Geographic Information System-based Analysis of Fish Diversity Trends of River Meenachil, Southern Western Ghats, Kerala.
1
Department of Zoology, Mar Thoma College, Thiruvalla,
Pathanamthitta, Mahatma Gandhi University,
Kottayam,
Kerala
India
2
Department of Geology,
University of Kerala,
Thiruvananthapuram,
Kerala
India
3
Department of Zoology,
Catholicate College, Pathanamthitta, Mahatma Gandhi University,
Kerala
India
Corresponding author Email: lethapc@hotmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.1.26
Copy the following to cite this article:
Cheriyan L. P, Appukuttan A, Oommen M. Geographic Information System-based Analysis of Fish Diversity Trends of River Meenachil, Southern Western Ghats, Kerala. Curr World Environ 2023;18(1). DOI:http://dx.doi.org/10.12944/CWE.18.1.26
Copy the following to cite this URL:
Cheriyan L. P, Appukuttan A, Oommen M. Geographic Information System-based Analysis of Fish Diversity Trends of River Meenachil, Southern Western Ghats, Kerala. Curr World Environ 2023;18(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-11-03 |
|---|---|
| Accepted: | 2023-02-11 |
| Reviewed by: | 
 Sadar Aslam
Sadar Aslam
|
| Second Review by: |

 Izolda Matchutadze
Izolda Matchutadze
|
| Final Approval by: | Dr. Hiren B. Soni |
Introduction
Gradual changes in fish community composition along the linear gradient of a river from upstream to downstream are better predicted by the longitudinal zonation hypothesis1. Tropical and temperate rivers usually exhibit a fairly homogenous river zonation pattern with upstream rithral areas of low species diversity followed by transitional areas with comparatively high diversity and downstream potamal areas with maximum species richness and abundance.2 Reference 3 mentions the anthropogenic impacts on the downstream of rivers and the deviating trends in fish diversity gradients. The Meenachil River of Kerala is the only river of the Western Ghats, having human inhabitancy right from its root source till its mouth, where it confluence to Vembanad Lake.4 The riverine ecosystem is under multiple stressors, which include catchment disturbances, water diversions, habitat loss and fragmentation, loss of riparian, water pollution, and overfishing exacerbated by climatic fluctuations, which have resulted in substantial shifts in diversity and distribution patterns of many endemic fishes of the region.5,6
Ecological patterns, changes in community composition, and diversity trends can be examined critically using diversity and similarity indices. Distribution and abundance are the main criteria used to ascertain species’ status as “threatened” or “endangered” for the implementation of species-targeted conservation measures. The conservation strategy should be more effective by integrating the complementary use of the various methodologies and the improved utilization of the available information7. Despite the fact that GIS is getting extra attention in the fields of hydrology and aquaculture management, their adoption for spatial decision support in this area is still moving extremely slowly.8. The addition of the geographic dimension in the form of GIS provides a better perspective on the diverse data and contributes effectively to global conservation efforts enhancing the conservation of biodiversity by providing integration of information in spatial overlays.9 GIS has been used in conservation biology not just for identifying and mapping a region's biodiversity but also for identifying and prioritizing the conservation areas by examining the habitat features and alterations for the implementation of proper restoration strategies.10,11 In order to triangulate its own position, the GPS recovery system uses at least three satellites and between 24 and 32 microwave-transmitting medium earth orbit satellites.12 To positionize deforestation, river pollution, substratum habitat structure, fish faunal variety, and interpolation, those satellites are currently being used 8. Several researchers have taken biophysical data from satellite photography and incorporated them into simulation models.13
By using the tools for deriving scientific output from the gathered data, GIS and its technologies have added additional flexibility to marine fisheries to produce marine environmental data useful to detect contaminants, keep tabs on fishing activity, map habitats on the sea floor, and quantify the physical and biological characteristics of the water column14. For the first time in India, a project including the use of GIS in marine fishing is being carried out along the coast of Karnataka state15.
A few GIS-based studies have been conducted on the fish distribution and abundance pattern of marine fish species of the Arabian sea16, but no such works have been conducted on the riverine fishes of Kerala.17 suggested that the application of RS for studies on the environmental characteristics of the oceans could provide a comprehensive picture on fish distribution, abundance, migration, and other information required for monitoring and managing the ocean ecosystems. The International Symposium on Remote Sensing and Fisheries was hosted in 2010 by the Project "Social Applications in Fisheries and Aquaculture using Remotely Sensed Imagery" (SAFARI) and took place in Kochi, Kerala covered the most recent applications to improve fisheries and aquaculture research, particularly in the creation of possible fishing zones.17
Studies on freshwater ichthyology in Kerala can be traced back to Bloch's work in the late 18th century, followed by 18-24 made substantial contributions after Francis Day's work to the study of freshwater fishes in Travancore. Despite the several fish diversity studies conducted in the rivers of Kerala, no works have been documented so far on GIS-based zonation and the patterns of fish assemblage in Kerala's rivers. Fish diversity measurements from the Upper Ganges were analysed and mapped utilising spatial interpolation techniques of geographic information systems by 25.A study was done by 26 using Kriging spatial interpolation methods for the spatial analysis and geographical information system mapping of fish diversity in the Pong reservoir in Himachal Pradesh. From seven locations along the Cauvery River basin in Tamilnadu, India, fish and water samples were collected, examined, and the results were entered into a GIS platform.8
Rivers of the Southern Western Ghats are hotspots of many threatened and endemic species of fishes, which emphasize an urgent need to consider their conservation.27 The fish fauna of the rivers of Southern Western Ghats documented was based on the taxonomy, geographical distribution, and ecological aspects.28 Due to the growing trend of site-specific distinct threats, such information is insufficient to address the essential concerns pertaining to the regional conservation and management of fish biodiversity 29 conducted a geomorphic assessment of the Meenachil river basin using the Geographic Information system. There are no thoroughgoing efforts to assess the present conservation status of the fishes of Meenachil River, their distribution, and ecological requirements utilizing the spatial interpolation techniques of geographic information systems, other than a few baseline inventories.
This study uses GIS technology to explore how fish diversity changed along the longitudinal gradient during 2016-2020 along the river and to assess the river’s present conservation and fish distribution status.
Material and Methods
Study area
Meenachil River (Fig. 1) with a length of 78 km has a basin area of 1272 km², with a watershed extending from northern latitudes of 90.51’ to 90.55’ and east longitudes of 76020’ to 76.55’.41 The major tributary originates from Annakunnumudi at an elevation of +922m above MSL, confluence to Vembanad lake.42
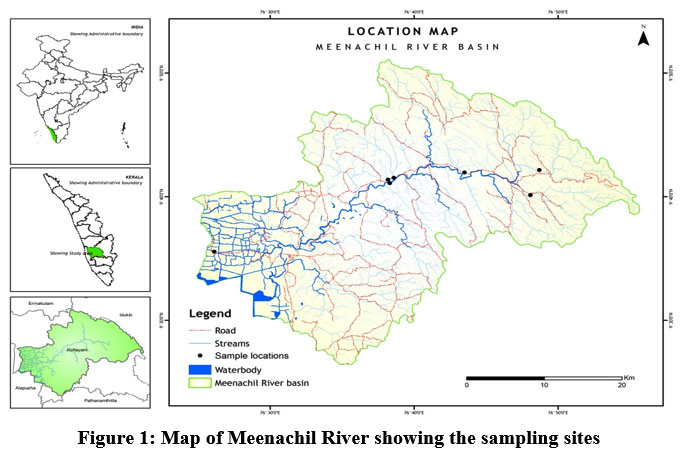 | Figure 1: Map of Meenachil River showing the sampling sites
|
Sampling sites
Seven different sampling sites were chosen from the upper, middle, and lower stretches of the Meenachil River. The study sites selected were Teekoy (TKY), Poonjar (PNJ), Bharananganam (BGM), Mutholy (MLY), Cherpunkal (CPL), Kattachira (KTA), and Kumarakom (KUM) (Table 1). The study period ranged from January 2016 to December 2020.
Table 1: Details of the sampling sites with geographic co- ordinates
| Sample site | Geographical location | Shoreline | |||
| Latitude | Longitude | Altitude | Riparian | Recreation | |
| TKY | 76.8115oE | 9.7028 oN | 274m | High- Partially covered by forest, tea plantation, coconut and pineapple plantation | Tourism destination |
| PNJ | 76.8012 oE | 9.6690 oN | 98m | Medium- Rubber, coconut and pineapple plantation | Not a tourist site |
| BGM | 76.7250 oE | 9.6994 oN | 35m | Medium - Rubber, coconut, cocoa and nutmeg | Not a tourist site
|
| MLY | 76.6431 oE | 9.6923 oN | 31m | High- Bamboo, Rubber, coconut, cocoa and nutmeg | Not a tourist site |
| CPL | 76.6384 oE | 9.6852 oN | 22m | High- Bamboo, Rubber, coconut, nutmeg and paddy | Not a tourist site |
| KTA | 76.6364 oE | 9.6899 oN | 19m | Very High- “Reserve Riparian Forest” with rich bamboo and Madhuca nerifolia plantations. | Not a tourist site
|
| KUM | 76.2607 oE | 9.3534 oN | 9m | Very low- Paddy cultivation, coconut, mixed vegetation | Backwater Tourism destination |
Sampling methods
Fish samples were collected from seven different sites falling in the up, mid, and downstream of the river (Figs. 2, 3 & 4). Collections were done from January 2016 to December 2020. Fish sampling was executed with the help of fishing experts using different gears based on the physical nature of the habitat. In addition, to cast nets, gill nets, and bag nets, specially designed traps were used to collect fish (Fig. 5, 6 & 7). Species for identification were collected and preserved in 70% ethyl alcohol. Identification was done using standard literature by 43,44 and the conservation status was documented according to IUCN red list 2022 with modifications based on updated literature.45.
Sampling Sites
 | Figure 2: Upstream
|
 | Figure 3: Midstream
|
 | Figure 4: Downstream
|
Sampling Methods
 | Figure 5: Trap - perumkoodu
|
 | Figure 6: Midstream casting
|
 | Figure 7: Gill net
|
Ethical Statement
The least number of fish was used for the study and the remaining ones caught were immediately released into the river without harming them. The study was carried out in accordance with the regulation of the Animal Ethics Institutional Committee.
Water quality parameters
Temperature, conductivity, total dissolved solids (TDS mg/L), dissolved oxygen (DO), Salinity (ppt) and pH were evaluated from different sites during different seasons from 2016 to 2020 using a Multiparameter portable meter, HANNA, Model HI 2020-02.
Species richness and diversity
Species richness and diversity of the different zones were assessed by Shannon’s diversity index, similarities between different zones of the river were compared using by Neighborhood Based Clustering using the software, PAST 46.
GIS Analysis
Study area boundaries were prepared from DEM (Digital elevation model) downloaded from the USGS website.47 The data has a resolution of 30m. DEM was used for the delineation of river basin boundaries and morphometric attributes like drainages and their tributaries. The waterbody (River) in the study area was digitized from the Survey of India Toposheets. The data pertaining to fish were collected from different stretches of the river using GPS and cross-checked with google imagery and toposheet for further verification.42 All the data layers were cross-checked and processed in WGS 1984 datum. Final data sets were converted and plotted over the ArcGIS Platform for further analysis. After the preliminary data entry process, the dataset was subjected to spatial variation analysis using the Inverse distance weighted (IDW) technique interpolation process.48 IDW software forecasts the values that greatly influence more than those with less influence.49 DEM and Vectorised GIS layers were used to prepare the various thematic layers depicting the spatial variation and related aspects in the current study.
Result
Fish Species distribution and conservation status
Sixty-seven fish species belonging to 15 orders, 29 families, and 46 genera were recorded from the river during the period. Cypriniformes was the most abundant order having 23 species, and Siluriformes second with 11 species. Cyprinidae was the most dominant family comprising 14 species, followed by Bagridae, comprising six species (Table 2). Twenty-five species were endemic to the Western Ghats (WG), three were exclusive to Kerala (KL), five were categorized as nearly threatened (NT), and three were vulnerable (VU) according to the IUCN criteria. The exotic species reported were distributed in the lower stretch of the river (Fig. 8 & Fig.9 a, b, c & d).
Table 2: Fish species collected from the upstream, midstream, and downstream sites of Meenachil River during different seasons (2016-2020).
| UP | MID | DOWN | IUCN STATUS | Endemicity | |||||
| TKY | PNJ | BGM | MLY | CPL | KAT | KUM | |||
| ANGUILLIFORMES | |||||||||
| Anguillidae | |||||||||
| Anguilla bengalensis (Gray, 1831) | ? | ? | ? | ? | ? | ? | ? | NT | |
| Anguilla bicolor (McClelland, 184I) | ? | ? | ? | ? | ? | ? | ? | NT | |
| CYPRINIFORMES | |||||||||
| Danionidae | |||||||||
| Amblypharyngodon melettinus (Val, 1844) | ? | ? | ? | ? | LC | ||||
| Devario aequipinnatus (McClelland, 1839) | ? | ? | ? | ? | ? | ? | DD | WG | |
| Barilius bakeri (Day, 1865) | ? | ? | ? | ? | ? | ? | LC | WG | |
| Rasbora daniconius (Hamilton, 1822) | ? | ? | ? | ? | ? | ? | LC | ||
| Horadandia brittani (Menon & RemaDevi, 1992) | ? | ? | ? | ? | LC | WG | |||
| Cyprinidae | |||||||||
| Hypselobarbus kurali (Menon & RemaDevi, 1995) | ? | ? | ? | ? | ? | ? | ? | LC | WG |
| Labeo dussumieri (Val, 1842) | ? | ? | ? | ? | LC | ||||
| Dawkinsia filamentosa (Val, 1844) | ? | ? | ? | ? | ? | ? | ? | LC | WG |
| Puntius mahecola (Val, 1844) | ? | ? | ? | ? | DD | KL | |||
| Pethia punctata (Day, 1865) | ? | ? | ? | ? | ? | ? | LC | WG | |
| Pethia ticto(Hamilton, 1822) | ? | ? | ? | ? | LC | ||||
| Puntius vittatus (Day, 1865) | ? | ? | ? | ? | LC | WG | |||
| Systomus sarana (Hamilton, 1822) | ? | ? | ? | ? | LC | ||||
| Puntius parrah (Day, 1865) | ? | ? | ? | ? | LC | WG | |||
| Haludaria fasciata (Jerdon, 1849) | ? | ? | ? | LC | WG | ||||
| Puntius bimaculatus (Bleeker, 1863) | ? | NE | |||||||
| Cyprinus carpio (Linnaeus, 1873) | ? | ? | ? | ? | LC | EX | |||
| Osteobrama bakeri (Day, 1873) | ? | ? | ? | LC | KL | ||||
| Gibelion catla(Hamilton, 1822) | ? | ? | ? | ? | LC | EX | |||
| Balitoridae | |||||||||
| Garra mullya (Sykes, 1839) | ? | ? | ? | LC | WG | ||||
| Cobitidae | |||||||||
| Lepidocephalichthys thermalis (Val, 1846) | ? | LC | |||||||
| Nemacheilidae | |||||||||
| Nemacheilus triangular (Day,1865) | ? | ? | ? | LC | WG | ||||
| Schistura scaturigina (McClelland, 1839) | ? | ? | ? | LC | |||||
| PLEURONECTIFORMES | |||||||||
| Soleidae | |||||||||
| Brachirus orientalis (Bloch & Schneider, 1801) | ? | NT | |||||||
| SILURIFORMES | |||||||||
| Horabagridae | |||||||||
| Horabagrus brachysoma (Günther, 1864) | ? | ? | ? | ? | VU | WG | |||
| Bagridae | |||||||||
| Mystus oculatus (Val, 1840) | ? | LC | WG | ||||||
| Mystus cavasius (Hamilton, 1822) | ? | LC | |||||||
| Mystus montanus (Jerdon,1849) | ? | ? | ? | ? | ? | ? | LC | WG | |
| Mystus gulio (Hamilton, 1822) | ? | LC | |||||||
| Mystus malabaricus (Jerdon, 1849) | ? | ? | ? | NT | WG | ||||
| Mystus atrifasciatus (Fowler, 1937) | ? | LC | |||||||
| Siluridae (buttercatfishes) | |||||||||
| Ompok bimaculatus (Bloch, 1794) | ? | ? | ? | ? | NT | ||||
| Ompok malabaricus (Val, 1840) | ? | ? | ? | ? | ? | ? | LC | WG | |
| Wallago attu (Bloch & Schneider, 1801) | ? | ? | ? | ? | NT | ||||
| Heteropneustidae (stinging catfishes) | |||||||||
| Heteropneustes fossilis (Bloch, 1794) | ? | ? | ? | ? | LC | ||||
| CYPRINODONTIFORMES | |||||||||
| Aplocheilidae (panchax) | |||||||||
| Aplocheilus lineatus (Val, 1846) | ? | ? | ? | ? | ? | ? | ? | LC | |
| Aplocheilus blockii (Arnold, 1911) | ? | ? | ? | ? | ? | ? | ? | LC | |
| BELONIFORMES | |||||||||
| Belonidae (Needlefihes) | |||||||||
| Xenentodon cancila (Hamilton, 1822) | ? | ? | ? | LC | |||||
| Hemiramphidae (Halfbeaks) | |||||||||
| Hyporhamphus limbatus (Val, 1847) | ? | LC | WG | ||||||
| Hyporhamphus quoyi(Val, 1847) | ? | NE | |||||||
| SYNBRANCHIFORMES | |||||||||
| Mastacembelidae(spiny eels) | |||||||||
| Mastacembelus armatus (Lacepède, 1800) | ? | ? | ? | LC | |||||
| Macrognathus guentheri (Day, 1865) | ? | ? | ? | ? | ? | ? | LC | WG | |
| Macrognathus aral (Bloch & Schneider, 1801) | ? | LC | |||||||
| PERCIFORMES | |||||||||
Ambassidae (Asiatic glassfihes/perchlets) | |||||||||
| Parambassis ranga (Hamilton, 1822) | ? | LC | |||||||
| Parambassis dayi (Bleeker, 1874) | ? | ? | ? | ? | LC | WG | |||
| Parambassis thomassi (Day, 1870) | ? | ? | ? | ? | ? | ? | ? | LC | WG |
| Scatophagidae | |||||||||
| Scatophagus argus (Linnaeus, 1766) | ? | LC | |||||||
| Gerreidae | |||||||||
| Gerres setifer (Hamilton, 1822) | ? | NE | |||||||
| Sillaginidae | |||||||||
| Sillago sihama (Forsskal, 1775) | ? | NE | |||||||
| CICHLIFORMES | |||||||||
| Cichlidae (pearl spot) | |||||||||
| Etroplus suratensis (Bloch, 1790) | ? | ? | ? | ? | LC | ||||
| Etroplus maculatus (Bloch, 1795) | ? | ? | ? | ? | ? | ? | ? | LC | |
| Oreochromis mossambicus (Peters, 1852) | ? | ? | ? | ? | LC | EX | |||
| GOBIIFORMES | |||||||||
| Gobiidae (gobies) | |||||||||
| Glossogobius giuris (Hamilton, 1822) | ? | LC | |||||||
| Glossogobius aureus (Akhito & Meguro, 1975) | ? | LC | |||||||
| ANABANTIFORMES | |||||||||
| Nandidae (Leaf fishes) | |||||||||
| Nandus nandus (Hamilton, 1822) | ? | ? | ? | ? | ? | ? | ? | LC | |
| Pristolepididae | |||||||||
| Pristolepis rubripinnis (Britz & Kumar, 2012) | ? | ? | ? | NE | KL | ||||
| Anabantiae (climbing perch) | |||||||||
| Anabas testudineus (Bloch, 1792) | ? | ? | ? | ? | DD | ||||
| Channidae (snakeheads) | |||||||||
| Channa marulius (Hamilton, 1822) | ? | ? | ? | ? | LC | WG | |||
| Channa striata (Bloch, 1793) | ? | ? | ? | ? | LC | WG | |||
| Channa diplogramma (Day, 1865) | ? | ? | ? | ? | VU | WG | |||
| Channa gachua (Hamilton, 1822) | ? | ? | ? | ? | ? | ? | LC | WG | |
| TETRAODONTIFORMES | |||||||||
Tetraodontiae (puffer fish) | |||||||||
| Carinotetraodon travancoricus (Hora & Nair, 1941) | ? | ? | ? | VU | WG | ||||
| ELOPIFORMES | |||||||||
| Megalopidae | |||||||||
| Megalops cyprinoides (Broussonet, 1782) | ? | DD | |||||||
| CLUPEIFORMES | |||||||||
| Clupeidae | |||||||||
| Ehirava fluviatilis (Deraniyagala, 1929) | ? | ? | ? | ? | DD | ||||
| CHARACIFORMES | |||||||||
| Serrasalmidae | |||||||||
| Piaractus brachypomus (Cuvier, 1818) | ? | ? | ? | ? | NE | EX | |||
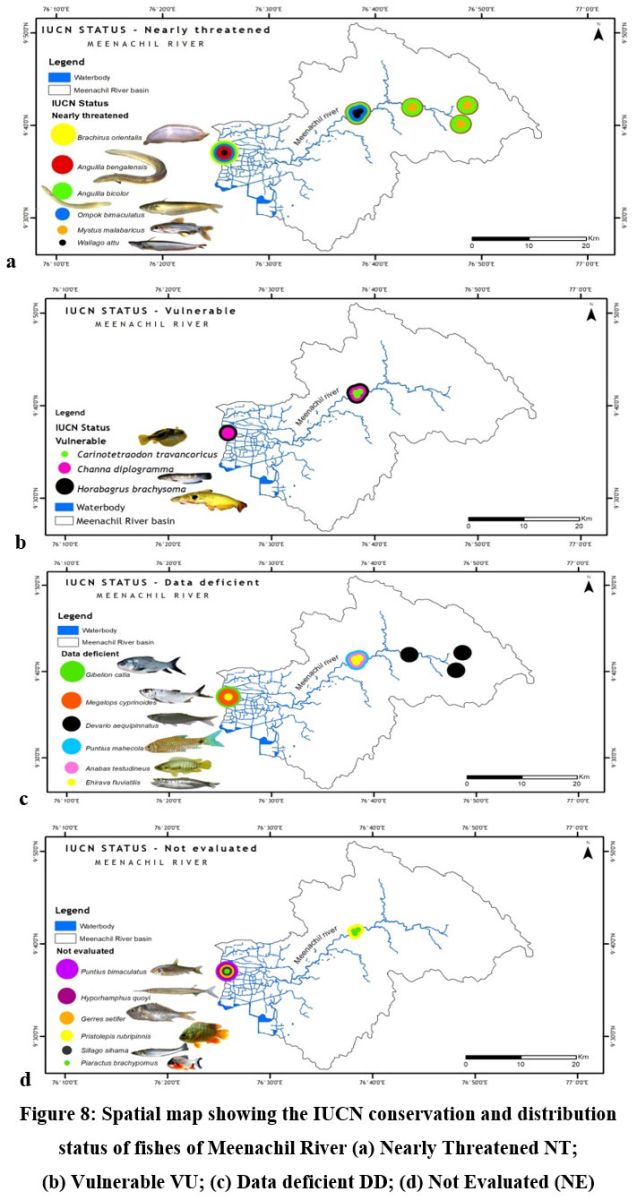 | Figure 8: Spatial map showing the IUCN conservation and distribution status of fishes of Meenachil River (a) Nearly Threatened NT; (b) Vulnerable VU; (c) Data deficient DD; (d) Not Evaluated (NE)
|
Fish diversity along the longitudinal gradient
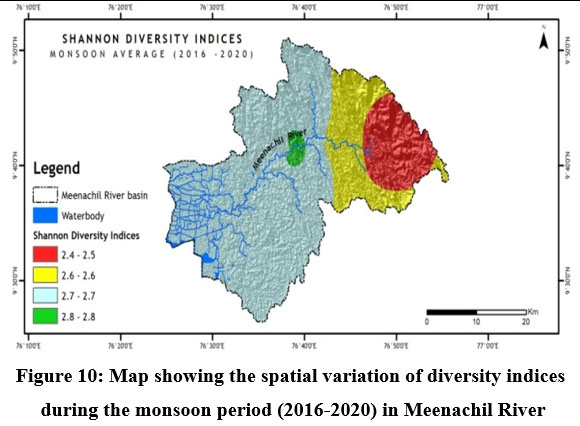
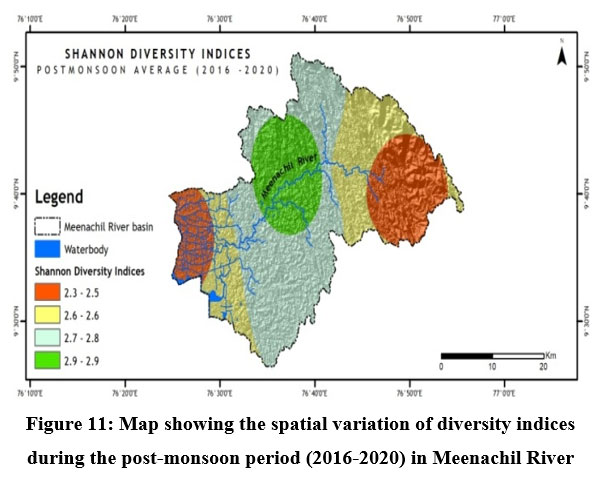
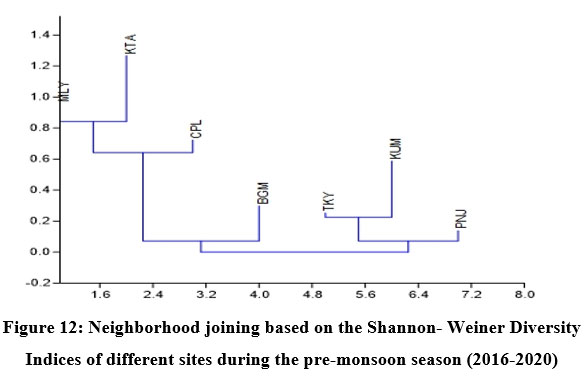
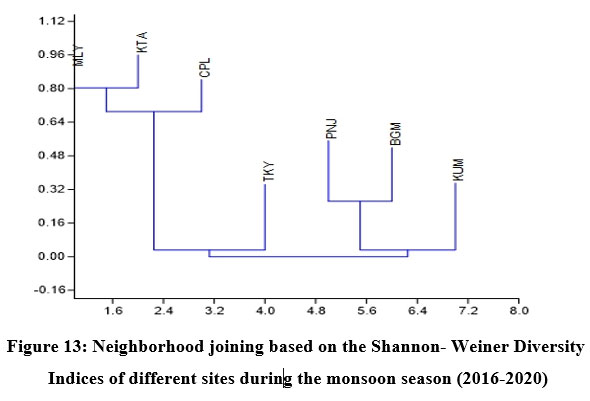
Fish diversity assessed by Shannon- Weiner Diversity Indices as in Table 3 showed significant seasonal variations in seven different sites along the longitudinal gradient of the river and was depicted through GIS mapping (Fig. 9-11). Neighborhood-based clustering of sampling sites based on the similarities in their diversity indices is given in Fig. 12-14.
Table 3: Shannon- Weiner Diversity Indices (H’) of fishes from the different sampling sites from 2016- 2020.
| YEAR | SEASON | SITE | ||||||
| TKY | PNJ | BGM | MLY | CPL | KTA | KUM | ||
| 2016 | PRM | 2.622 | 2.552 | 2.693 | 2.777 | 2.988 | 3.217 | 2.567 |
| M0N | 2.354 | 2.624 | 2.827 | 3.019 | 2.8799 | 2.783 | 2.609 | |
| POM | 2.348 | 2.237 | 2.576 | 3.077 | 3.087 | 2.993 | 2.499 | |
| 2017 | PRM | 2.551 | 2.635 | 2.498 | 3.011 | 2.967 | 3.22 | 2.377 |
| M0N | 2.48 | 2.526 | 2.623 | 3.115 | 2.836 | 2.988 | 2.729 | |
| POM | 2.451 | 2.397 | 2.217 | 2.924 | 2.781 | 2.962 | 2.284 | |
| 2018 | PRM | 2.498 | 2.501 | 2.233 | 2.951 | 2.851 | 3.197 | 2.638 |
| M0N | 2.523 | 2.385 | 2.356 | 2.962 | 3.011 | 3.059 | 2.713 | |
| POM | 2.784 | 1.926 | 2.079 | 2.766 | 2.913 | 2.881 | 2.342 | |
| 2019 | PRM | 2.554 | 2.481 | 2.554 | 2.943 | 2.616 | 2.992 | 2.538 |
| M0N | 2.504 | 2.645 | 2.484 | 2.517 | 2.528 | 2.062 | 2.719 | |
| POM | 2.346 | 2.459 | 2.743 | 3.216 | 3.072 | 3.172 | 2.493 | |
| 2020 | PRM | 2.283 | 2.482 | 2.752 | 3.232 | 3.024 | 3.249 | 1.968 |
| M0N | 2.357 | 2.494 | 2.803 | 2.578 | 2.654 | 2.149 | 2.447 | |
| POM | 2.298 | 2.488 | 2.698 | 2.499 | 2.299 | 2.692 | 1.909 | |
| AVERAGE | PRM | 2.501 | 2.533 | 2.5465 | 2.982 | 2.889 | 3.171 | 2.417 |
| M0N | 2.443 | 2.534 | 2.618 | 2.838 | 2.781 | 2.608 | 2.643 | |
| POM | 2.445 | 2.301 | 2.462 | 2.893 | 2.828 | 2.944 | 2.305 | |
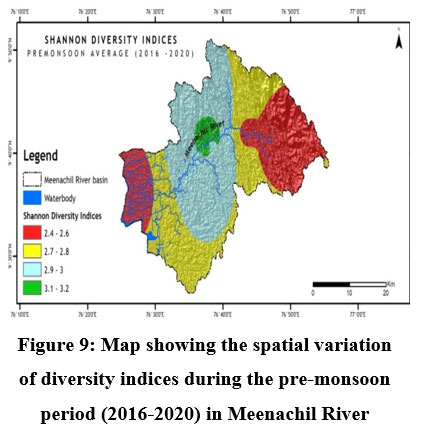 | Figure 9: Map showing the spatial variation of diversity indices during the pre-monsoon period (2016-2020) in Meenachil River
|
 | Figure 10: Map showing the spatial variation of diversity indices during the monsoon period (2016-2020) in Meenachil River
|
 | Figure 11: Map showing the spatial variation of diversity indices during the post-monsoon period (2016-2020) in Meenachil River
|
 | Figure 12: Neighborhood joining based on the Shannon- Weiner Diversity Indices of different sites during the pre-monsoon season (2016-2020).
|
 | Figure 13: Neighborhood joining based on the Shannon- Weiner Diversity Indices of different sites during the monsoon season (2016-2020).
|
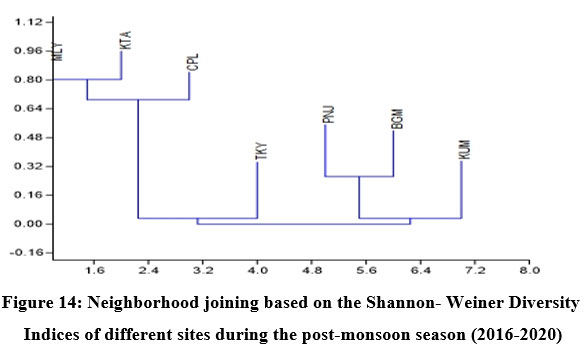 | Figure 14: Neighborhood joining based on the Shannon- Weiner Diversity Indices of different sites during the post-monsoon season (2016-2020).
|
The Shannon diversity indices of seven different sampling sites (Table 3) for the study period indicated a strong relationship with overall species richness, having considerable variations ranging from a minimum of 1.909 at Kumarakom during the post-monsoon of 2019 to a maximum of 3.249 at Kattachira during the monsoon of 2019. Kattachira zone showed maximum diversity, consistently maintained over the three seasons from 2016-2020, peaking during the post-monsoon period (Fig. 9-11).
In Neighborhood joining based on Shannon diversity indices, the midstream sites formed a cluster during all three seasons, with the Kattachira site out-grouping the rest, having the highest diversity index of all other locations throughout the seasons. The upstream site Teekoy clustered with the downstream site Kumarakom showing dwindling diversity at the headwaters and estuarine zone. Poonjar and Bharananganam clustered together due to their similar diversities (Fig 12-14).
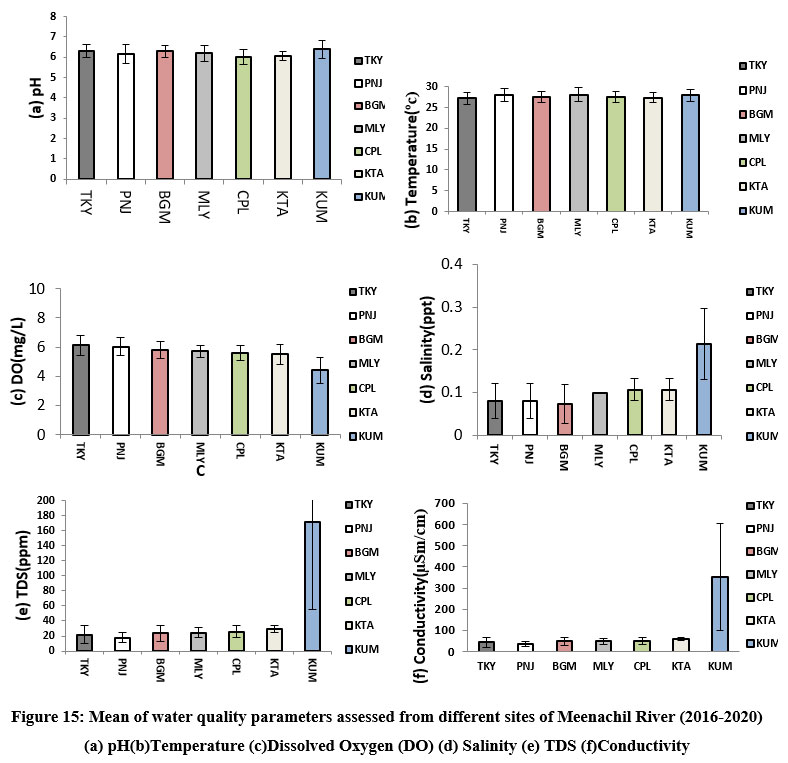 | Figure 15: Mean of water quality parameters assessed from different sites of Meenachil River (2016-2020) (a) pH(b)Temperature (c)Dissolved Oxygen (DO) (d) Salinity (e) TDS (f)Conductivity.
|
Among the water quality parameters, total dissolved solids, conductivity, salinity, and dissolved oxygen varied considerably from upstream to downstream stream stretches of the river. Their means showed maximum fluctuations in the downstream Kumarakom station. A more or less uniform temperature and pH were maintained at different sites from 2016-2020 (Fig. 15 a-f).
Discussion
Conservation of riverine fish diversity at the regional level needs spatially mapped information on the current trends of fish diversity and site-specific conservation targets at a relatively accurate scale.50 GIS-based research comprised a breakthrough in revealing the trends of riverine fish diversity and prioritizing global conservation.51 In Meenachil River, the lowest fish species heterogeneity was recorded at the Teekoy headwaters, increasing towards Poonjar and Bharananganam down the longitudinal gradient. Diversity increased towards the midstream stations, Mutholy and Cherpunkal, with maximum diversity recorded at the Kattachira site (Table 3 & Fig. 9-11). From the midstream, diversity and abundance were declining towards the estuarine downstream zone of the river at Kumarakom station, where the river confluences to Vembanadu Lake. Contrary to forecasts of higher species richness at the downstream reaches of tropical rivers, the Meenachil River's unexpected species richness and abundance were found in the midstream stretch rather than the downstream estuarine zone.3 The decline in fish diversity and abundance in rivers due to habitat destruction and deterioration in water quality was reported.52
The present study results were consistent with Huet’s longitudinal fish zonation concept53, emphasizing different river zones along the longitudinal gradient with specific community structures. The headwater system of rivers with low diversity was usually occupied by small-sized nektonic fishes restricted to the region mainly dependent on allochthonous resources, N. triangularis, S.scaturigina, G. mullya, P. fasciata, B. bakeri, and M. guntheri, while the downstream is home to bigger species that are sustained by autochthonous resources.1,54
The midstream geographical zone of Meenachil River was found to be the most diverse region, which maintained consistently high diversity throughout the study period from 2016 to 2020 despite seasonal variations (Table. 3 & Fig.9-11). Species richness generally increased with the area sampled 55. Leaving the headwater tributaries Teekoy and Poonjar, the river gradually widens from Bharananganam and forms the open channel of the midstream zone. The sites Mutholy, Cherpunkal, and Kattachira, falling within the wide-open channel of the river, showed maximum diversity (Table 3), and shared almost similar taxa representations (Fig. 12-14). The order Cypriniformes was 17 in number representing the highest number of taxa (Table. 2). Nearly threatened species (NT) Anguilla bengalensis, Anguilla bicolor, Ompok bimaculatus and Wallago attu; vulnerable species (VU) Horabagrus brachysoma, Channa diplogramma and Carinotetraodon travancoricus; data deficient species (DD) Devario aequipinnatus, Puntius mahecola, Anabas testudineus and Ehirava fluviatilis; and the not evaluated species (NE) Pristolepis rubripennis and Piaractus brachypomus were found in the midstream zone (Fig. 8a-d). Species distribution pattern showed more addition than a replacement from the upstream to downstream,56,57 with a positive relationship between fish size and river width.1,54,58 The highest heterogeneity, maximum abundance, and a maximum number of large-sized fish species were characteristic of the midstream zone of the Meenachil River. The larger species were the W. attu, C. diplogramma, C. marulius, Mastacembelus armatus, A.bengalensis, and Gibelion catla. As the stream size increases, more resources and different niches become available, allowing the co-existence of species from the same trophic level, which resulted in increasing species richness.59-61 The midstream stretch receives several tributaries linked to the major flood plains of the river basin.62 reported the proximity of rivers to flood plains as another significant factor contributing to the higher species richness and diversity of the river’s middle reaches than the upper stretches.
“The fish diversity hotspot” Kattachira station of Meenachil River consistently maintained high diversity throughout the study period. The region is ecologically unique, with a ‘Reserve Riparian Forest’ belt and lateral connectivity to the floodplains of the river. The higher fish diversity and unusual abundance of species were in line with the findings of 63, describing the riparian zone as one of the most influencing “in-stream diversifier” elements that provide leaves, branches, and wood debris yielding to complex microhabitat patterns, including the riffles, pools, and runs which sustained high species richness.
From the midstream, diversity and abundance were declining towards the downstream estuarine zone of the river, the Kumarakom station, where the river confluences to Vembanadu Lake (Table.3 & Fig.9-11). The wide-open channel of the river splits into distributaries of smaller size in the Kumarakom station before its confluences (Fig. 1). In addition to the true freshwater fishes, the downstream fauna includes secondary species, Brachirus orientalis, Megalops cyprinoides, Scatophagus argus, Ehirava fluviatilis, Gerres setifer, and Sillago sihama which are the anadromous migrants to the estuarine zone of the river from the Arabian Sea. The affected downstream stretch homes five nearly threatened species Anguilla bengalensis, Anguilla bicolor, Brachirus orientalis, Ompok bimaculatus and Wallago attu; two vulnerable species, Horabagrus brachysoma and Channa diplogramma; four data deficient species Anabas testudineus, Megalops cyprinoides, Ehirava fluviatilis, and Puntius mahecola; and five not evaluated species Hyporhamphus quoyi, Gerres setifer, Silago sihama, Puntius bimaculatus and Piaractus brachypomus (Fig. 8 a-d).
A major ecological problem affecting the downstream of the Meenachil River is the temporary retention of water in the lower stretches due to the closure of the barrage, Thanneermukkam Bund. The barrage was constructed in 1974 across Vembanad Lake and the Arabian Sea to prevent saltwater intrusion into the low-lying paddy fields.6 Closing the bund for six months every year prevents the easy discharge of waters from the rivers before emptying into the Arabian Sea. Organic pollution of the Lake due to the closure of the bund and the drastic decline of the fish populations in Vembanad backwaters and associated water bodies was documented by.64,5 Retention of water in the interconnected distributaries has resulted in the water quality deterioration in the river’s lower stretches. This was indicated by the extremely high TDS, conductivity, varying salinity, and very low levels of dissolved oxygen recorded from the Kumarakom station during the present study (Fig. 15 e, f, d & c). In addition, in the past two decades, there has been significant growth in backwater tourism, specifically in the estuarine zone of the river and Vembanadu Lake reported.65 Approximately 187 tons/day of solid waste ultimately reach the Lake from the houseboats, resorts, and hotels without adequate treatment. The downstream fish fauna gets regularly exposed to fluctuating environmental attributes, particularly salinity, conductivity, and TDS, along with the accumulation of high levels of pollutants from various sources. Pollutants from various sources have exacerbated the region’s water quality deterioration.66 The riparian belt has also been cleared away for tourism development.67
Analysis and Geographic Information System-based mapping by 25 using the spatial interpolation methods of the many assessments of the Upper Ganges basin's freshwater fish biodiversity showed disparities in spatial overlays which is in agreement with the present study results of varying diversity trends in the different zones of Meenachil River. The upper northern section of the Ganga1 and the mid and lower southern parts of the Alaknanda/Pindar subbasins were found to have increased species abundance and diversity, according to the composite evaluation of species abundance and index of fish diversity. Contradictory to the above findings, in the present study, the mid-stream zone of the Meenachil River maintained consistently high diversity throughout the study period when compared to the upstream and midstream geographical zones of the river. The abundance of threatened fishes was also found to be fairly distributed among the tributaries of the main waterways of all three subbasins of the Upper Ganges whereas the highest distribution of the threatened species was confined to the lower stretches of the Meenachil River. GIS-based fish distribution analysis of Meenachil River reveals the significance of extending the local conservation efforts to the river’s most pollution-affected zones to protect the threatened endemic fish species of the river.
Conclusion
The midstream zone of the Meenachil River supported diversified fish species and a high degree of endemic, nearly threatened, and vulnerable species. The declining native fish diversity from downstream stretches of the Meenachil River has reflected the extent of habitat alterations and pollution due to anthropogenic interventions. Higher diversity recorded at the midstream stretches of the Meenachil River reflected the outcome of local conservation efforts initiated by “Meenachil Nadee Samrakshana Samithi,” which was acknowledged by the Government of India as the “Bhaghirath Prayas Samman” on the ‘River Day’ of 2017.68 Similar efforts should also be extended to the tourism-affected downstream stretches to restore its rich fish faunal diversity. The integration of geographic features with the help of GIS provided a better perspective on the fish diversity data of Meenachil River contributing effectively to regional and global biodiversity conservation efforts. The present study recommends that local conservation efforts should continue along with the bio-banking to check further species loss, adoption of sustainable management policies for riverine habitat and biodiversity conservation, ecosystem functioning and resilience, and the livelihood of humans, to provide a better long-term basis for the conservation of freshwaters and its unique fish resources considering the present global biodiversity crisis.
Acknowledgement
The authors are grateful to the Principal, Dr Icy K John of Mar Thoma College Thiruvalla, for providing the necessary work facilities. We are greatly indebted to the fishing craft and gear operators and “Meenachil River Restoration Samithi” members for their support during the fieldwork and data collection during the study period.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding sources
No funding was received for the work.
Reference
- Schlosser I. Stream fish ecology. A landscape perspective. Bio Science. 1991;41(10):704-712. https://doi.org/10.2307/1311765
CrossRef - Habit E., Belk M., Tuckfield C., Parra O. Response of the fish community to human?induced changes in the Biobío River in Chile. Freshwater Biology. 2006; 51: 1-11. http://dx.doi.org/10.1111/j.1365-2427.2005.01461.x
CrossRef - Oberdorff T., Dias M. S., Jézéquel C., Albert J. S., Arantes C.C., Bigorne R., Carvajal-Valleros F. M., De Wever A., Frederico R. G., Hidalgo M., Hugueny B., Leprieur F., Maldonado M., Maldonado-Ocampo J., Martens K., Ortega H., Sarmiento J., Tedesco P. A., Torrente-Vilara G., Zuanon, J. Unexpected fish diversity gradients in the Amazon basin. Science Advances. 2019;5(9):eaav8681 https://doi.org/10.1126/sciadv.aav8681.
CrossRef - Vincy M. A watershed approach for sustainable ecosystem management of Meenachil river basin with emphasis on remote sensing and GIS, Kottayam; 2014. http://hdl.handle.net/10603/79194
- Padmakumar K. G., Bindu L., Joseph N., Sreekumar B., Sunil G., Unnikrishnan N., Kurien J. S. River Fish Inventory under Participatory Approaches ‘Meenachil Fish Count 2004. Environment and Ecology. 2006;24(3):584-590.
- Padmakumar K. G., Santhosh N. K., Shanu R., Rakesh C. G., Bindusha K. B., Jasmine B. A study on the fish catch from Vembanadlake, south of Thannermukkom barrage during the period from March, 2011 to February 2012, Natl. Sem., Biodiv. Farming Syst.Wetland ecology. 2017;22-23:35-36.
- Salem B. B. Application of GIS to biodiversity monitoring Department of Environmental Sciences, Faculty of Science, Alexandria University, 21511 Moharram Bey, Alexandria, Egypt Journal of Arid Environments. 2003;54:91-114 https://doi.org/10.1006/jare.2001.0887
CrossRef - Arunkumar, A. A., Manimekalan, A., Manikandan, V., & Velmurugan, P. (2015). Fish species richness and habitat quality mapping with geographical information system across Cauvery River in Tamil Nadu, India. Journal of Aridland Agriculture? Vol, 1, 1.
- Marqules C. R., Austin M. P. Nature Conservation: Cost Effective Biological Surveys and Data Analysis. CSIRO, Melbourne; 1991.
CrossRef - Raibley P. T., Hara O., Irons T. M., Blodgett K. D. Largemouth bass distributions under varying hydrologic regimes in the Illinois River. Transactions of the American Fisheries Society. 1997;126(5):850-856. http://dx.doi.org/10.1577/1548-8659(1997)126<0850:NLBSDU>2.3.CO;2
CrossRef - Pathak A. K., Kantharajan G., Saini V. P., Kumar R., Dayal R., Mohindra V., Lal K. K. Fish community and habitat diversity profiling of Luni, an ephemeral saline river from Thar Desert of India for conservation and management. Community Ecology. 2020;21(3):303-316.
CrossRef - Gumma, M. K., Kadiyala, M. D. M., Panjala, P., Ray, S. S., Akuraju, V. R., Dubey, S., ... & Whitbread, A. M. (2022). Assimilation of remote sensing data into crop growth model for yield estimation: A case study from India. Journal of the Indian Society of Remote Sensing, 50(2), 257-270.
CrossRef - Zhao, T., Shi, J., Lv, L., Xu, H., Chen, D., Cui, Q., ... & Zhang, Z. (2020). Soil moisture experiment in the Luan River supporting new satellite mission opportunities. Remote Sensing of Environment, 240, 111680.
CrossRef - Carocci, F., Bianchi, G., Eastwood, P. and G. Meaden.2009. Geographic Information Systems to support the ecosystem approach to fisheries. FAO Fisheries and AquacultureTechnical Paper No. 532. Rome, FAO. 2009.120 p.
- Ap, Dineshbabu & Thomas, Sujitha. (2016). GIS and its applications in Marine Fisheries Conservation and Management in Karnataka, India.
- Saha, P., Thomas, S., Shailaja, S., Dineshbabu, A. P., Rohit, P., & Nataraja, G. D. (2019). Fishery and GIS based spatio-temporal distribution analysis of smooth blaasop, Lagocephalus inermis, in south-Eastern Arabian Sea. Turkish Journal of Fisheries and Aquatic Sciences, 20(4), 267-278.
- Stuart, V., Platt, T., Sathyendranath, S., & Pravin, P. (2011). Remote sensing and fisheries-An introduction.
CrossRef - Day, F. (1865). The Fishes of Malabar. Bernard Quaritch. London
- Jerdon, T.C. (1849). On the freshwater fishes of southern India. Madras Journal of Literature and Science, 15(2), 302–346
- Hora. S.L. (1923). Notes on fishes in the Indian museum. V: On the composite genus Glyptosternon. Records of the Indian Museum, 25, 1-44.
CrossRef - Menon, A. G. K. (1997). Rare and endangered fishes of Malabar, India. Zoos Print, 12 (11), 6-19.
- Devi, K.R., T.J., Indra., & Knight, J.D. (2010). Puntius rohani (Teleostei: Cyprinidae), A New Species of Barb In The Puntius filamentosus group from the Southern Western Ghats Of India. J. Threatened Taxa, (2), 1121-1129.
CrossRef - Easa, P. S., & Basha, S. C. (1995). A Survey on the Habitat and Distribution of stream Fishes in the Kerala Part of Nilgiri Biosphere Reserve. K.F.R.I.
- Bijukumar, A., & Raghavan, R. (2015). A checklist of fishes of Kerala, India. Journal of Threatened Taxa, 7(13), 8036-8080. https://doi.org/10.11609/jott.2004.7.13.8036-8080
CrossRef - Pathak, Ajey. (2014). Spatial gradients in freshwater fish diversity, abundance and current pattern in the Himalayan region of Upper Ganges Basin, India. Biodiversitas, Journal of Biological Diversity. 15. 186-194. 10.13057/biodiv/d150210.
CrossRef - Chakraborty, H., Kayal, T., Lianthuamluaia, L., Sarkar, U. K., Das, A. K., Chakraborty, S., ... & Das, B. K. (2022). Use of geographical information systems (GIS) in assessing ecological profile, fish community structure and production of a large reservoir of Himachal Pradesh. Environmental Monitoring and Assessment, 194(9), 643.
CrossRef - Raghavan R., Dahanukar N., Kumar K. K., Ali, A., Solomon, S., Ramprasanth, M. R., Baby, F., Perera, B., Tharian, J., Philip, S. Western Ghats fish fauna in Peril: are pseudo conservationist attitude to be blamed? Current Science. 2012;102:835-837.
- Bloch M. E. Naturgeschichte Der Ausländischen Fische.Natural History of Foreign Fishes. Berlin; 1795.
- Vincy, M. & Rajan, Brilliant & A.P., Pradeepkumar. (2012). Geographic information system–based morphometric characterization of sub-watersheds of Meenachil river basin, Kottayam district, Kerala, India. Geocarto International. 27. 1-24. 10.1080/10106049.2012.657694
CrossRef - Hamilton F. An Account of the Fishes of River Ganges and its Branches. George Ramsay And Co, London; 1822.
CrossRef - Jerdon T. C. On the freshwater fishes of southern India. Madras. Journal of Literature and Science. 1849;15(2):302-346.
- Gunther A. Catalogue of Fishes in the British Museum. Nature. 1864; 3: 342-344. https://doi.org/10.1038/003342a0
CrossRef - Day F. The Fishes of Malabar. Bernard Quaritch. London; 1865.
- Silas E. G. On a collection of fish from travancore. Journal of Bombay Natural History Society. 1950;48:792-795.
- Silas E.G. 1951. On a collection of fish from the Anamalai and Nelliampathi Hill ranges (Western Ghats) with notes on its zoogeographical significances. Journal of Bombay Natural History Society. 49;670-681.
- Devi K. R., Menon A. G. Horalabiosa palaniensis, A New Cyprinid Fish from Palani hills, Western Ghats, South India. Journal of Bombay Natural History & Society. 1994;91:110-111.
- Shaji C. P., Easa P. S., Gopalakrishnan A. Freshwater fish diversity of Western Ghats. In Ponniah, A.G. and Gopalakrishnan, A. (Eds.). Endemic Fish Diversity of Western Ghats (pp. 33-35).NBFGR – NATP Publication – 1. National Bureau of Fish Genetic Resources, Lucknow, U.P., India; 2000.
- Dahanukar N., Raghavan R., Ali A., Abraham R., Shaji C. P. The status and distribution of freshwater fishes of the Western Ghats. The status and distribution of freshwater biodiversity in the Western Ghats. India. 2011;3:21-48.
- Dahanukar N., Kumar P., Katwate U., Raghavan R. Badisbritzi, a new percomorph fish (Teleostei: Badidae) from the Western Ghats of India. Zootaxa. 2015;3941(3):429-436. http://dx.doi.org/10.11646/zootaxa.3941.3.9
CrossRef - Kumar B, A., Raghavan R. A checklist of fishes of Kerala, India. Journal of Threatened Taxa. 2015;7(13);8036-8080. https://doi.org/10.11609/jott.2004.7.13.8036-8080
CrossRef - Kerala State Land Use Board [KSLUB]. National resource data bank, Kottayam; 2013:99-177.
- Survey of India SO1 Toposheets (Scale-1:50,000) Number- 58C-05,58C-06,58C-09,58C-10,58C-13 and 58C-14
- Jayaram K. C. The Freshwater Fishes of the Indian Region.Narendra Publishing House, Delhi; 2010.
- Talwar P. K., Jhingran A. G. Inland Fishes of India and Adjacent Countries. Oxford and IBH Co., Pvt. Ltd., New Delhi; 1991.
- IUCN (International Union for Conservation of Nature). The IUCN Red List of Threatened Species Version. 2022; 1. http://www.iucnredlist.org
- Hammer O., Harper D. A., Ryan P. D. Past: Paleontological Statistic software package for education and data analysis. Paleontologia Eletronica. 2001;4(1):1-9. http://palaeoelectronica.org/2001_1/past/issue1_01.htm Accessed: 08/09/2022
- https://earthexplorer.usgs.gov/
- Appukuttan, A, Reghunath R. Identification of groundwater potential zones in a tropical lateritic terrain by analytical hierarchy process?based multi?criteria analysis and geospatial technology. Arabian Journal of Geoscience 2022;15:1323. https://doi.org/10.1007/s12517-022-10587-4
CrossRef - Bronowicka-Mielniczuk U., Mielniczuk J. A comparison of some interpolation techniques for determining spatial distribution of nitrogen compounds in groundwater. International Journal of Environmental Research. 2019;13:679-687. https://doi.org/10.1007/s41742-019-00208-6
CrossRef - Fitz-Hugh T. W. GIS tools for freshwater biodiversity conservation planning. Trans in GIS. 2005; 9(2): 247-263.
CrossRef - Januchowski-Hartley S. R, Pearson R. G, Puschendrf R, Rayner T. Freshwaters and Fish diversity: Distribution, protection and disturbance in tropical Australia. PLOS One. 2011;6(10):e25846. https://doi.org/10.1371/journal.pone.0025846
CrossRef - Patten D. T. Riparian ecosytems of semi-arid North America: Diversity and human impacts. Wetlands. 1998;18(4):498-512.
CrossRef - Huet M. Profiles and biology of western European streams as related to fish management. Transactions of the American Fisheries Society. 1959;88(3):155-163.
CrossRef - Welcomme R. L., Winemiller K. O., Cowx I. G. Fish environmental guilds as a tool for assessment of ecological condition of rivers. River Research & Applications. 2006; 22:377–396.
CrossRef - Mc Arthur R.H., Wilson E.O. The theory of island biogeography. Princeton University Press, Princeton; 1967.
CrossRef - Illies J., Botosaneanu L. Problèmes et méthodes de la classification et de la zonation écologique des eauxcourantes, consideréessurtout du point de vue faunistique. Internationale Vereinigung für Theoretische und Angewandte Limnologie: Mitteilungen. 1963;12:1-57.
CrossRef - Horwitz R. J. Temporal variability patterns and the distributional patterns of stream fishes. Ecological Monographs. 1978;48:307-321. https://doi.org/10.2307/2937233
CrossRef - Santos M., Canas A., Lino P. Length-girth relationships for 30 marine fish species. Fisheries research. 2006;78:368-373. https://doi.org/10.1016/j.fishres.2006.01.008.
CrossRef - Tedesco P., Hugueny B., Oberdorff T., Durr H. H., Merigoux S., de Merona B. River hydrological seasonality influences life history strategies of tropical riverine fishes. Oecologia. 2008;156:691-702.
CrossRef - Villéger S., Ramos M. J., Flores H.D., Mouillot D. Contrasted changes in taxonomic and functional diversity of tropical fish communities after habitat degradation. Ecological Applications, in press; 2010. https://doi.org/10.1890/09-1310.1
CrossRef - Pease A. A., Gonzalez-Diaz A. A., Rodiles-Hernandez R., Winemiller K. O. Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshwater Biology. 2012;57:1060-1075. https://doi.org/10.1111/j.1365-2427.2012.02768.x
CrossRef - Stegmann L. F., Leitão R. P., Zuanon J., Magnusson W. E. Distance to large rivers affects fish diversity patterns in highly dynamic streams of central Amazonia. PLOS ONE. 2019;14(10):E0223880. Https://Doi.Org/10.1371/Journal.Pone.0223880
CrossRef - Casatti L., Ferreira C., Carvalho F. Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: An assessment of two tropical pasture river basins. Hydrobiologia. 2009;632:273-283.https://doi.org/10.1007/s10750-009-9849-y
CrossRef - Kurup B. M., Samuel C. T. Systematics and distribution of the fishes of the family Leignathidae (Pisces) of the Vembanad Lake, Kerala, S. India.Rec. Zool. Surv. India. 1983;80:387-411.
CrossRef - Safoora Beevi K.H., Devadas V. Impact of tourism on Vembanad lake system in Alappuzha district. International journal of research. 2014;1(5):542-551.
- Joseph C, Reghunathan R. Impacts of Backwater Tourism in Kerala. International Journal of Advanced Engineering and Research Development. 2017;4(7):227-232.
- Remani K.N., Jayakumar P., Jalaja T.K. Environmental problems and management aspects of Vembanad kol wetland in south west coast of India. Nature environment and pollution technology. 2010;9(2):257-254.
- South Asia Network on Dams, Rivers and People (SANDRP). BPS Awards to Meenachil Samiti in Kerala; 2017.







