Experimental Studies on the Removal of Chromium from Tannery Wastewater using Chemical Precipitation and Adsorption Techniques
1
Department of Civil Engineering,
National Institute of Technology,
Warangal,
Telangana
India
2
Corresponding author Email: mcs@nitw.ac.in
DOI: http://dx.doi.org/10.12944/CWE.18.1.15
Copy the following to cite this article:
Ankareddy S, Matli C. S. Experimental Studies on the Removal of Chromium from Tannery Wastewater using Chemical Precipitation and Adsorption Techniques. Curr World Environ 2023;18(1). DOI:http://dx.doi.org/10.12944/CWE.18.1.15
Copy the following to cite this URL:
Ankareddy S, Matli C. S. Experimental Studies on the Removal of Chromium from Tannery Wastewater using Chemical Precipitation and Adsorption Techniques. Curr World Environ 2023;18(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-04-08 |
|---|---|
| Accepted: | 2023-01-06 |
| Reviewed by: | 
 Mohammad Yusuf
Mohammad Yusuf
|
| Second Review by: |

 Ashish Khanal
Ashish Khanal
|
| Final Approval by: | Dr. Gangadhar Andaluri |
Introduction
Chromium (Cr) is predominantly present in hexavalent (Cr+6) and trivalent forms (Cr+3)1,2. Chromium finds its importance in several industries like steel manufacturing, alloy production, leather tanneries, industrial catalysts and pigments manufacturing, plating, and glass treatment because of its resistance to corrosion and its hardness 3–5. Out of the two predominant states of chromium, the +6 state is hazardous and the +3 state is essential for human beings to some extent. It has been seen that exposure to hexavalent chromium (Cr(VI)) causes dermatitis, organ damage, respiratory impairment, and gastrointestinal ulcers. It has also been reported to be a carcinogen and a teratogen 6–8. Negative impacts of hexavalent chromium on plants include, a decrease in germination, impairment of photosynthesis, inhibition of root growth, and reduction in the number of leaves and leaf area 9. Hexavalent chromium is a priority pollutant of concern at several hazardous and waste dump sites and is reported to be harmful to flora, fauna, and human beings 10.
Whereas, trivalent chromium is considered a vital nutrient for the metabolism of insulin, sugar, and lipids in human beings in developed countries like the United States, Canada, and Japan. However, in 2014, the European Food Safety Authority (ESFA) stated that this heavy metal cannot be considered an essential nutrient because of inadequate evidence 11. In 2013, the Dietary Reference Intakes (DRI) Committees from the United States and Canada accepted nutrient nominations for review, and chromium was one of the 16 nutrients that was nominated but was not one of the four nutrients chosen for further consideration 12.
It has been observed that the oxidation of chromium oxide (Cr2O3) by oxygen and oxidation of chromium hydroxide (Cr(OH)3) by manganese dioxide (MnO2) are thermodynamically possible reactions in the natural environment indicating the chances of non-toxic trivalent chromium (Cr(III)) getting converted to toxic Cr(VI) as incidents involving the dumping of fresh tannery sludge containing trivalent chromium as the predominant species have been revealed to have shown the presence of hexavalent chromium in the tannery sludge and the soil at tannery sites 13. Improper disposal of chromium-bearing wastes by a chromium salts manufacturing unit located in an industrial area in India had caused severe soil and groundwater contamination in that area and in the surrounding villages of that area 14.
Considering the detrimental impacts of chromium on human health and the environment caused by industrial activities of manufacturing and production, it is necessary to meet the effluent discharge standards of 2 ppm set by the regulatory authorities 15. To meet the discharge criteria, the treatment and handling practices at the industry level should be highly efficient.
There are several reported and practiced methods for the treatment of wastes containing chromium. Some of the widely practiced methods are chemical precipitation, membrane filtration, adsorption, and reverse osmosis 16. There has also been a rise in the usage of electrocoagulation, electrodialysis, bioremediation techniques, and adsorption by low-cost adsorbents such as rice husk, rice bran, activated carbon, and other biosorbents from biowastes, etc.17,18. However, technology transfer from lab-scale or pilot-scale studies to an industrial scale is complex involving several variables such as initial concentration of metal ions, capital investment economic feasibility, demand for technical expertise, regulatory requirements and time constraints, etc. In addition, certain conventional technologies for heavy metals removal from wastewater such as coagulation and flocculation, although widely used for their low costs can pose difficulties in thorough removal of the heavy metals; the common adsorbents like activated carbon and zeolites tend to be expensive for treating large volumes of wastewater; and membrane technology, despite its practicality can be very challenging concerning membrane fouling issues. Likewise, higher sludge volume getting generated during the treatment process is a drawback to the chemical precipitation technique.19,20 However, the experimental studies presented here conducted were majorly focused on improving the current industrial practice towards its optimization, and this paper puts forth the inferences from comparative studies conducted using chemical precipitation and biochar adsorption to ascertain if the current chromium removal practice in the study area is an optimal method or not.
There have been several reported studies on the removal of chromium from tannery wastewater using chemical precipitation techniques, however, either the studies were focused on using synthetic samples, administering heavy dosages of precipitants like lime and sodium hydroxide individually, or using a combination of two different precipitants but on tannery wastewater with lower concentrations of chromium, i.e., diluted wastewater. Therefore, the current paper focused on using two different precipitants in a combination in varying ratios on the chrome line/ tannery wastewater instead of using synthetic samples or wastewater with lowered concentrations of chromium. In addition, the study also concentrated on understanding the efficacy of inactivated biochar on raw tannery wastewater instead of experimenting with diluted wastewater.
Hence, an attempt was made in this paper to compare the common industrial practice of chromium removal using chemical precipitation with magnesium oxide (MgO) in small to medium-scale tanneries with a combination of sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)2) in varying ratios, and adsorption by biochar. Comparison of both chemical precipitation and biochar adsorption methods based on optimal dosages at different pH ranges, efficiencies of removal, amount of sludge generated, the concentration of chromium in the supernatant, time and costs involved, reliability of each method based on long-term environmental benefits are presented in this paper.
Materials and Methods
One of the three tanneries located in a cluster in the Warangal district of Telangana state was chosen for experimental studies. The cluster is equipped with Common Effluent Treatment Plant (CETP) and a solar evaporation pond. The treated effluent from CETP is used for irrigational purposes and the sludge is sent to a nearby Treatment, Storage, and Disposal Facility. The supernatant after the chemical precipitation using MgO at the tannery was repurposed for other manufacturing steps and the sludge was sent to chrome drying beds and later to a nearby tannery for the recovery of chromium basic sulphate for re-tanning purposes. As per the operations adopted at tanneries in general 21–23 and the study area, approximately 44800 litres/ day of wastewater is generated at the chrome tanning stage in the tannery under consideration.
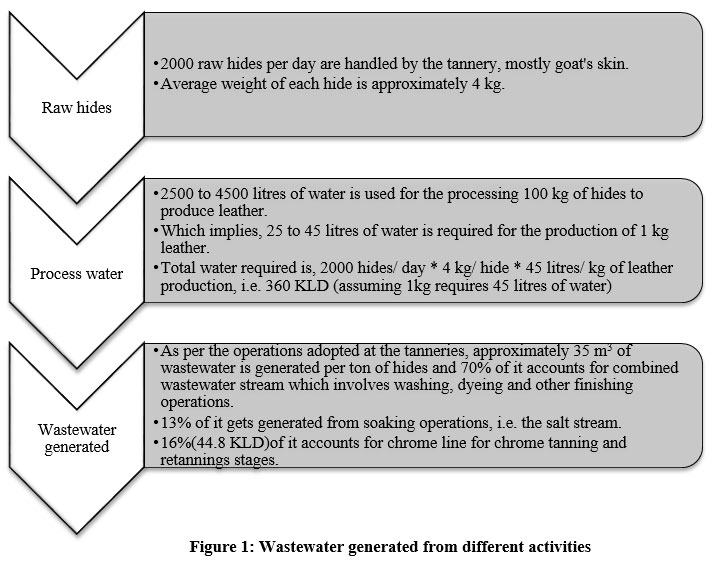 | Figure 1: Wastewater generated from different activities.
|
Materials
Analytical Reagent (AR) grade chemicals, specifically, magnesium oxide (MgO), calcium hydroxide (Ca(OH)2), sodium hydroxide (NaOH), chromium basic sulphate (Cr4(SO4)5(OH)2), sodium bicarbonate (NaHCO3) and biochar for the experimental work were procured. As per the manufacturer’s description provided, biochar was made from the pyrolysis of woody biomass of Prosopisjuliflora and it can be used as a soil-enhancer for growing food organically. A representative sample from the chrome stream of the study area was collected in a 5-litre polyethylene container with appropriate PPE (Personal Protective Equipment) for subsequent laboratory studies.
Methods
Chromium in the sample was analyzed by MP-AES (Microwave Plasma Atomic Emission Spectrometer) instrument and Standard Methods for Examination of Water Wastewater by the American Public Health Association (APHA) was used for the sample preparation and analysis. Hexavalent chromium was determined by using a UV-Visible spectrophotometer (Colorimetric method) as per the APHA Standard Methods for Examination of Water and Wastewater. The experimental work carried out aimed at creating field conditions and industry protocols were adopted for the chemical precipitation process. A fresh sample was collected for analysis after each iteration of precipitation with different agents.
In the industry under study, 10 kg of MgO is added to every 5000 L of wastewater for chemical precipitation, which is equivalent to a dose of 2000 mg/L of MgO. As per Schedule I: Standards for Emission or Discharge of Environmental Pollutants from Tanneries, published by the Central Pollution Control Board (CPCB) of India, the maximum permissible limit for total chromium is 2 mg/L and for hexavalent chromium, it is 0.1 mg/L 15. Hence, the industry should meet these discharge criteria prior to the disposal of the effluent generated from the tannery.
For the current project, batch studies were carried out with MgO and NaOH + Ca(OH)2 at dosages ranging from 1500 mg/L to 2500 mg/L to find the optimum dose of chemical precipitant for precipitation. Chemical precipitants selectively, NaOH and Ca(OH)2 were added in varying ratios of 1:1, 1:2, 2:1, 1:3, 3:1 so that the total dosage of the combination was equal to that of 1500 mg/L, 1800 mg/L, 2000 mg/L, 2200mg/L, and 2500 mg/L by weight respectively. Synthetic samples having similar concentrations of chromium as that of tannery wastewater samples were used for experimental studies to understand the differences in the behaviour of the two samples, i.e., tannery wastewater and synthetic samples, and the rate of precipitation. As per literature, the chemical precipitation of Chromium is highly efficient in neutral to alkaline conditions24,25. Therefore, experiments were conducted at a pH of 7 and 8 respectively by raising the pH using sodium bicarbonate (NaHCO3).
For adsorption studies, biochar was used in similar dosages as that of chemical precipitants and the dosage was increased to check for its efficacy in removing chromium when added at higher dosages. Experiments were conducted at a pH of 6, which is the pH of the raw chrome line sampled from the study area and an attempt was made to inspect the removal capacity of biochar which is not physically or chemically modified. Hence, no chemical agents were added to increase the pH unlike in the case of chemical precipitation. As per literature 26, the pH of tannery wastewater varies from 6.6 to 10.72.
The details of experimental conditions adopted in the study are presented in Table 1.
Table 1: Summary of Test Conditions for Chemical Precipitation and Adsorption.
| |||||||||
Precipitating agent: MgO | |||||||||
| Initial chromium conc. (mg/L) | 2405 | 2405 | 2405 | 2405 | 2405 | ||||
| Dosage (mg/L) | 1500 | 1800 | 2000 | 2200 | 2500 | ||||
Precipitating agent: NaOH + Ca(OH)2 | |||||||||
| Initial chromium conc. (mg/L) | 2610.28 | 2610.28 | 2610.28 | 2610.28 | 2610.28 | ||||
| Dosage by weight (mg/L) | 1500 | 1800 | 2000 | 2200 | 2500 | ||||
| Ratio [NaOH : Ca(OH)2 ] | 1:1 | 1:2 | 2:1 | 1:3 | 3:1 | ||||
| Dosage of NaOH (mg/L) | 750 | 600 | 1333.33 | 550 | 1875 | ||||
| Dosage of Ca(OH)2 (mg/L) | 750 | 1200 | 666.67 | 1650 | 625 | ||||
Adsorption using Biochar | |||||||||
| Initial chromium conc. (mg/L) | 2405 | 2405 | 2405 | 2405 | 2405 | 2405 | |||
| Dosage (mg/L) | 1500 | 2000 | 2500 | 5000 | 20000 | 48000 | |||
A fresh sample was taken for each analysis round, because of which the initial chromium concentrations varied for the tannery samples as shown in Table 1. The initial chromium concentration of samples for MgO precipitation and Biochar adsorption studies was 2405 mg/L and for a combination of NaOH and Ca(OH)2 in varying ratios, it was 2610.28 mg/L. Synthetic samples (identical to the tannery sample’s chromium concentrations) were prepared by dissolving 8.35 g and 9.06 g of chromium basic sulphate (Cr4(SO4)5(OH)2) in 1 litre of deionized water to achieve 2405 mg/L and 2610.28 mg/L concentrations respectively.
The costs of various commercial-grade dosing agents per 25 kg are as follows: MgO is 875 INR (Indian Rupee), NaOH is 1000 INR, Ca(OH)2 is 162.5 INR, NaHCO3 is 575 INR, and Biochar’s price varies from 134.25 INR to 5500 INR.
Results and discussions
An analysis for finding out the hexavalent chromium concentration in both the tannery samples using UV-Visible Spectrophotometry showed non-detectable values indicating the absence of the same and the total chromium present in the samples is of the trivalent form. The absence of hexavalent chromium can be attributed to the addition of chromium basic sulphate by the tannery in the form of Cr2(SO4)3.x(H2O), chromium (III) sulphate. An investigation carried out to understand the increased renal damage caused by exposure to trivalent chromium in workers at tanneries in Bangladesh revealed that greater than 99.99% of the chromium species in tannery wastewater are in trivalent form with undetectably low levels of hexavalent chromium in it 27. Another experimental study conducted to remove chromium from tannery wastewater using agricultural and industrial wastes also showed chromium in trivalent form upon characterization analysis of tannery wastewater 28.
Chemical Precipitation
MgO-Precipitation
MgO when in contact with water forms magnesium hydroxide, Mg(OH)2. This magnesium hydroxide in turn reacts with chromium basic sulphate to produce chromium hydroxide, Cr(OH)3, which is a precipitate that is insoluble in water and settles down in the form of sludge. The experimental results of chromium removal using MgO as a precipitant at pH 7 and pH 8 for both synthetic and tannery wastewater samples are tabulated below in Table 2.
Table 2: Chromium removal using MgO
Initial concentration of sample (mg/L) | Dosage of MgO (mg/L) | Removal efficiency (%) | Sludge Volume (mL/ 200 mL) | ||||||
Synthetic sample | Tannery wastewater sample | Synthetic sample | Tannery wastewater sample | ||||||
pH7 | pH8 | pH7 | pH8 | pH7 | pH8 | pH7 | pH8 | ||
2405 | 1500 | 99.58 | 99.66 | 95.1 | 96.49 | 10.5 | 20 | 21.5 | 64 |
2405 | 1800 | 99.74 | 99.49 | 95.32 | 98.59 | 11.5 | 20.5 | 22 | 72.5 |
2405 | 2000 | 99.76 | 99.62 | 95.67 | 99.2 | 13.5 | 23 | 21.5 | 71.5 |
2405 | 2200 | 99.68 | 99.65 | 95.64 | 98.9 | 12.5 | 25 | 21 | 74.4 |
2405 | 2500 | 99.61 | 99.75 | 96.4 | 98.18 | 14 | 30 | 17.5 | 87 |
Synthetic samples yielded better chromium removal efficiencies and lower volumes of sludge as compared with the tannery wastewater samples, which can be attributed to the presence of interfering substances like solids (TSS and TDS) in the wastewater samples. Because of different unit processes adopted in industry, real wastewater consists of concomitant compounds 29 as opposed to the synthetic sample which contains only the inorganic chromium basic sulphate in deionized water. Maximum removal efficiency of 96.4 % was observed at a dosage of 2500 mg/L of MgO at a pH of 7 and 99.2% removal efficiency was observed at 2000 mg/L of MgO at pH8. However, the sludge volume generated at this maximum efficiency observed at pH8 (71.5 mL / 200 mL) was approximately 4 times greater than that observed at pH 7 (17.5 mL /200 mL). The removal efficiencies obtained were higher than the industry reported range of 86 ~ 90% removal at a pH of 6 for a dosage of 2000 mg/L indicating that the current practice followed by the industry is not an optimal method and increasing the pH to alkaline conditions would allow for better removal efficiencies24,25.
In a similar study, 83.35 % and 93.64 % removal efficiencies, and 88 mL and 90 mL sludge volumes at a pH of 6.9 and 8.6 respectively in tannery wastewater samples having an initial effluent chromium concentration of 5010 mg/L for a dosage of 10% (w/w) MgO solution, which is equivalent to 100 g/L were reported. Increasing the pH from 8.6 to 9.1 and then to 10.3 yielded removal efficiencies greater than 99% with sludge volumes in the range of 80 to 85 mL 30.
NaOH + Ca(OH)2- Precipitation
Experimental studies were carried out with a combination of NaOH and Ca(OH)2 in varying proportions such that the total weight of the agents added up to that of MgO dosages. The following table, Table 3, depicts the results obtained from an attempt to observe the behaviour of the precipitation reaction when these two agents are added in combination.
Table 3: Chromium removal using NaOH and Ca(OH)2
| Initial concentration (mg/L) | Dosage of combination (mg/L) | Ratio | NaOH (mg/L) | Ca(OH)2 | Removal efficiency (%) | Sludge Volume (mL/ 200 mL) | ||||||
| Synthetic sample | Tannery wastewater sample | Synthetic sample | Tannery wastewater sample | |||||||||
| at pH7 | at pH8 | at pH7 | at pH8 | at pH7 | at pH8 | at pH7 | at pH8 | |||||
2610.28 | 1500 | 01:01 | 750 | 750 | 99.69 | 99.72 | 80.72 | 99.08 | 42 | 42 | 51 | 148 |
2610.28 | 1800 | 01:02 | 600 | 1200 | 99.8 | 99.6 | 83.23 | 99.23 | 40 | 39 | 49 | 154 |
2610.28 | 2000 | 02:01 | 1333.33 | 666.67 | 99.8 | 98.71 | 96.64 | 99.77 | 54 | 46 | 171 | 162 |
2610.28 | 2200 | 01:03 | 550 | 1650 | 99.7 | 99.83 | 95.96 | 99.71 | 44 | 43 | 60 | 136 |
2610.28 | 2500 | 03:01 | 1875 | 625 | 99.7 | 99.8 | 95.25 | 99.84 | 62.5 | 50 | 148 | 152 |
In contrast to the higher removal efficiencies achieved with synthetic samples when compared to tannery wastewater samples during MgO precipitation, both the samples behaved analogously to each other at higher dosages at a pH of 8 during the combination reaction. Maximum removal efficiency of 96.64 % was observed at a dosage of 2000 mg/L, i.e., NaOH and Ca(OH)2 when added in a 2:1 ratio at pH 7, and 99.84 % removal efficiency was obtained at a dosage of 2500 mg/L, with NaOH and Ca(OH)2 in 3:1 ratio at a pH of 8. However, the sludge volumes obtained with tannery samples at pH 8 and those at higher dosages at pH 7 were the highest among all the experimental trials indicating the poor settling capacity of NaOH. Overall, a reaction with a dosage of 2200 mg/L with NaOH and Ca(OH)2 in a 1:3 ratio at a pH of 7 could be considered an optimal option considering the lower sludge volume (60 mL per 200 mL) obtained with a removal efficiency of 95.96 % which is higher than the industry reported range of 86~90% with MgO.
A similar chemical precipitation study conducted on tannery wastewater with an initial concentration of trivalent chromium ~2131 mg/L reported 98-99% removal of Cr (III) at a pH of ~4 by administering a heavy dose of lime, 20000 to 30000 mg/L 16. Removal efficiencies of 96.4% and 99.9% at a pH of 6.9 and 8.6 respectively with NaOH (15% w/w) solely and removal efficiencies of 96.1% and 99.9% at pH 6.9 and pH 8.6 with hydrated lime (12% w/w) were observed in experiments conducted by a team in Ethiopia to chemically precipitate and recover chromium from tannery wastewater 30. Another study conducted with a combination of NaOH and Ca(OH)2 at a dosage of 100 mg/L on synthetic samples at pH 7 reported 99.7% removal efficiency and the results were on par with the values obtained for industrial samples containing trivalent chromium concentration in the range of 10 mg/L31,32.
Adsorption with Biochar
The removal of chromium was checked under the same agitation period of 1hr at 100rpm and an idle period of 2hrs condition. Table 4 below summarizes the experiment carried out with biochar for chromium removal from tannery wastewater.
Table 4: Adsorption using Biochar
Initial concentration (Cini) (mg/L) | pH | Dosage of biochar (mg/L) | Residual concentration (Cres) (mg/L) | Removal (Crem) (mg/L) | Removal efficiency or % Removal |
2405 | Slightly greater than 6 (> 6) | 1500 | 2366 | 39 | 1.62 |
2405 | 2000 | 2317 | 88 | 3.66 | |
2405 | 2500 | 2222 | 183 | 7.61 | |
2405 | 5000 | 2212 | 193 | 8.025 | |
2405 | 20000 | 1438 | 967 | 40.2 | |
2405 | 47408 (theoretical) | 14.05 | 2380.95 | 99.0 | |
2405 | 48000 | 644.54 | 1760.46 | 73.2 |
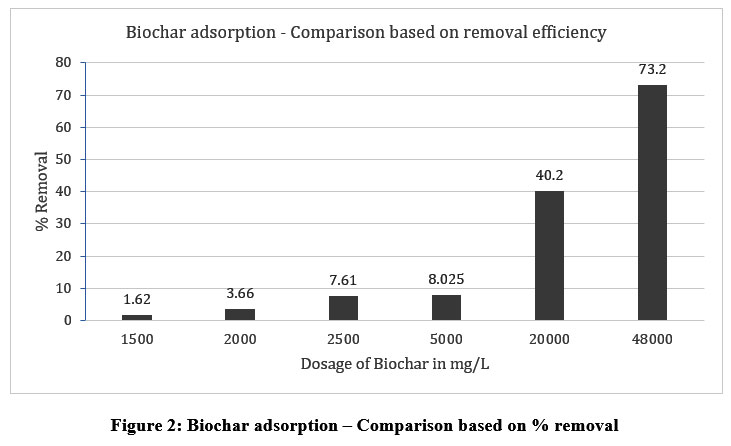 | Figure 2: Biochar adsorption – Comparison based on % removal.
|
Creditable results were not achieved with the adsorption study carried out with biochar at the same dosages as that of chemical precipitation as shown in Figure 2. Increasing the dosage certainly increased the removal efficiency and a theoretical value of 47408 mg/L was extrapolated to give 99% removal efficiency but when the experiment was carried out with 48000mg/L, only 73.2% of removal efficiency was achieved. This could have been due to the initial pH conditions of the tannery wastewater sample which was slightly ?6. Also, the biochar was prepared using hardwood and the temperature for pyrolysis was between 300 °C to 400 °C only as mentioned by the manufacturer. So, by changing the pyrolysis conditions, and by modifying the biochar, noteworthy results can be achieved. The higher the pyrolysis temperatures, the higher will be the porosity, the higher will be the efficiency of removal and the lower will be the sludge volumes 33.
A team in China worked on chromium removal and adsorption using biochar derived from municipal sludge. The municipal sludge was air-dried and pyrolyzed at 900 °C. Results indicated that Cr (III) was more likely to get removed compared to Cr (VI) and the removal efficiencies were higher for lower initial chromium concentrations. That is, for an initial Cr (III) concentration of 50 mg/L, the removal efficiency was as high as 85% and that at 200 mg/L was around 20% 34. In a similar adsorption study with unmodified water hyacinth shoot powder, for an initial chromium concentration of 10.475 mg/L, a removal efficiency of 98.83 % was achieved with untreated tannery effluent further indicating that adsorption with unadulterated biochar/bio sorbents is highly effective and sustainable at lower chromium concentrations 35.
Comparison of different dosing agents
Figure 3 below shows the analysis results achieved based on the experimental work carried out in comparing different chemical precipitating agents and a low-cost adsorbent in chromium removal from tannery wastewater.
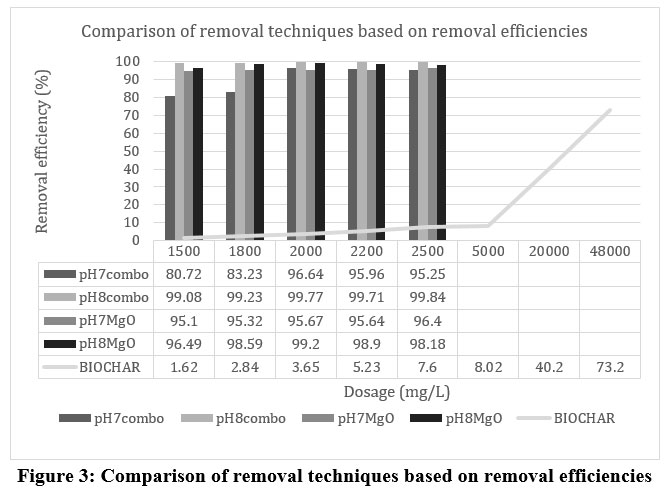 | Figure 3: Comparison of removal techniques based on removal efficiencies.
|
Table 5 below summarizes details on optimum efficiencies (here, optimal efficiency is considered as the one that is not only greater than other values comparatively but also yields a reasonable amount of sludge volume instead of very high sludge volume) achieved with a particular dosing agent/ combination, and the treatment costs involved in achieving these efficiencies.
Table 5: Maximum efficiencies achieved and costs involved
Dosing agent | pH | # Optimum dosage (mg/L) | Initial chromium concentration (mg/L) | % Removal @optimum efficiency | Sludge volume generated @optimum efficiency (mL per 200 mL) | Costs for treating 44800 L/ day of chrome line |
MgO | 7 | 2500 | 2405 | 96.4 | 17.5 | 3920 INR |
MgO | 8 | 2000 | 2405 | 99.2 | 71.5 | 3136 INR |
NaOH+Ca(OH)2 [1:3] | 7 | 2200 | 2610.28 | 95.96 | 60 | 1466.08 INR |
NaOH+Ca(OH)2 [1:3] | 8 | 2200 | 2610.28 | 99.71 | 136 | 1466.08 INR |
Biochar | > 6 | 48000 | 2405 | 73.2 | - | 12200 INR |
*Approximately 0.2-0.5 g/L of NaHCO3 was used in raising the pH of the tannery wastewater samples. Hence, in addition to the above-mentioned costs, approximately 220-550 INR/ day should be considered for raising the pH. | ||||||
Conclusions
The current industrial practice of chemical precipitation with MgO at a pH of 6 is not an optimal method and considering the detrimental effects of chromium on our environment and on human health, it should be carried out at neutral pH with a dosage of 2500 mg/L or at a slightly alkaline pH at a dosage of 2000 mg/L for better removal efficiencies than that reported by the industry under study. The residual chromium concentrations at these dosages are 86.58 mg/L and 19.24 mg/L, which are higher than the effluent discharge limit of 2 mg/L. Hence, after this primary treatment, additional handling of the residual chromium is necessary to achieve final disposal standards or the supernatant can be repurposed within the industries for re-tanning.
The chemical precipitation with a combination of NaOH and Ca(OH)2 at a dosage of 2200 mg/L in a 1:3 ratio gave analogous removal efficiencies to that of the MgO precipitation. However, the sludge volume generated with a combination at pH 7 is 3 times greater than that with MgO and 2 times greater than that generated at pH8 with MgO. Further research can be conducted in enhancing the settling nature of a combination reaction considering the higher removal efficiencies, lower residual chromium, and very low treatment costs compared to that of MgO precipitation.
Recommendation
The chemical precipitation done with the combination of chemicals in ratios can be adopted in the industry provided, the treatment of sludge generated, cost of treatment of sludge, and purity analysis of the recovered tanning agent is done beforehand and checked if the advantages greatly outweigh the disadvantages of MgO precipitation. Studies on using a combination of MgO and lime can be done to understand if lime could complement the treatability of tannery wastewater and thus reduce the treatment costs as lime is an inexpensive chemical.
If not for the complete treatment of tannery wastewater with biochar, at least the chromium present in the residual after treatment should be treated with biochar as it could be effective in treating lower concentrations of heavy metals. If the biochar used is of sludge generated from water treatment plants or other wastes like dried leaves, sugarcane crushed waste, etc., then the problem of sludge handling in these plants or handling of such wastes generated will be easier, beneficial for the biochar industry, and can lead to sustainable industrial practices.
Acknowledgments
None
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Sources
This research received no specific grant from any funding agency to carry out the experimental work.
References
- Shahid M, Shamshad S, Rafiq M, et al. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere. 2017;178:513-533. doi:10.1016/j.chemosphere.2017.03.074
CrossRef - Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environmental Monitoring and Assessment. 2019;191(7). doi:10.1007/s10661-019-7528-7
CrossRef - Viczek SA, Aldrian A, Pomberger R, Sarc R. Origins and carriers of Sb, As, Cd, Cl, Cr, Co, Pb, Hg, and Ni in mixed solid waste – A literature-based evaluation. Waste Management. 2020;103:87-112. doi:10.1016/j.wasman.2019.12.009
CrossRef - Ren S, Li H, Wang Y, Guo C, Feng S, Wang X. Comparative study of the China and U.S. import trade structure based on the global chromium ore trade network. Resources Policy. 2021;73. doi:10.1016/j.resourpol.2021.102198
CrossRef - Seragadam P, Rai A, Ghanta KC, Srinivas B, Lahiri SK, Dutta S. Bioremediation of hexavalent chromium from wastewater using bacteria-a green technology. Biodegradation. 2021;32(4):449-466. doi:10.1007/s10532-021-09947-w
CrossRef - Tseng CH, Lee IH, Chen YC. Evaluation of hexavalent chromium concentration in water and its health risk with a system dynamics model. Science of the Total Environment. 2019;669:103-111. doi:10.1016/j.scitotenv.2019.03.103
CrossRef - Vaiopoulou E, Gikas P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere. 2020;254. doi:10.1016/j.chemosphere.2020.126876
CrossRef - Alvarez CC, Bravo Gómez ME, Hernández Zavala A. Hexavalent chromium: Regulation and health effects. Journal of Trace Elements in Medicine and Biology. 2021;65. doi:10.1016/j.jtemb.2021.126729
CrossRef - Ertani A, Mietto A, Borin M, Nardi S. Chromium in Agricultural Soils and Crops: A Review. Water, Air, and Soil Pollution. 2017;228(5). doi:10.1007/s11270-017-3356-y
CrossRef - Ahamed MIN, Rajeshkumar S, Ragul V, Anand S, Kaviyarasu K. Chromium remediation and toxicity assessment of nano zerovalent iron against contaminated lake water sample (Puliyanthangal Lake, Tamilnadu, India). South African Journal of Chemical Engineering. 2018;25:128-132. doi:10.1016/j.sajce.2018.04.004
CrossRef - Vincent JB. New evidence against chromium as an essential trace element. Journal of Nutrition. 2017;147(12):2212-2219. doi:10.3945/jn.117.255901
CrossRef - U.S. Department of Health and Human services. Nutrient Assessment for DRI Review. https://health.gov/our-work/food-nutrition/dietary-reference-intakes-dris/nutrient-assessment-dri-review.
- Xu T, Jiang X, Tang Y, Zeng Y, Zhang W, Shi B. Oxidation of trivalent chromium induced by unsaturated oils: A pathway for hexavalent chromium formation in soil. Journal of Hazardous Materials. 2021;405. doi:10.1016/j.jhazmat.2020.124699
CrossRef - Manoj S, RamyaPriya R, Elango L. Long-term exposure to chromium contaminated waters and the associated human health risk in a highly contaminated industrialised region. Environmental Science and Pollution Research. 2021;28(4):4276-4288. doi:10.1007/s11356-020-10762-8
CrossRef - Ministry of Environment F and CCI. Standards for discharge of effluents from tannery industry. https://cpcb.nic.in/displaypdf.php?id=SW5kdXN0cnktU3BlY2lmaWMtU3RhbmRhcmRzL0VmZmx 1ZW50LzQ0OS5wZGY=.
- Ahmed E, Abdulla HM, Mohamed AH, El-Bassuony AD. Remediation and recycling of chromium from tannery wastewater using combined chemical–biological treatment system. Process Safety and Environmental Protection. 2016;104:1-10. doi:10.1016/j.psep.2016.08.004
CrossRef - GracePavithra K, Jaikumar V, Kumar PS, SundarRajan PS. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. Journal of Cleaner Production. 2019;228:580-593. doi:10.1016/j.jclepro.2019.04.117
CrossRef - Ashraf A, Bibi I, Niazi NK, et al. Chromium(VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. International Journal of Phytoremediation. 2017;19(7):605-613. doi:10.1080/15226514.2016.1256372
CrossRef - Qasem NAA, Mohammed RH, Lawal DU. Removal of heavy metal ions from wastewater: a comprehensive and critical review. npj Clean Water. 2021;4(1). doi:10.1038/s41545-021-00127-0
CrossRef - Xu Z, Gu S, Rana D, Matsuura T, Lan CQ. Chemical precipitation enabled UF and MF filtration for lead removal. Journal of Water Process Engineering. 2021;41. doi:10.1016/j.jwpe.2021.101987
CrossRef - FAO (Food and Agricultural Organization); UNIDO (United Nations Industrial Development Organization). Tanneries-Emissions-Wastewater. http://www.fao.org/3/X6114E/x6114e05.htm#b5-3.2.2.%20Wastewater.
- Sawalha H, Al-Jabari M, Elhamouz A, Abusafa A, Rene ER. Tannery wastewater treatment and resource recovery options. In: Waste Biorefinery. Elsevier; 2020:679-705. doi:10.1016/b978-0-12-818228-4.00025-3
CrossRef - Pal M, Malhotra M, Mandal MK, Paine TK, Pal P. Recycling of wastewater from tannery industry through membrane-integrated hybrid treatment using a novel graphene oxide nanocomposite. Journal of Water Process Engineering. 2020;36. doi:10.1016/j.jwpe.2020.101324
CrossRef - Yadav M, Gupta R, Sharma RK. Chapter 14 - Green and Sustainable Pathways for Wastewater Purification. In: Advances in Water Purification Techniques: Meeting the Needs of Developed and Developing Countries. Elsevier; 2018:355-383. doi:10.1016/B978-0-12-814790-0.00014-4
CrossRef - Dahman Y. Nanopolymers**By Yaser Dahman, Kevin Deonanan, Timothy Dontsos, and Andrew Iammatteo. In: Nanotechnology and Functional Materials for Engineers. Elsevier; 2017:121-144. doi:10.1016/b978-0-323-51256-5.00006-x
CrossRef - Saxena G, Purchase D, Bharagava RN. Environmental Hazards and Toxicity Profile of Organic and Inorganic Pollutants of Tannery Wastewater and Bioremediation Approaches. In: Bioremediation of Industrial Waste for Environmental Safety. Springer Singapore; 2020:381-398. doi:10.1007/978-981-13-1891-7_17
CrossRef - Tsuchiyama T, Tazaki A, al Hossain MA, et al. Increased levels of renal damage biomarkers caused by excess exposure to trivalent chromium in workers in tanneries. Environmental Research. 2020;188. doi:10.1016/j.envres.2020.109770
CrossRef - Abdelkader SE, El-Gendy AS, El-Haggar S. Removal of trivalent chromium from tannery wastewater using solid wastes. Innovative Infrastructure Solutions. 2021;6(2). doi:10.1007/s41062-020-00414-8
CrossRef - Vasquez-Medrano R, Prato-Garcia D, Vedrenne M. Ferrioxalate-Mediated Processes. In: Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology. Elsevier Inc.; 2018:89-113. doi:10.1016/B978-0-12-810499-6.00004-8
CrossRef - Chandravanshi BS, Leta S. Chemical Precipitation Method for Chromium Removal and Its Recovery from Tannery Wastewater in Ethiopia Chemical International. 2017;3(4):291-305
- Basavaraju P, Ramakrishnaiah CR. Hexavalent Chromium Removal from Industrial Waste Water by Chemical Precipitation Method. International Journal of Engineering Research and Applications.2012;2(2):599-603.
- Nur-E-Alam Md, Mia MdAS, Ahmad F, Rahman MdM. An overview of chromium removal techniques from tannery effluent. Applied Water Science. 2020;10(9). doi:10.1007/s13201-020-01286-0
CrossRef - Callegari A, Capodaglio AG. Properties and beneficial uses of (bio)chars, with special attention to products from sewage sludge pyrolysis. Resources. 2018;7(1). doi:10.3390/resources7010020
CrossRef - Chen T, Zhou Z, Xu S, Wang H, Lu W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresource Technology. 2015;190:388-394. doi:10.1016/j.biortech.2015.04.115
CrossRef - Sarkar M, Rahman AKML, Bhoumik NC. Remediation of chromium and copper on water hyacinth (E. crassipes) shoot powder. Water Resources and Industry. 2017;17:1-6. doi:10.1016/j.wri.2016.12.003
CrossRef







