Alteration in the Antioxidant Enzymes activities as Potential biomarkers for Identification of Stress Caused by Afidopyropen Intoxication in Cyprinus carpio.
1
Environmental and Molecular Toxicology Research Laboratory,
Department of Studies in Zoology,
Karnatak University,
Dharwad,
Karnataka
India
Corresponding author Email: davidkcd@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.1.5
Copy the following to cite this article:
Dodamani M, David M. Alteration in the Antioxidant Enzymes activities as Potential biomarkers for Identification of Stress Caused by Afidopyropen Intoxication in Cyprinus carpio. Curr World Environ 2023;18(1). DOI:http://dx.doi.org/10.12944/CWE.18.1.5
Copy the following to cite this URL:
Dodamani M, David M. Alteration in the Antioxidant Enzymes activities as Potential biomarkers for Identification of Stress Caused by Afidopyropen Intoxication in Cyprinus carpio. Curr World Environ 2023;18(1).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-10-18 |
|---|---|
| Accepted: | 2023-01-04 |
| Reviewed by: | 
 Vijaya Kumara
Vijaya Kumara
|
| Second Review by: |

 Rama Subramanian
Rama Subramanian
|
| Final Approval by: | Dr. Hiren B Soni |
Introduction
Nowadays agrochemical market is a flamboyant field for pharmaceuticals and chemicals, which consists of natural products and semi-synthetic products from natural sources and their derivatives that are derived from plants and microbes1,2,3. This is because it has an increasing demand for pest management products to ensure sustainable use by promoting efforts in the discovery and development of new chemicals which are made of living things1. We can see Wide production and extensive use of pesticides throughout the world to control various pests in crops. Since 1991 it has represented a beginning of a new era for new insecticides with many improved environmental concerns2, which means concerned with the protection of aquatic animals, soil animals, and land animals. The development strategy for novel pesticides has been extremely low dosage, and pesticides resulted in a significant reduction in the active ingredient needed for the control of pests and weeds, but there is no such research evidence on these insecticides which are derived from natural sources that do not prove to have an impact on non-target organisms. And new chemical related to crop protection is still being reported4. In the present study, we used one such pesticide which is afidopyropen, a novel insecticide, and an excellent insecticidal activity against common aphid species, that damage a variety of fruit plants, vegetables, and ornamentals plants, and also against mealybugs, leafhoppers, whiteflies3. And afidopyropen has been previously reported to be harmful to Cyprinus Carpio fish5.
For animals, pesticides act as a stressor. Pesticides enter the aquatic ecosystem and interact with various targets in the cells of fish and other living organisms. Oxidative stress in aquatic living things, essentially fish has the most important animal model for aquatic toxicology. Since oxidative stress is incited by various manufactured compounds including a few pesticides, these xenobiotics might change the antioxidant status in the fish body6, and disturbances in the production of free radicles and antioxidant activity also result in oxidative stress.
Living cells have highly active enzyme defense systems against reactive oxygen species, and enzyme activity varies according to species and muscle type7. When ROS increases in cells it leads to a lack of balance between the production of ROS and antioxidant defense that alters the physiological processes in the body, finally which impacts and alters the DNA material as previously it was8. In cells, antioxidant defense systems play an important role in maintaining the cellular homeostasis of organisms against reactive oxygen species (ROS), which are continuously detoxified and removed within cells9. And also, different types of antioxidants protect cells against damage10 such as Vitamin E, and Vitamin C, and known antioxidant enzymes that fight against them. Oxidative stress is evoked by many chemicals including some pesticides, organochlorine, organophosphates, carbamates, pyrethroids, bipyridyl, triazine, chloroacetanilide, and other pesticides11, all these pesticides have been shown to cause oxidative stress. As a result, reactive oxygen species are a typical outcome of xenobiotic metabolism, and many contaminants exhibit some of their toxicity through the production of reactive oxygen species12 and lead to DNA damage, and reduced protein synthesis, which may cause cellular integrity 13.
The common carp Cyprinus carpio is widely used for aquaculture and also used as a food source this fish is the best aquatic toxicology model because of its sustainability and availability throughout the season and good adaptation capacity to laboratory conditions. Therefore, several investigations about pesticide toxicity in different fishes have been conducted so far. However, no study on the blood antioxidant enzymes of Cyprinus carpio exposed to afidopyropen has yet to be reported. Because animal blood is having abundant antioxidants and also serves as a transporter of oxygen, antioxidants including superoxide dismutase, catalase, and other antioxidant enzymes14. Hence, this current study aims at highlighting the biochemical changes in Cyprinus carpio exposed to afidopyropen.
Material and methods
Test Animal and maintenance
Healthy Cyprinus carpio were obtained from local fishermen and were acclimatized for a week in a huge cement tank containing 5000L of tap water with continuous aeration. During this period commercial pallet was given and health status was monitored, before the test. Healthy fishes were exposed to Afidopyropen and throughout the experimental duration, the oxygen concentration and photoperiods 12 hrs dark and 12 hrs light were maintained. Fish Cyprinus carpio (mean body length 18.18 ± 1.62 cm, mean weight 100.64 ± 10.25 g). The physicochemical properties of the water15 in all aquaria were controlled as follows: Dissolved oxygen-7.8 ± 0.9 mg/L. Total hardness-32.2 ± 3.1 mg as CaCO3/L. Ph-7.05 ± 0.2. Salinity; Nil. Specific gravity-1.001. Calcium-18.29 ± 1.52 mg/L. Magnesium-0.76 ± 0.3 mg/L. Phosphate-0.032 ± 0.005 mg/L and oxygen saturation was 90–98%. Temperature-26 ± 2 °C.
Test Chemicals
Insecticide afidopyropen volume was procured from Garg Agro Center Malout, Panjab., The remaining chemicals used in the present study were procured from SRL. Pvt. Ltd. Company Mumbai.
Experimental Design and Exposure
The experiment was a semi-static assay conducted over 1,10,20,30 days. Fish have been divided into five groups including control held in an aquarium. Sub-lethal concentration was selected based on 1/5th of the Lc50 value. Fish were exposed to 0.2 mg/L afidopyropen and throughout the experimental duration, the oxygen concentration and photoperiods 12 hrs dark and 12 hrs light were maintained in Control and treated groups in the test chamber.
Sample Collection and biochemical Assays
Fish were starved for 24 hours before sampling. The blood sample was collected from the caudal vein, centrifuged at 6000 rpm for 10 min at 4°C, and stored at ?25 °C until further use. Measurement of biochemical parameters of the CAT, SOD, MDA, and GPx activities was determined by using a commercial kit in a microplate reader16 (RANSEL by Randox Laboratories, UK), and was expressed in international units per liter, MDA was expressed in mMol/ml. The estimation of plasma levels of ALT, GGT, and ALP was determined according to the modified IFCC method (International Federation of Clinical Chemistry) by using a commercial kit. Protein concentrations were measured according to lowry et al17 by using a UV-visible spectrophotometer (Systronic) and values were expressed in g/dL.
Ethical statement
Throughout the experiments, the animals were used and maintained according to the guidelines imposed forth by the committee for control and supervision of experiments on animals. Delhi, India.
Statistical Analysis
To determine antioxidant enzyme activities in the blood are presented as mean ± SE. The statistical significance of the difference between the control and treatment was analyzed by analysis of variance followed by Duncan’s post hoc test was performed using the SPSS software version 25. P <0.05 is considered to be statistically significant.
Results
In the present investigation, significant alteration in the antioxidant status of fish Cyprinus carpio was found to be dose-duration manner. The results are summarized in 1-7 graphs. The plasma CAT activity in fish showed a significant (p < 0.05) maximum percent inhibition of 46.31% on the 30th day of exposure (Figure.1). While 1-day exposed fish showed a slight increase of 1.12%, and fish treated for 10 and 20 days showed a significant decrease of 17.25%, and 34.32% respectively compared to the control.
Under the influence of afidopyropen, the plasma SOD activity was significantly elevated by 63.21% on the 30th day of exposure. Whereas 1-day exposed fish showed an increase of 26.81%, fish exposed to 10- and 20-days showed a significant increase of 46.72%, and 50.75% respectively compared to the control (Figure.2).
The plasma LPO activity showed an increase of 64.73% on the 30th day of exposure. Also, 10 and 20 days showed a significant increase of 33.15%, and 50.48% respectively. While 1-day exposure indicated an increase of 1.16% compared to the control (Figure.3).
The sub-lethal concentration depicted decreased levels in the plasma GPx activity in a dose-dependent manner. The plasma GPx activity of 1- day exposed fish revealed a negligible decline of 0.13% (Figure.4), however, it suggested relatively less harm as compared to the control. It was noticed that the highest decrement level of GPx activity was 58.18% on the 30th day. Also, decreased GPx activity was observed on day 10 and day 20 with 11.27%, and 26.54% respectively compared to the control.
The effect of afidopyropen on ALP activity (Figure.5) was higher compared to the control. Fish exposed for 1-day showed a 2.08% increase in ALP activities in blood plasma compared to the control. The 30th day of exposure had the highest level of activity by 30.88%, whereas the 10- and 20-day exposed groups showed an increase of 12.61%, and 21.29% respectively compared to the control. The ALP levels were remarkably increased with the increasing duration of treated groups.
Although ALT activity significantly increased at 62.97% in fish exposed to afidopyropen on the 30th day. The fish exposed for 1-day had the least increment of 0.71% and did not much differ from the control group (Figure 6). Whereas, 10 and 20 days of exposure showed 34.74%, and 51.85%, and were significantly higher compared to the control.
In addition, on the 30th day of exposure, fish depicted the lowest GGT activity of 59.95% percent on the 30th day. The 1-day exposed fish showed a slight detectable fall in the plasma GGT activity of 0.98% without much difference from the control. 10 and 20-day exposed fish showed a significant decrease of 9.82%, and 20.96% (Figure.7), respectively compared to the control. Protein content significantly lowered in all exposure durations, 1 day (1.56%), 10 days (16.62%), 20 days (26.58%), and gradually decreased on the 30th day (57.27%) respectively compared to the control.
 | Figure 1: Effect of Afidopyropen on CAT activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
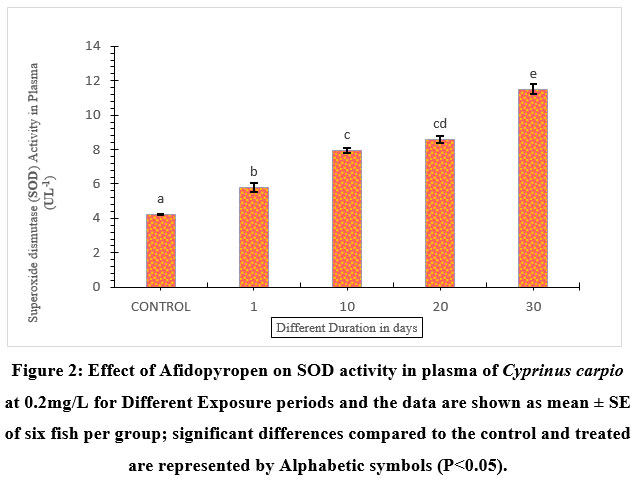 | Figure 2: Effect of Afidopyropen on SOD activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
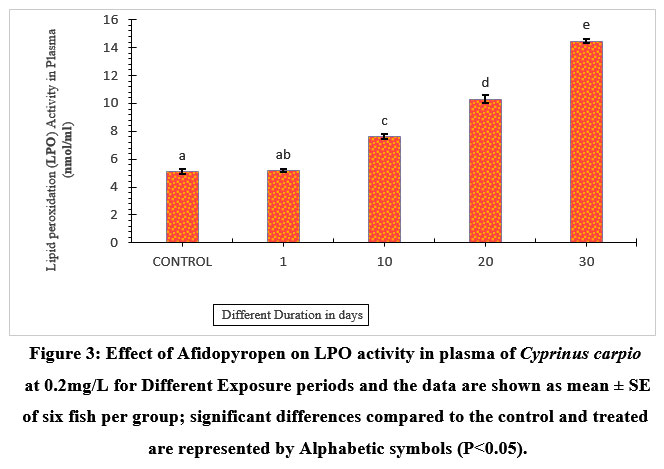 | Figure 3: Effect of Afidopyropen on LPO activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
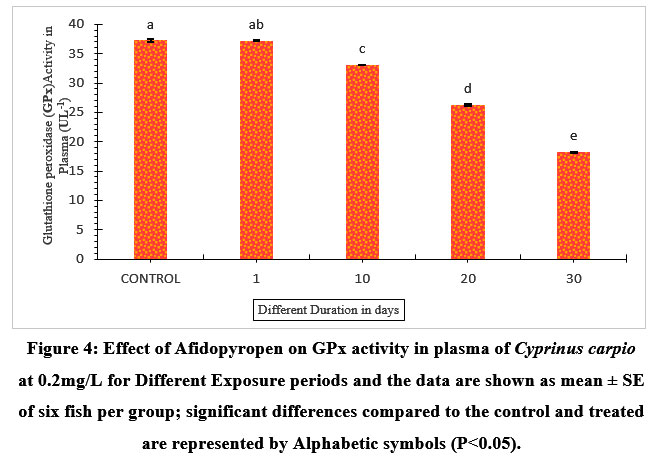 | Figure 4: Effect of Afidopyropen on GPx activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
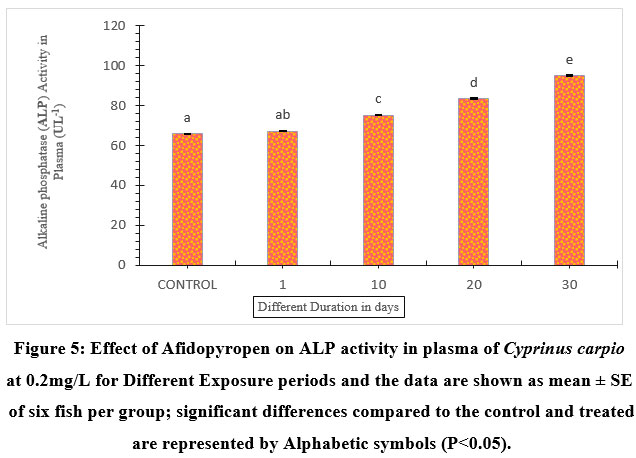 | Figure 5: Effect of Afidopyropen on ALP activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
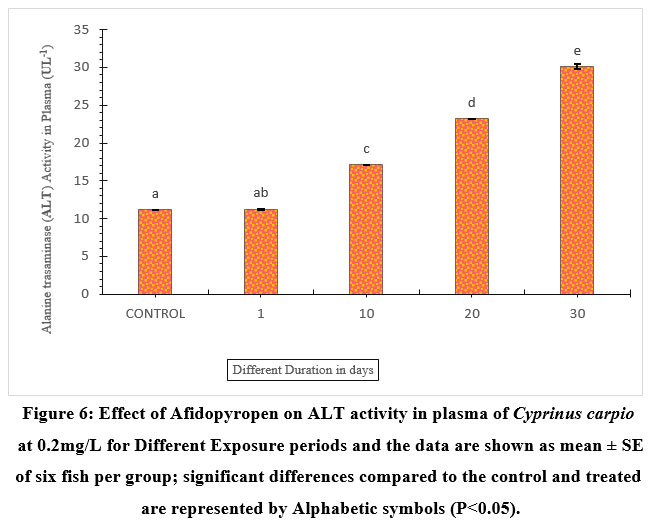 | Figure 6: Effect of Afidopyropen on ALT activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
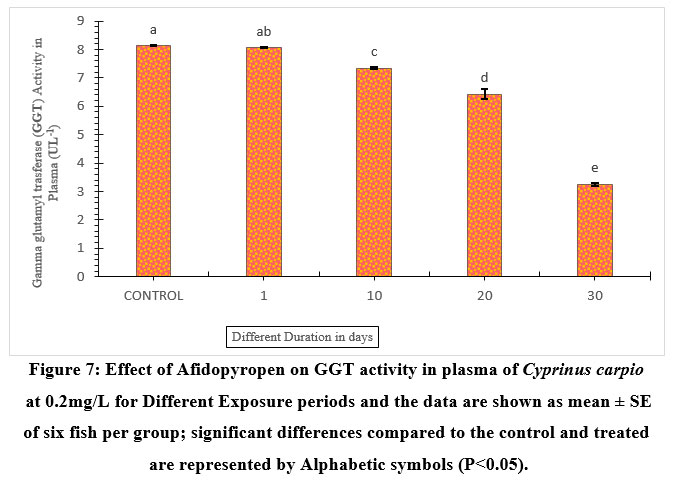 | Figure 7: Effect of Afidopyropen on GGT activity in plasma of Cyprinus carpio at 0.2mg/L for Different Exposure periods and the data are shown as mean ± SE of six fish per group; significant differences compared to the control and treated are represented by Alphabetic symbols (P<0.05).
|
Discussion
Blood provides an important profile for studying the toxicological effect on animals. Different blood parameters frequently undergo changes depending on stress conditions and various environmental factors. An increase or decrease in certain enzymes depends on the nature of the species and xenobiotics. In the present study, the antioxidant enzymes are expected to form an antioxidant defense system to increase under the exposure of afidopyropen to detoxify ROS, the enzymes are intrinsically linked and dependent upon the activity of one another.
Catalase is an antioxidant enzyme that helps to separate water and oxygen from hydrogen peroxide. It avoids the excess accumulation of hydrogen peroxide that is present within a cell Jeeva et al18. This enzyme is in charge of neutralizing hydrogen peroxide by breakdown, ensuring an optimum level of the molecule in the cell, which is also important for cellular signaling activities Nandi et al19. On 1 day of exposure, fish showed the defensive mechanism by increasing CAT activity. The present study showed that the plasma CAT activity is decreased may be due to a detoxifying mechanism failure against afidopyropen toxicity. On days 10 and 20 the activity decreased and further decreased on the 30th day of exposure, it showing reduced activity in protecting the cells against afidopyropen. similar work was reported by Vinodhini and Narayan20 who exposed Cyprinus carpio to heavy metals. In this study, afidopyropen decreases the metabolic rate, which in turn results in lower CAT, and GGT enzyme activity at sub-lethal concentration, the decreased activity of CAT may indicate failure to scavenge H2O2 by increased ROS production under sub-lethal concentration.
SOD is one of the antioxidants that act to prevent the formation of reactive species in cells. It's a key endogenous antioxidant. This enzyme helps in making the potentially damaging superoxide anion less dangerous to the living cells Ighodaro and Akinloye21. In this study SOD activity in plasma was significantly increased by afidopyropen exposure and this was dependent on the duration of the exposure and leads to damage to the gills and liver. Vinodhini and Narayanan20 found that the SOD levels found to be were increased and decreased for 16- and 32-day exposure in the liver and kidney which triggers induction response in heavy metal intoxicated groups. Our results have consisted of the findings of Abhijith et al22 reported that a significant increase in SOD activity leads to leakage of these enzymes into plasma with damage to the liver, and gills of Catla catla exposed to methyl parathion. which also reflects similar findings noticed by afidopyropen increases the SOD activity in the plasma noted in the present study and shows a possibility of a defense mechanism itself against the toxic effect by increasing its activity. Hence, SOD and glutathione levels can be considered indicators of oxidative stress.
MDA levels either increase or decrease it’s usually a key point in estimating the lipid peroxidation parameter Rui et al23, and its primary step of cellular damage caused by pesticides and other chemicals Yonar and Sakin24. In this study, MDA levels in the blood plasma of Cyprinus Carpio exposed to afidopyropen showed a marked increase in all exposed duration except 1-day exposure duration which slight changes were observed as compared to the control. Similar results were reported by Banaee, et al25 in Alburnus mossulensis, exposed to fenpropathrin. A similar increase in plasma LPO level was noted in cypermethrin-treated freshwater mussel unio Elongatulus eucirrus by koprucu et al26 and in Cyprinus carpio exposed to dichlorvos by hai et al27. The increase in plasma and liver LPO levels in Cyprinus carpio is due to the failure defense mechanism against afidopyropen toxicity, The current study indicated that the imbalance of MDA levels causes oxidative stress.
Glutathione peroxidase has to suppose protect the blood from damage by H2O2 and also protect tissue against oxidative damage due to lipid peroxidation20. In the current study we observed reduced GPx activity in exposed fish which leads to tissue damage our findings are in agreement with Espinoza et al28, who reported that When GPx activity is reduced, more hydrogen peroxide is produced in a cell, resulting in tissue damage.
Alkaline phosphatase removes the phosphate group from molecules using a phosphatase enzyme. Haghi, Banaee29 reported that 0.2 mg /L microplastic exposed to Cyprinus carpio for a period of 30 days leads to damage to the liver cells membrane, and other digestive and reproductive organs due to a significant increase. In the present study, fish exhibited an increase in plasma ALP, and ALT activities (Figure.5 and 6), which could be one of the reasons for liver damage and thus. In addition, Demerdash et al30 determined that enzyme leakage compared with is a result of cell damage. Hence, toxic substances make damage the membrane function at the cellular level and are often associated with increased cell membrane permeability.
In the present study, GGT activity was a significant decrease, similar results were observed in Cyprinus carpio treated with chlorpyrifos and polyethylene glycol, TiO2-NPs, and paraquat Hatami et al 31; Banaee et al32. Monteiro et al33; Yonar and Sakin24 reported that enzyme activity can decrease when some biochemical processes are slowed down by excess substrate. Thus, a reduction in the activity of GGT might reflect a possible failure of the antioxidant system in the blood plasma of exposed fish. Protein is important in a living organism it provides energy and the breakdown of protein to reduce the toxic effect of xenobiotics on the body David et al34. In this study, the protein content of Cyprinus carpio was found to be decreased significantly. Hence, exposed fish shows a decrease in the total protein as compared to the control. Our results consisted of Amin and Hashem35 who reported that a decrease in protein could lead to alters protein synthesis which led to increasing ALT and AST activities.
Conclusion
Enzymes are responsible for catalyzing chemical reactions within the cell. If chemicals, interrupts the biological system it results in the cause of severe damage to the cells, and an imbalance in the activity of an enzyme leads to aberrant physiological conditions and slows down biochemical events in the body, which may be harmful. The present study of afidopyropen showed responsible for the altered antioxidant enzymes are the witness to the unhealthy status of the fish Cyprinus carpio and the changes in these enzymes are the root cause of DNA damage. As This study greatly fills a gap in the scientific literature about afidopyropen.
Acknowledgement
The authors are grateful to the Head of the Department of Zoology, Karnatak university for permission to access the laboratory facilities.
Conflict of Interest
I declare there is no conflict of interest in this article.
Funding Sources
The author’s received no financial support for the publication of this article.
References
- Cantrell CL, Franck ED, and Stephen OD. Natural Products as Sources for New Pesticides. J. Nat. Prod. 2012, 75, 1231?1242. dx.doi.org/10.1021/np300024u.
CrossRef - Sparks TC, Wessels FJ, Lorsbach BA, et al. New age of insecticide discovery-the crop protection industry and the impact of natural products. Pestic. Biochem. Phys. 2019 161, 12–22. 2309.
CrossRef - Horikoshi R, et al. Afdopyropen, a novel insecticide originating from microbial secondary extracts. Scientifc Reports. 2022. 12:2827.
CrossRef - Lorsbach BA, et al. Natural products: A strategic lead generation approach in crop protection discovery. Pest Manag. Sci. 2019; 75, 2301– 2310.
CrossRef - Mahantesh Dodamani, Muniswamy David. Acute Toxicity, and Neurobehavioral Responses of Afidopyropen on Exposure to Freshwater Edible Fish, Cyprinus carpio (Linnaeus). Research Journal of Pharmacy and Technology. ‘In press’.
- Srinivas BN, Muniswamy D, and Rajeshwari DS. Studies on fenaxoprop-p-ethyl induced antioxidant response in Cyprinus carpio. Research journal of agriculture science. 2021; 12; 2185-2192.
- Subhendu Datta. Fish as source of antioxidants, Editors: Radha C Das, Archana Sinha, A. K. Pal, Subhendu Datta, Parimal Sardar. In book: Nutritional and Biochemical techniques in Fisheries,1st ed, KOLKATA, Publisher: Director, CIFE, May 2003. https://www.researchgate.net/publication/259263595_Fish_As_a_Source_of_Antioxidants, Accessed October 13, 2022.
- Seyed HH. Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. reviews in fisheries science & aquaculture. 2020; 198-217 https://doi.org/10.1080/23308249.2020.1795616.
CrossRef - Ozmen I, Bayir A, Cengiz M, Sirkecioglu AN, Atamanalp M. Effects of water reuse system on antioxidant enzymes of rainbow trout (Oncorhynchus mykiss w., 1792).Veterinární Medicína; Prague 2004: 49, 10 , 373-378. DOI:10.17221/5726-VETMED.
CrossRef - Halliwell B , Aeschbach R, Loliger J, Aruoma. The characterization of antioxidants. Fd chem. Toxk., 1995. Vol. 33, no. 7, pp. 601-617.
CrossRef - Slaninova A, et al. A review: Oxidative stress in fish induced by pesticides. Neuroendocrinol Lett 2009; 30(Suppl 1): 2–12.
- Lackner, R. “Oxidative stress” in fish by environmental pollutants. Fish ecotoxicology,1998; 203–224. Doi:10.1007/978-3-0348-8853-0_6.
CrossRef - Rosa M, et al. Antioxidant defenses in fish: biotic and abiotic factors. Reviews in fish biology and fisheries. 2005; 15: 75–88. Doi 10.1007/s11160-005-7846-4.
CrossRef - Chengyi tu. Extraction of antioxidants from animal blood and its potential application as a pet food preservative. Thesis. Clemson University 2013. Accessed August 15, 2022. https://tigerprints.clemson.edu/all_theses/2307.
- Rodger Baird (Author), Andrew D. Eaton, Eugene W. Rice, Laura Bridgewater (Editor). Standard methods for the examination of water and wastewater, 21st ed. Washington, DC, USA: APHA, AWWA, WEF (publisher). 2005.
- Seyyed MH, Morteza Y. Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline?induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss) Aquaculture Nutrition. 2019;25:298–309.
CrossRef - Lowry, OH, Rosenbrough NJ, Farr AL, Randal RJ. Proteins estimation by folin phenol method. J Biol Chem. 1951. 193:265–275.DOI:10.1016/S0021-9258(19)52451-6.
CrossRef - Sathiya J. J. Enzymatic antioxidants and its role in oral diseases. J pharm bioallied sci. 2015. 7suppl 2: 331–333. Doi: 10.4103/0975-7406.16343819.
CrossRef - Ankita N, et al. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid med cell longev., 2019: 961;3090. Doi: 10.1155/2019/9613090.
CrossRef - Vinodhini R, and Narayana M. Biochemical changes of antioxidant enzymes in common carp (Cyprinus carpio L.) after heavy metal exposure. Turk J. Vet. Anim. Sci. 2009; 33 (4); 273-278.
CrossRef - Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (sod), catalase (cat) and glutathione peroxidase (gpx): their fundamental role in the entire antioxidant defence grid. Alexandria journal of medicine. 2018; 54 287–293; https://doi.org/10.1016/j.ajme.2017.09.001.
CrossRef - Abhijith BD, Ramesh M, Poopal RK, Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. The Journal of Basic & Applied Zoology. 2016, 77, 31-40.
CrossRef - Rui ji,. et al. (in vitro and in vivo hepatoprotective and antioxidant effects of astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (cyprinus carpio) fish physiol biochem. 2012; 38:871 –881 doi 10.1007/s10695-011-9575-z.
CrossRef - Enis YM, Fatih S. Ameliorative effect of lycopene on antioxidant status in cyprinus carpio during pyrethroid deltamethrin exposure. Pesticide biochemistry and physiology. 2011; 99, 3, 226-231 doi: 10.1093/gerona/63.5.505.
CrossRef - Banaee M, et al. Alteration in biochemical parameters of the freshwater fish, alburnus mossulensis exposed to sub-lethal concentrations of fenpropathrin. Int j a quat boil.,2014; 2 [2], 58-68.
- Koprucu SS, Yonar E, and Seker E. Effect of deltamethrin on antioxidant status and oxidative stress biomarkers in fresswater mussel, unio elongatulus eucirrus. Bull environ contam toxicol. 2008; 81, 253-257. http://doi.org/10.1007/s00128-008-9474-x.
CrossRef - Hai DQ, Varga SI, and Matkovic B. Organophosphate effects on antioxidant system of carp (Cyprinus carpio) and catfish (Ictalurus nebulosus). Comparative biochemistry and physiology part c: pharmacology, toxicology, and endocrinology,1997; 117(1), 83–88. Doi:10.1016/s0742-8413(96)00234-4 10.1016/s0742-8413(96)00234-4.
CrossRef - Sara EE, Hongfei G, Neal F, et al. GlutathionePeroxidase Enzyme Activity in Aging. J Gerontol A Biol Sci Med Sci. 2008; 63(5): 505–509.
CrossRef - Nematdoost HB, & Banaee M. Effects of micro-plastic particles on paraquat toxicity to common carp (cyprinus carpio): biochemical changes. International journal of environmental science and technology. 2016; 14(3), 521–530. Doi:10.1007/s13762-016-1171-4 10.1007/s13762-016-1171-4.
CrossRef - El-demerdash, et al. Role of alpha-tocopherol and b-carotene in ameliorating the fenvalerate induced changes in oxidative stress, hemato-biochemical parameters, and semen quality of male rats. J environ sci health b. 2004; 39:443–459.
CrossRef - Mahdiye H, Mahdi B, Behzad NH. Sub-lethal toxicity of chlorpyrifos alone and in combination with polyethylene glycol to common carp (cyprinus carpio) chemospere. 2019; 219:981-988. Doi: 10.1016/j.chemosphere.2018.12.077.
CrossRef - Banaee M, et al. Blood biochemical changes in common carp (Cyprinus carpio) upon co-exposure to titanium dioxide nanoparticles and paraquat. Iranian journal of fisheries sciences., 2019. 18. 242-255. Doi;10.22092/ijfs.2019.118174.
- Monteiro, et al. Oxidative stress biomarkers in the freshwater characid fish, brycon cephalus, exposed to organophosphorus insecticide folisuper 600 (methyl parathion). Comparative biochemistry and physiology part c: toxicology & pharmacology. 2006. 143, 2, 141-149.
CrossRef - David M, et al. Response of cyprinus carpio (linn) to sublethal concentration of cypermethrin: alterations in protein metabolic profiles. Chemosphere. 2008; 4, 347-352 https://doi.org/10.1016/j.chemosphere.2004.02.024.
CrossRef - Kamal AA, and khalid SM. deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (clarias gariepinus): antioxidant defense and role of alpha-tocopherol. bmc vet res. 2012; 8: 45. Doi: 10.1186/1746-6148-8-45/.
CrossRef







