) revealed that, until approximately 2006, there were notable similarities between the fluctuations in nutcracker occurrence and the cone yield in the last year (Fig. 5). This observation is supported by a positive correlation (R2 = 0.55) (Fig. 6a). Similar delays in the relationships between other plants and their primary seed consumers, exhibiting comparable patterns of seed production fluctuations, have been observed 47,48.This situation is expressed by a famous trophic relationship in the "producer-consumer" or "prey-predator" system 49,50,51, where the predator's population naturally increases in the following year after a surge in the main food resource, fueled by the presence of young individuals. Since 2006, the dynamics of yield fluctuations and nutcracker occurrence have become synchronized (Fig. 5). Consequently, there is a strong relationship (R2 = 0.84) between nutcracker occurrence and the yield in a given year (Fig. 6b).
 | Figure 5: Dynamics of the relative crops of mature cones (Nc, c/s) of Pinus sibirica (1) and the occurrence of nutcrackers per hour (Nn, birds/hour) on the accounting rout (2) in the period of seed caching in the Northern Urals.
|
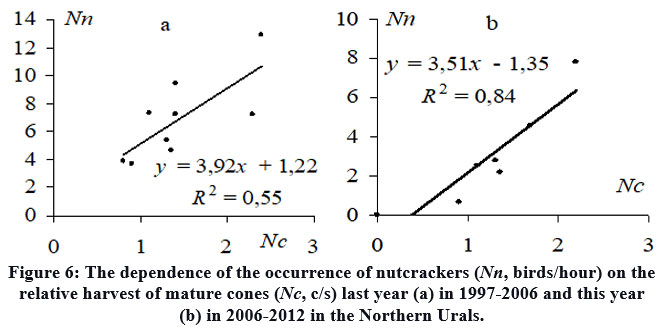 | Figure 6: The dependence of the occurrence of nutcrackers (Nn, birds/hour) on the relative harvest of mature cones (Nc, c/s) last year (a) in 1997-2006 and this year (b) in 2006-2012 in the Northern Urals.
|
Simultaneously, we can observe a consistent synchronization between fluctuations in the renewal of Pinus sibirica and the variations in the relative nutcracker population of the previous year (Fig. 7). Consequently, an upsurge in the generation of one-year-old seedlings occurs in the next year after an increase in nutcracker presence during the seed caching period, while a falling in renewal follows the subsequent year after a decrease in their population. This relationship expressed in a strong positive correlation (R2 = 0.81) between the relative abundance of one-year-old seedlings in a given year and the occurrence of nutcrackers (Fig. 8) in the last year’s seed caching season.
 | Figure 7: Dynamics of regeneration of Pinus sibirica (Ns) and the occurrence of nutcrackers (Nn) in the season of seed caching in the Northern Urals.
|
 | Figure 8: The dependence of the abundance (Ns, thous. spec./ha) of one-year-old seedlings on the occurrence of nutcrackers (Nn, birds/hour) in the last year’s seed caching season in the Northern Urals
|
By comparing our findings with previously published data on yield in Siberian stone pine forests from various regions6,7,8,10,31, it becomes evident that each region demonstrates its unique pattern of seed yield dynamics. Interestingly, abundant or poor harvests, as well as their duration, can either align (such as in the Urals and Khamar-Daban from 2004 to 2007; Fig. 2b, c) or exhibit complete opposition. Some previous studies reported that the dynamics of Siberian stone pine seed yields in the foothill and mountain in the Urals and the nearest regions in Western Siberia were nearly identical 7. However, it is possible that in some nearby regions the fluctuations in yield dynamics have ceased to coincide, leading to changes in the quantitative nutcracker dynamics (Fig. 5) and the appearance of its dependence on the current year's yield (Fig. 6b). Similar synchronicity with yields is manifested in the dynamics of the abundance of mouse-like rodents in Western Siberian forests of Pinus sibirica 34 or squirrels in the subalpine woodlands of Pinus Cembrae L.9 in the Alps, as a result of their migrations due to changes in the main food supply or the search for alternative more abundant and satisfying food. In this case, the nutcracker population in a specific region depends on both last year's harvest, which impacts the number of young in that year, and this year's harvest, including in some nearby areas, which triggers bird migrations. The first case exhibits a typical "producer-consumer" relationship (Fig. 6a), possibly supplemented by migratory nutcrackers in certain years. In the other case, fluctuations in nutcracker dynamics align with cone yields (Fig. 6b). Despite these changes and significant variations in nutcracker numbers over the years (Fig. 7), the consistent positive association between Siberian stone pine renewal and the previous year's nutcracker population during the seed caches period remains unchanged (Fig. 8).
It is highly likely that significant fluctuations in nutcracker dynamics and changes in their dependence on yields are influenced as much by the quantitative variations in the local population as by the number of migrants from other regions. Nutcrackers are dependent on the availability of cone-bearing trees and the quantity of cones in their area during the harvest season 15,16,32,52. They travel long distances in search of their primary food source, with some individuals reportedly moving up to 650 kilometers away from their natal territories33. For instance, there were sharp increases in bird numbers in 2000 following a high-yielding year in 1999, followed by a subsequent decline in 2001 (Fig. 5). The scarcity of food sources can also result in a decline in the population size and even the refusal of birds to breed 53,54,55,56,57. A significant reduction in the nutcrackers occurrence was observed in the region during a lean year in 2011, when they failed to appear in the autumn and did not breed in the following year, but began to return in August in 2012 [Fig. 5]. These fluctuations can be attributed not only to low crop yields but also to increased competition among nutcrackers, resulting from a surge in bird numbers following a high crop in the last year and limited food availability this year 23,28,30,53. This, in turn, appears to be connected to the subsequent decrease in nutcracker numbers for the following year. After a period of limited productivity seeds, a high yield remains unused as animal feed by the remaining few consumers. As a result, the following year sees a proliferation of young plants 58,59. However, this phenomenon is applicable only to species that are dispersed by wind (anemochoric species).
To ensure the regeneration of five-needle pines, it is crucial to have a sufficient population of nutcrackers (Nucifraga sp.) 10, as they are the sole distributors of their seeds. If the population of nutcrackers in a specific region is relatively small, the overall quantity of seed caches will be low60, even if the crop yield is high. Consequently, the number of one-year-old seedlings will be low for the subsequent year. For instance, despite a bountiful crop (2.3 c/s) in 2004, accompanied by a relatively low occurrence of nutcrackers (3.7 birds/hour), the Siberian stone pine forest in the Northern Urals only produced 0.7 thous. spec./ha of seedlings in 2005 (Fig. 3b, Fig. 7). Conversely, if this year's crop is lower than last year's, there is a notable increase in seed utilization, facilitated by a higher numbers of birds [53]. In our context, this can be perceived as seed caching. Following abundant harvests in 1999 (2.4 c/s) and 2004 (2.3 c/s), there was an increase in the number of birds in 2000 (12.9 birds/hour) and 2005 (7.2 birds/hour), coupled with an average crop (1.4-1.6 c/s). Consequently, a significantly larger amount of seeds was stored, leading to more than a twofold increase in renewal during 2001 and 2006 (1.7-1.8 thous. seedlings/ha). The extremely low number of seedlings (less than 0.1 thous. spec./ha) in 2000 (Fig. 3b) after a high crop (2.4 c/s) in 1999, with a relatively high occurrence of nutcrackers (7.3 birds/hour), is likely explained by the near complete depletion of food caches for feeding a higher number of chicks and its may be due to low natural loss of birds (Fig. 7). A similar situation occurred in Khamar-Daban (Fig. 3c), where there was only 0.2 thous. spec./ha of seedlings in 2012 after a high crop (1.9 c/s) in 2011. Overall, by examining the relationship between Siberian stone pine renewal and cone yields, while considering the fluctuation in nutcracker populations in the Northern Urals, we can explain a similar pattern observed in Khamar-Daban. After a year of high yields, the abundance of seedlings is two to three times lower compared to years with average or low yields (Fig. 3c).
According to some studies, nutcrackers consume up to 85% of their caches during the entire autumn-winter-spring season for both their own sustenance and feeding their chicks 2,15,24. Additionally, snow cover up to 60 cm high does not hinder nutcrackers from effectively excavating their caches 21. Nutcrackers exclusively feed their chicks with Siberian stone pine seeds during the nesting period. The number of chicks in a brood, typically ranging from 1 to 3 (occasionally up to 5), depends on the quantity of stored seeds and the crop yield 12,61. Since nutcrackers only retrieve their individual caches 15,27,29, it can be assumed that most of the seedlings emerge from unutilized caches due to natural loss during the winter. Ornithologists suggest that a common characteristic among all bird species is that, as their numbers increase, their caution decreases, making them more vulnerable to predators23,53. In other words, as the population of nutcrackers increases, the number of bird deaths also rises. Even if the natural mortality rate remains relatively constant in percentage terms, it will increase in absolute quantitative terms alongside the bird population. Therefore, the greater the abundance of nutcrackers in a region at the time of seed harvest, the greater the number of bird deaths during the winter, resulting in more unutilized seed caches that can germinate. It would be reasonable to assume that if a nutcracker were to die early in winter, the number of unused caches (potential seedlings) would be greater compared to its death later in winter. This may explain why, after a rise in the nutcracker population in the region, there was an increase in the seedling abundance the following year.
The presence of a direct positive relationship between the renewal of Siberian stone pine and the previous year's cone abundance in the Aldan Highlands suggests that in this region, where there are consistently high cone yields over several years, the local nutcracker population is likely to be relatively stable and acts as a consistent agent of seed dispersal, similar to the role of wind. Similarly, in the subalpine woodlands of the Khamar-Daban ridge, where the abundance of Siberian stone pine seedlings is also positively correlated with the cone yield from two years ago, the fluctuations in the previous year's nutcracker population are likely responsible, which in turn correlates with the seed crop from the previous year, as observed in the Northern Urals.
Conclusion
The various regions within the Siberian stone pine growth area exhibit distinct patterns in seed production dynamics. Each of the three regions discussed in the article demonstrates its own relationship between Siberian stone pine regeneration and seed yields. Based on our examination of Pinus sibirica natural regeneration in the Northern Urals, considering the long-term dynamics of nutcracker populations, we conclude that the key factor influencing the quantitative renewal of Siberian stone pine is the activity of nutcrackers and their number in the preceding year’s seed caching season. Conversely, the nutcracker population is influenced by seed yields from both the previous and current year and possibly also by the yield levels in neighboring regions. Given the varying seed production dynamics in the three regions studied and the established diverse relationships between seedling numbers and seed yields, each region is likely to have its unique dynamics in nutcracker populations, which play a crucial role in seed dispersal. The reasons behind changes in nutcracker population dynamics in the Urals and alterations in the relationship between nutcracker numbers and seed yields remain unclear, along with several other unanswered questions. Therefore, further research is necessary to shed light on these matters.
Acknowledgment
We would like to express our sincere appreciation to Professor YU Ruide for their valuable insights, support, and expertise, which greatly contributed to the research.
Funding Sources
This research was funded by the state assignment of the Institute Botanic Garden Ural Branch of Russian Academy of Sciences. The research was supported by the Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, China, specifically through the Tianchi Talents Project of Xinjiang (E3350107), the National Natural Science Foundation of China (Grant No.42107084), and the Key Research and Development Program of Xinjiang (2022B01032-4). The Institute Botanical Garden of Ural Branch of RAS, located in Yekaterinburg, Russia, also provided assistance and platforms for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Contribution:
Nikolai V. Tantsyrev: Article writing and data sources
Seyed Omid Reza Shobairi: Editing and modification, correcting parallelism and grammar and submission, validation
Vladimir A. Usoltsev: Editing, writing and proofreading
Sun Lingxiao: Validation and review of the article
Zhang Haiyan: Validation and review of the article
Li Chunlan: Validation and review of the article
He Jing: Validation and review of the article
Samira Hemmati Roudbari: Validation and editing
Behnam Asghari Beirami: Validation and editing and correction of text similarity
Qirghizbek Ayombekov: Validation and study of the article
Data Availability Statement
Not applicable
Ethics Approval Statement
It has not been studied on humans or animals
References
- ASSMANN E. WALDERTRAGSKUNDE, ORGANISCHE PRODUKTION, STRUKTUR, ZUWACHS UND ERTRAG VON WALDBESTÄNDEN. (No Title). 1961.
- BALDA RP. CONRADS K. Freilandbeobachtungen an Sibirischen Tannenhähern (Nucifraga caryocatactes macrorhynchos) 1977/78 in Bielefeld. Bericht des Naturwissenschaftlichen Vereins fu?r Bielefeld und Umgegend. 31, 1-31, 1990.
- BEKH IA. VOROB’YEV VN. Potential Siberian stone pine forests. Siberian stone pine problems. Tomsk, SB RAS, [Institute of Ecology of Natural Complexes - Branch of the Institute of Forest]. 6, 123, 1998 [In Russian].
- LORENZ TJ, SULLIVAN KA, BAKIAN AV, AUBRY CA. Cache-site selection in Clark’s nutcracker (Nucifraga columbiana). Auk. 128, 237–247, 2011.
CrossRef - MCLANE AJ. SEMENIUK K. MCDERMID GJ. TOMBACK DF. LORENZ T. MARCEAU D. Energetic behavioural-strategy prioritization of Clark’s nutcrackers in whitebark pine communities: An agent-based modeling approach. Ecological Modeling. 354, 123–139, 2017.
CrossRef - VOROB’YEV VN. CHERKASHIN VP. Kuz’michev VV. Cyclicity of growth and seed-bearing in Pinus sibirica. Russian Forest Science. 4, 38–48, 1982 [In Russin].
- SMOLONOGOV EP. Ecological and geographical differentiation and dynamics of Siberian stone pine forests of the Urals and West Siberian Plain (ecological and forestry bases of optimization of the economy). Sverdlovsk: RISO, Ur.B RAS. 288, 1990 [In Russin].
- ZEMLYANOY AI. BARANOVSKY VI. Features of seed production of Siberian stone pine on the northern border of the range. Conifers of the boreal zone. 24 (2–3), 183–186, 2007 [In Russian].
- ZONG C, WAUTERS LA, VAN DONGEN S, MARI V, ROMEO C, MARTINOLI A, PREATONI D, TOSI G. Annual variation in predation and dispersal of Arolla pine (Pinus cembra L.) seeds by Eurasian red squirrels and other seed-eaters. Forest Ecology and Management. 260 (1), 587–594, 2010.
CrossRef - GOROSHKEVICH S, VELISEVICH S, POPOV A, KHUTORNOY O, VASILYEVA G. 30-year cone production dynamics in Siberian stone pine (Pinus sibirica) in the southern boreal zone: a causal interpretation. Plant Ecology and Evolution. 154 (3), 321-331, 2021.
CrossRef - MEZHENNY AA. Biology of the nutcracker in South Yakutia. Zoological journal. 43 (2), 1679–1687, 1964 [In Russin].
- VANDER WALL SB. BALDA RP. Coadaptations of the Clark´s Nutcracker and the pinon pine for efficient seed harvest and dispersal. Ecological Monographs. 47, 89-111, 1977.
CrossRef - HUTCHINS HE, LANNER RM. The central role of Clark’s nutcracker in the dispersal and establishment of whitebark pine. Oecologia. 55, 192–201, 1982.
CrossRef - TOMBACK DF. Dispersal of whitebark pine seeds by Clark’s nutcracker: a mutualism hypothesis. J. Anim. Ecol.51, 451–467, 1982.
CrossRef - VOROB’YOV VN. Nutcracker and its interrelations with Siberian stone pine (Experience of Quantitative Analysis). Novosibirsk, Nauka Publ. 1982 [In Russian].
- SCHAMING TD, Sutherland CS. Landscape- and local-scale habitat influences on occurrence and detection probability of Clark’s nutcrackers: Implications for conservation. PLoS ONE. 15 (5), e0233726, 2020.
CrossRef - KRYLOV GV. Talantsev NK. Kozakova NF. Siberian stone pine. Moscow, Lesnaya promyshlennost’, 216, 1983 [In Russian].
- SEMECHKIN IV. Structure and dynamic of Siberian stone pine forests in Siberia. Novosibirsk, Publishing Hous SB RAS. 253, 2002 [In Russian].
- POLYAKOV VI, SEMECHKIN IV. Dynamics and stability siberin stone pine forests of Western Sayan. Russian Forest Science. 2, 12-19, 2004 [In Russian].
- DEBKOV N?, PANEVIN VS. The estimate of natural regeneration of Siberian pine in middle taiga (Western Siberia) under forest canopy and on clearings. Vestnik of Volga State University of Technology. Ser.: Forest. Ecology. Nature Management. 4 (40), 5–20, 2018 [In Russin].
CrossRef - TANTSYREV NV. Analysis of placement of Siberian stone pine seeds storage by nutcracker in traces of their winter use. Vestnik of Buryat State Academy of Agriculture. Ulan-Ude, 3 (60), 117-125, 2020 [In Russin].
- SOTSKAYA MN. Zoopsychology and comparative psychology. 1. Moscow, Yurayt Publ, 2014 [In Russian].
- REIMERS NF. Birds and mammals of the southern taiga of Western Siberia. Mscow, "Nauka" Publ. 1966 [In Russian].
- LANNER RM. Made for each other. A symbiosis of birds and pines. New York, Oxford, Oxford University Press, 1996.
CrossRef - SCHAMING TD. Clark’s nutcracker breeding season space use and foraging behavior. PLoS ONE. 11, e0149116, 2016.
CrossRef - HUTCHINS HE, HUTCHINS SA, LIU B. The role of birds and mammals in Korean pine (Pinus koraensis) regeneration dynamics. Oecologia. 107 (1), 120–130, 1996.
CrossRef - BEDNEKOF PA, BALDA RP. Clark’s nutcracker spatial memory: The importance of large, structural cues. Behavioural Processes. 102, 12-17, 2014.
CrossRef - TORNICK JK, RUSHIA SN, GIBSON BM. Clark's nutcrackers (Nucifraga columbiana) are sensitive to distance, but not lighting when caching in the presence of a conspecific. Behavioural Processes.123, 125-133, 2016.
CrossRef - OMEL’KO AM, OMEL’KO MM. Creating caches of nuts by nutcracker (Nucifraga caryocatactes L.) and using them in winter time in secondary broadleaved forests with plantations of Korean pine (Pinus koraiensis Sieb. Et Zucc .). Amurian Zoological Journal. 9 (2), 102-111, 2017 [In Russian].
CrossRef - CLARY D, KELLY DM. Cache protection strategies of a non-social food-caching corvid, Clark's nutcracker (Nucifraga columbiana). Animal Cognition. 14 (5), 735-744, 2011.
CrossRef - TANTSYREV NV, SANNIKOV SN. Analysis of consortive relationships between the Siberian stone pine and the nutcracker in the Northern Urals. Russian Journal of Ecology. 42 (1), 17-21, 2011 [In Russian].
CrossRef - Barringer LE, Tomback DF, Wunder MB, McKinney ST. Whitebark pine stand condition, tree abundance, and cone production as predictors of visitation by Clark’s nutcracker. Newsom LA, editor. PLoS ONE. 7, e37663, 2012.
CrossRef - HOLMGREN ML, WILKERSON RL, SIEGEL RB. Assessing trends and vulnerabilities in the mutualism between whitebark pine (Pinus albicaulis) and Clark's nutcracker (Nucifraga columbiana) in national parks of the Sierra-Cascade region. PLoS One. 15 (10), e0227161, 2020.
CrossRef - SEMECHKIN IV. The dependence of the renewal of Siberian stone pine on the nut yield of Siberian stone pine forests and the number of mouse-like rodents. In: Proceedings of the Commission for Nature Protection. Nature and forest vegetation of the northern part of the Sverdlovsk region. Sverdlovsk, Ural Branch of the USSR Academy of Sciences. 1, 141-149, 1964 [In Russian].
- STASHKEVICH NY, SHISHIKIN AS. Zoogenic factor of Siberian pine restoration in the mountain taiga forests of East Sayan. Contemporary Problems of Ecology. 2, 313–318, 2014 [In Russian].
- NIKOLAEVA SA, SAVCHUK DA. The Siberian stone pine regeneration dynamics in the Ket-Chulym interfluve (West Siberian Plain). Interexpo Geo-Siberia. 3 (4), 64-68, 2015 [In Russin].
- TANTSYREV NV, SANNIKOV SN. Dynamics of environmental factors and Siberian stone pine regeneration in burned-out and clear-cut forest areas in the Urals. Russian Journal of Ecology. 39 (2), 151-154, 2008 [In Russian].
CrossRef - SANNIKOV SN, TANTSYREV NV. Survival curves of Siberian pine undergrowth as a basis for reconstructing its population dynamics. Russian Forest Science. 4, 275-281, 2015 [In Russin].
- GORCHAKOVSKY PL. New in the methodology of research of coniferous seed bearing. Botanical Journal. 43 (10), 1445-1459, 1958 [In Russian].
- ARMAND DL. Functional and correlation relations in physical geography. Izvestiya Vsesoyuznogo Geograficheskogo Obshchestva. 1, 81-94, 1949 [In Russian].
- KARPACHEVSKY LO. Forest and Forest Soils. Moscow, Lesnaya Promyshlennost’ Publ, 1981 [In Russian].
- KOLMOGOROV AN. On the question of the suitability of the forecast formulas found by statistical means. Zavodskaya Laboratoriya. 1, 164-167, 1993 [In Russian].
- SANNIKOV SN, TANTSYREV NV, PETROVA IV. Invasion of Siberian pine populations in mountain tundra in the Northern Urals. Contemporary Problems of Ecology. 11 (4), 396-405, 2018 [In Russian].
CrossRef - KAJIMOTO T, ONODERA H, IKEDA S, DAIMARU H, SEKI T. Seedling establishment of subalpine stone pine (Pinus pumila) by nutcracker (Nucifraga) seed dispersal on Mt. Yumori, Northern Japan. Arctic and Alpine Research. 30 (4), 408-417, 1998.
CrossRef - TOMBACK DF, ANDERIES AJ, CARSEY KS, POWELL ML, MELLMANN-BROWN S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology. 82 (9), 2587-2600, 2001.
CrossRef - TILLMAN-SUTELA E, KAUPPI A, KARPPINEN K, TOMBACK D. Variant maturity in seed structures of Pinus albicaulis (Engelm.) and Pinus sibirica (Du Tour): Key to a soil seed bank, unusual among conifers? Trees. 22 (2), 225-236, 2008.
CrossRef - BOUTIN S, WAUTERS LA, MCADAM AG, HUMPHRIES MM, TOSI G, DHONDT AA. Anticipatory reproduction and population growth in seed predators. Science. 314 (5807), 1928–1930, 2006.
CrossRef - WAUTERS LA, GITHIRU M, BERTOLINO S, MOLINARI A, TOSI G, LENS L. Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography. 31 (1), 104–114, 2008.
CrossRef - TIMOFEEV-RESSOVSKY NV, YABLOKOV AV, GLOTOV NV. An essay of the doctrine of the population. Moscow, Nauka Publ, 1973 [In Russian].
- HARPER JL. Population Biology of plants. L; N.Y., Acad. Press, 1977.
- BIGON M, HARPER D, TOWNSEND K. Ecology. Individuals, populations and communities. Moscow, Mir Publ, 1989. [In Russian].
- MCKINNEY ST, FIEDLER CE, TOMBACK DF. Invasive pathogen threatens bird-pine mutualism: implications for sustaining a high-elevation ecosystem. Ecol Appl. 19, 597–607, 2009.
CrossRef - VLADYSHEVSKY DV. Ecology of forest animals and birds. Forage extraction and its biogeocenotic significance. Novosibirsk, Nauka Publ, 1980 [In Russin].
- KATAEV GD. Population monitoring of small mammals in the Kola peninsula over 75 years. Russian Journal of Ecology. 43 (5), 406–408, 2012 [In Russian].
CrossRef - KREBS CJ, BOONSTRA R, BOUTIN S, ET AL. Trophic dynamics of the boreal forests of the Kluane region. Arctic. 67 (1), 71–81, 2014.
CrossRef - BOGDZIEWICZ M, ZWOLAK R, CRONE EE. How do vertebrates respond to mast seeding? Oikos, 125 (3), 300–307, 2015.
CrossRef - SCHAMING TD. Population-Wide failure to breed in the Clark’s nutcracker (Nucifraga columbiana). PLoS ONE. 10, e0123917, 2015.
CrossRef - VISSER MD, JONGEJANS E, VAN BREUGEL M. ET AL. Strict mast fruiting for a tropical dipterocarp tree: a demographic cost benefit analysis of delayed reproduction and seed predation. Journal of Ecology. 99 (4),1033–1044, 2001.
CrossRef - CRONE EE. RAPP JM. Resource depletion, pollen coupling, and the ecology of mast seeding. Annals of the New York Academy of Sciences. 1322, 21–34, 2014.
CrossRef - VANDER WALL SB. Masting in animal-dispersed pines facilitates seed dispersal. Ecology. 83 (12), 3508–3516, 2002.
CrossRef - SWANBERG PO. Territory in the thick-billed nutcracker Nucifraga c. caryocatactes. Ibis.56,98, 1956.
CrossRef







