Biofuel Formation from Microalgae: A Renewable Energy Source for Eco-Sustainability
Corresponding author Email: owais.micro@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.1.2
Copy the following to cite this article:
Oves M, Qari H. A, Ismail I. M. Biofuel Formation from Microalgae: A Renewable Energy Source for Eco-Sustainability. Curr World Environ 2022;17(1). DOI:http://dx.doi.org/10.12944/CWE.17.1.2
Copy the following to cite this URL:
Oves M, Qari H. A, Ismail I. M. Biofuel Formation from Microalgae: A Renewable Energy Source for Eco-Sustainability. Curr World Environ 2022;17(1). Available From:
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 09-08-2021 |
|---|---|
| Accepted: | 04-03-2022 |
| Reviewed by: | 
 Debajyoti Kundu
Debajyoti Kundu
|
| Second Review by: |

 Shemaa Fatih
Shemaa Fatih
|
| Final Approval by: | Dr. Umesh Chandra Kulshrestha |
Introduction
The manufacturing of eco-friendly and sustainable energy sources is the biggest challenge faced by the contemporary world 1,2. Thus, there is great significance in researching alternate energy sources that can produce energy eco-friendly and sustainable 3,4. Although adequate research had carried out on alternative energy sources; but, there is a break in knowledge nowadays, which needs to achieve sustainability 5,6. Several studies were conducted to determine the most effective and appropriate renewable source for energy production as an alternative to transporting fuels. A study estimated that the transportation sector consumes almost 28 % of the produced energy in the USA 7. Nearly 92 % of energy comes from fossil fuels out of vast proportions. Thus, alternative fossil fuels for energy production are being investigated 8. In recent years, biofuels have played a significant role in transforming the transportation sector, incorporating non-fossil fuel sources 9. The researchers examined that algal biomass cultivates biofuels locally to produce energy 10,11. With the help of this conversion, the revitalization of algal research is being carried worldwide. Thus, it is observed that several major technologies are carrying out the process of converting biomass into biofuels 10-12.
Nanotechnology applications have been implemented in the biofuel industry because conventional microalgae diversity production strategies have several restrictions, for example, incompatible industrial-scale cultivation, increased production costs, power consumption for microalgae biofuel fabrication, and a rise in greenhouse gas emissions. Some nanomaterials used as a catalyst high rate biofuel productions is known as nano-additives for example nano-droplets, nano-magnets, micro, nano-fibres, and other nano-additives have been created to increase biofuel productivity and effectiveness 13-15. Nanotechnology uses in various phases of cultivation to biofuel production and application in fuel engines due to reliability, catalytic efficiency, stability, purity, adsorption efficiency, high storage capacity, economic benefit, and eco-friendly features. According to earlier research, nanotechnology increased cultivation, the high rate production of different biofuels, and the consequences of biofuel used in gasoline and diesel engines. Nanofibers, nanoparticles, nanotubes, nanosheets, and other nanostructures have been examined as efficient nano-catalysts to enance the applicability 16-19. Magnetic nanoparticles, for example, have been successfully used for enzyme immobilization to boost bioethanol and biodiesel production. Magnetic nanoparticles were preferred for methanogenesis to produce biomethane because of their strong coactivity and robust paramagnetic characteristics 19.
It observed that technology is used to convert biomass into oil, promoted in diesel, hydrocarbon fuels, and gasoline 20. The cultivated algae's biomass production is placed within the container, which has been harvested to make biofuels an alternative liquid fuel. It was observed that algae produce more oil for each pound than conventional biological sources like soybean and corn 21. As algae can be cultivated within saltwater or sewage water, it cannot compete with freshwater sources or land space 22. Furthermore, algae are being acquired to produce energy because these are easy to grow. Harvesting of cultivated algae was carried out within ten days. It reveals that algae's biomass is almost 50 times more than conventional food crops 23. Thus, algae are being used to produce energy as a dominant and valuable alternative source for transportation 24.
In this article, the main focus is on alternative liquid fuels, which are being used to manufacture environmentally friendly and sustainable energy. According to the study of Kleinova et al. 25, biodiesel originated from oleaginous microbes and is being increasingly used as one of the significant renewable alternatives to petroleum and diesel. Hence, the study of Demirbas 26 suggested that microalgae stand as the only renewable biodiesel, which can meet the ever-increasing global demands of transportation energies because microalgae like plant use sunlight to create biofuel 27. However, creating oil from microalgae is far better than crop plants. The basic concept of the production of transport fuel from microalgae is not new; but, this idea has been considered in recent years due to the rising prices of petroleum and limitation of production and ease of availability with the environment's sustainability 28. In addition to this, the growing attention towards global warming also appears as one of the major concerns. The use of microalgae as substitute energy materials is being considered 22. With the help of energy production through microalgae, the burning of fossil fuels has been eliminated.
In this study, the primary aim is to determine the requirement of microalgae to produce energy. In doing so, the paper will emphasize the status of bio-fuel in the production of power. Types of bio-fuel and the significance of microalgae as an alternative energy source have been discussed.
Biofuels Generation
Three distinct biofuel generations have resulted from decades of study and development in the field of biofuels. It's impossible to generalize about the benefits and drawbacks of a generation's feedstock. There are three generation biofuels: first, second, and third. For example, corn ethanol or soy oil might be considered first-generation biofuels since they are developed from a conventional row crop, like corn or soybeans 23. The second-generation biofuels was develops from the cellulosic biomass, such as perennial grasses. Algae will be used to produce biofuels of the third generation 24. As a renewable energy source, they are limited by their supplies of biomass and technical advancements.
Ist-Generation
The use of Ist-generation biofuels symbolizes a step toward reducing emissions and eliminating fossil fuels. As a result of the increasing demand for crops, these biofuels also benefit agricultural sectors and rural communities 25. There are, however, several drawbacks to first-generation biofuels. Since they require food crops like maize and sugar beet for their biomass, they threaten food prices. Increases in the cost of food and animal feed throughout the world have been attributed to manufacturing first-generation biofuels. Vegetable oil, starch, or sucrose are the primary sources of first-generation biofuels. To turn vegetal oil into biodiesel and plant based starch and sucrose into ethanol, the constituents must be subjected to basic biochemical procedures 26. There is no longer a necessity for further research and development already creating carriage fuels because these procedures have already been made in the food business. Additionally, they may have a harmful influence on biodiversity and water resources in some locations. In addition, the area required to produce biomass for first-generation biofuels delivers only a minimal decrease in greenhouse gas emissions. In terms of greenhouse gas emissions, they are only marginally better than fossil fuels since they still take a lot of energy to produce, harvest, and process 27. Fossil fuels are now used to power current industrial methods. Moreover, Ist-generation biofuels are extra costly than gasoline 28. Because biodiesel nearly often originates from recycled restaurant oils rather than virgin oils, the supply is constrained by restaurants' oil use.
IInd-Generation
For the IInd generation biofuel development is require cellulosic biomass, it is comes from sources such as crop wastes, perennial grasses, and trees 29. Marginal farmland may cultivate these crops where row crop cultivation is unprofitable. This avoids competing with rich land that may be better suited to grow high demanding food crops by focusing on highly erodible regions or have poor soil quality. Once produced, many crops require further treatment despite their low initial input requirements to convert cellulose into a useful end product like liquid gasoline. In addition, producers face logistical and cost challenges when transporting large amounts of biomass. For example, organic waste, wood, food waste, and particular biomass crops are the primary biomass sources for 2nd generation biofuels. To get fuel from fast-growing trees like poplar, lignin, the "glue" of the plant, must be broken down through a sequence of chemical processes 30. The plant fibers are subjected to thermochemical or biochemical procedures in order to release the sugars contained inside. The design is similar to the first-generation ethanol process to produce plant ethanol. There is also a thermochemical process that makes syngas from straw and other forest waste (a mixture of carbon monoxide, hydrogen, and other hydrocarbons). In addition to gas oil, hydrogen and other hydrocarbons can be utilized as fuels.
Many of the problems with first-generation biofuels are addressed with second-generation biofuels. Due to their different biomass, they don't compete with fuels and food crops. The second-generation biofuels produce more energy per acre than Ist-generation biofuels. Because of this, land that would otherwise be unsuitable for growing food may be put to good use. Even though the technology is still in its infancy, future advancements in science may reduce costs and improve manufacturing efficiency. However, because part of the biomass for second-generation biofuels grows in the same environment as food crops, there is still competition with land usage. Farmers and policymakers are now faced with the difficult task of deciding which crop to cultivate. Biomass might be derived from cellulosic sources such as maize stover, which grows alongside food crops (leaves, stalk, and stem of corn). As a result, too many nutrients would be removed from the soil and replaced with fertilizer. To make 2nd generation fuels, the biomass must be pretreated to release the contained sugars, making the process more complicated than 1st generation biodiesel. As a result, more resources and energy are required.
IIIrd-Generation
The IIIrd -generation biofuels use crops like algae that have been genetically modified to produce energy. To extract the oil from these algae, they are grown and harvested. The oil can then be used to make biodiesel or other biofuels to replace petroleum-based fuels in the same way as first-generation biofuels 31. Algae biomass or oil is harvested to produce third-generation biofuels. Pretreatment isn't necessary for oil-producing algae (also known as Oilgae), and it cultivates rapidly. Although, environment conditions for optimal growth is challenging and expansive. Equipment and facilities are often required to keep strict environmental regulations in place. Figure 1 depicts the general steps involved in producing 3rd generation biofuels. The energy density per harvest area is higher in 3rd generation biofuels than first and second-generation biofuels. These organisms are cultivated for their ability to produce low-cost, high-energy, and renewable energy. Algae have the advantage of being able to grow in conditions that are not suitable for first- and second-generation crops, reducing the strain on available water and arable land. Sewage, wastewater, and saltwater, such as from the sea or a salt lake, can all be used to grow the plants in this way 32. That way, water that would otherwise be used to hydrate humans could be saved. In order to compete with petrodiesel and other petroleum-based fuels, further research is needed to improve the extraction process.
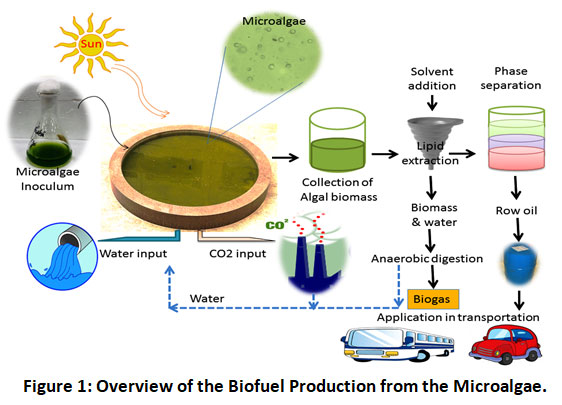 |
Figure 1: Overview of the Biofuel Production from the Microalgae. Click here to view Figure |
Characteristics of Microalgae
Algae cells are classified into two categories: eukaryotic and prokaryotic. As thallophytes and the oldest living species, microalgae are studied. Microalgal chlorophyll is made up primarily of photosynthetic pigments. The photosynthesis mechanism in microorganisms is the same as it is in higher plants. Furthermore, microalgae are cultivated as an aqueous suspension. These cells have more access to carbon dioxide, water, and other nutrients. Pigment types, cell wall constituents, the chemical composition of storage products, and morphological features are critical factors in microalgae classification.
Furthermore, microalgae may be autotrophic or heterotrophic if they obtain carbon from inorganic compounds. When microalgae use light as an energy source, they are photoautotrophic. On the other hand, microalgae are heterotrophic when organic compounds are used for development. Mixotrophic microalgae are photosynthetic microalgae that incorporate autotrophy and heterotrophy over photosynthesis 33.
Microalgae can also fix carbon dioxide from many sources, including factory waste gases, soluble carbonate salts, and the atmosphere. The most popular and effective method of carbon sinking is carbon dioxide fixation 34. That is due to the mass transformation of air into microalgae within the aquatic growth environment during photosynthesis. Because of their limited proportion, terrestrial plants' introduction does not seem to be an economically viable alternative.
Challenge of using Microalgae
It is found that fossil fuels are being deployed to generate liquid fuels and electrical power. Different types of renewable technologies are being deployed to create liquid fuels. The study of Jones & Mayfield revealed that it is expected that by the year 2030, 6 percent of the demand for total energy will be fulfilled by biofuels 35. However, there are also increasing chances of other biofuels contributing to the ever-increasing need for power. Although there are several advantages of using algae for producing biofuels; but, low competitiveness and high processing costs to fossil diesel are also critical barriers to widespread commercial utilization. The study has further identified that governments and researchers are trying to reduce operating costs and investments to produce commercially sustainable and feasible. Thus, the major challenge is establishing bio-fuel production industries through algae to ensure cost-competitive and sustainable energy. Still, biofuel processing and oil extraction improvements need to be introduced to ensure algal effectiveness. Development of friendly energy from microalgae should be developed sustainable environment. Further, some researchers recently applied microalgae for biofuel production and biosynthesize high amounts of lipids from the microalgae depicted in Table (1) 36-49.
Table 1: Microalgae for Lipid Production as the Primary Source of Biofuel Generation.
|
Microalgae |
Yield (g/l/d) |
Ref. |
|
|
Lipid |
Biomass |
||
|
Chlorella pyrenoidosa |
39.0 |
0.35 |
36 |
|
Chlorella vulgaris |
0.148 |
0.37 |
37 |
|
Chlorella vulgaris |
0.14 |
0.24 |
38 |
|
Chlorella emersonii |
0.122 |
0.36 |
38 |
|
Chlorella emersonii |
0.157 |
0.25 |
37 |
|
Chlorella minutissima |
0.0912 |
0.16 |
37 |
|
Chlorella protothecoides |
1.6–1.7 |
3.6–4.1 |
39 |
|
Chlorella protothecoides |
1.24–4.16 |
2.4–7.3 |
40 |
|
Chlorella protothecoides |
0.27–1.06 |
1.93 |
41 |
|
Chlorella protothecoides |
0.56 |
0.93 |
42 |
|
Chlorella protothecoides |
0.654 |
1.3 |
43 |
|
Botryococcus braunni |
0.029–0.064 |
0.16 |
44 |
|
Nannochloropsis sp. |
0.142 |
0.48 |
45 |
|
Nannochloropsis sp. |
0.204 |
0.3 |
46 |
|
Neochloris oleoabundans |
0.0126 |
0.055 |
47 |
|
Schizochytrium limacinum |
0.22–0.54 |
0.186–2.0 |
48 |
|
Scenedesmus obliquus |
0.27 |
0.15 |
49 |
Step Involved in Biofuel Production Microalgae
Cultivation of Algae
When compared to other traditional crops, including soybeans, rapeseeds, and palms, algae efficiently yield nearly 300 times more oil per acre. Algae have a growth cycle that lasts anywhere from one to ten days. Algae harvesting allows for several harvests to be carried out. Gouveia & Oliveira 52 discovered that such soil, which appears unsuitable for growing other crops, can grow algae. Algal development may also take place on arid and dry land and some algae has discovered they can grow ultra-faster upto 20 to 30 times higher than food crops.
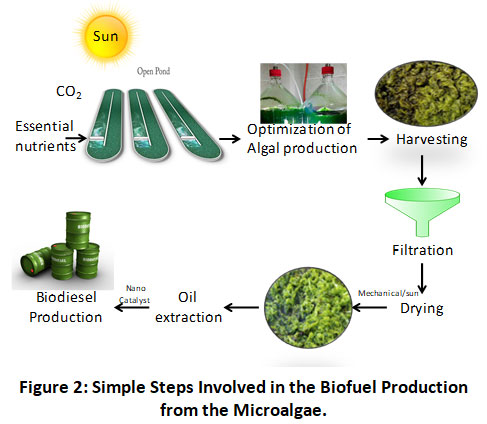 |
Figure 2: Simple Steps Involved in the Biofuel Production from the Microalgae. Click here to view Figure |
Cultivation Methods
Open pond for Cultivation
Choosing the right technology to cultivate and harvest algal biomass for energy generation is critical. Industrial-scale open ponds are now the only option due to economic issues. As a rule of thumb, their design is relatively straightforward. A paddle agitator is commonly used to agitate these tanks, which have vast surface areas and depths of up to 0.25 meters. They are constructed in the shape of a racetrack or a pond. The way of life Cultivation Methods for Microalgae Chemical substances or sewage with enough biogenic chemicals, supplemented if required with microelements, are the most often used media. It is possible to get carbon dioxide straight from the atmosphere using diffusion. The downsides of this technology include significant water losses due to evaporation, low biomass output, and the inability to grow certain algae species that are vulnerable to various illnesses, diseases, and parasites. In places with a lot of sunlight and easy access to water, such as along the coast, these kinds of systems work best. Spirulina and Chlorella genus algae are frequently grown in Asia, the United States, Europe, and Mexico 50. Here describe open systems because of the excellent outcomes with breeding ponds at a speedway. For this method, open ponds with a surface area of 1000–5000 square meters are constructed from PVC, clay, or concrete and range in width from 2.0–3.0 m and depth from 0.15–0.3 m 51. The concept of a pond-style racetrack is based on recirculation in closed-loop duct series. Mixing, and flow activities in a device can be sparked by the paddle wheel there. White plastic is used to line the pond's channels, which might be made of concrete or compacted earth. In the day tume when the culture is effectively radiated, the medium can be continuously supplied into the system 52.
Before the paddle wheel, the stream begins to breed, nutrients are injected. The paddlewheel at the other end of the loop receives the harvested biomass from the gadget. To keep algae from dropping, a stirrer is kept continually moving. Figure 3 depicts the Rays-Ways Reactor. In comparison to the 5–10 g BZT/m2 d (or the 10–15 g BOD/m3 d) accomplished in traditional stabilization ponds, the wastewater system can remove up to 35 g BZT/m2 d (or the 175 g BZT/m3/d pond at a depth of 0.2 m) 53. As a result, the system's hydraulic retention time (HRT) is reduced from 10–40 days to 2–6 days 54. Few of these systems are currently in use for wastewater treatment across the globe, despite having a substantially higher cleaning efficiency.
Three Primary Types of Open Systems are Described as Follows.
Unstirred Open System: The majority of these are freshwater bodies of water, such as lakes, ponds, and lagoons. Although the expense is modest, a lack of stirring can result in poor mixing for commercial-scale systems. The shallowness of these pools of water (less than 0.5 meters) allows light to permeate to the bottom. According to a prior study, these water bodies may be covered with plastic sheets for improved temperature management.
Circular Pond: The diameter of a circular pond is typically 40-50 meters, and the depth is typically 20-30 centimeters. The middle of the pond has a long revolving arm that works as a paddlewheel for mixing algae cells and culture medium. Even though unstirred systems are more efficient, environmental contamination is unavoidable when using a hybrid approach.
Raceway Pond: Concrete or plastic raceway ponds are typically between 15 and 50 centimeters deep. Pure or wastewater can be employed as the culture material in these ponds. CO2 collecting facilities can also be incorporated into this system's overall design. The presence of a paddlewheel, baffle, and channels prevent sedimentation and guarantee that the algae cells are floating in the medium. As a result, all algal cells receive the proper amount of sunlight and CO2 to develop appropriately. Microalgae growers choose this pond because it is more efficient than circular ponds or not agitated systems 55. Figure 3 is a schematic depiction of the same. Contamination of microalgae cultures by bacteria and fungus and pigments and other substances. Thus, while these bioreactors continue to be used for commercial microalgae growing, additional research is needed to improve light penetration and mixing and reduce water evaporation.
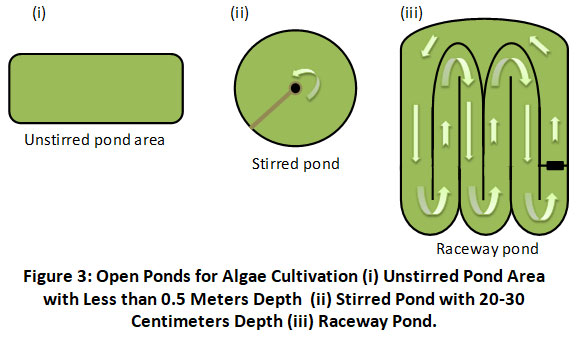 |
Figure 3: Open Ponds for Algae Cultivation (i) Unstirred Pond Area with Less than 0.5 Meters Depth (ii) Stirred Pond with 20-30 Centimeters Depth (iii) Raceway Pond. Click here to view Figure |
Photobioreactors for Algae Cultivation
It is a fundamentally new solution to the problem of algae biomass proliferation and cultivation that uses closed systems or photobioreactors. The photobioreactor types produced too far include horizontal tubular photoreactors, vertical tubular photoreactors, and photobioreactors sloped at any angle 56,57. On the other hand, photobioreactors are far more adaptable and may be used in a wide range of climates. In a closed bioreactor, evaporation is limited, parasites and predators are eliminated, and photosynthesis is ensured by artificial illumination. Growing cultures of particular algae species, such as those with a high oil concentration in biomass, is possible under these circumstances. A large gag photobioreactor was the first closed photobioreactor used in research 58,59. Plastic bags around 0.5 meters in diameter were used with an aeration system. Although most of the structures are built for batch mode operation, several semi-continuous systems exist.
The main issue with its use is that it must be done indoors because there is no way to adjust the temperature. There are also issues with culture lighting due to the bags' diameter, which reduces the system's output. Because this reactor has a high workload and does not guarantee proper mixing, it might collapse and decrease process efficiency. Most of the reactors are composed of polycarbonate or glass, and the gases supply and flow of medium are carried out by pumps or, more preferable, by airlift 51,55. Tube photobioreactors often employ air pumps or airlift systems to circulate and mix their cultures. This reactor may be used indoors and outdoors because it has a large light area. However, one of its key downsides is poor mass transfer due to increased oxygen levels and larger reactors. It has been shown that the tubular reactors may readily achieve significant dissolved oxygen levels.
Furthermore, the photoinhibition process in the tubular reactor under exterior (outside) circumstances is paramount. The ratio of lighting surface area to system volume decreases as the tubes' diameters increase. Unless a suitable mixing mechanism is in place, the cells at the bottom of the tube will not be able to get enough light for cell development (due to light shadowing). Then, light may be more effectively delivered to cells. The inability to regulate the temperature in a tubular photobioreactor is another challenge. Although a thermostat may be used, it is a costly and complicated option to execute. Algae cells can stick to the reactor's walls, which is something to keep in mind. Oxygen and carbon dioxide transport gradients run parallel to the tubes in a long tubular reactor. As pH rises, so does the cost of algae production because of the increased frequency of re-carbonization. For example, a tubular photobioreactor such as biocoil type is that consists of a plastic tube with a 2.4–5.0 cm modest diameter, with a screw wrapped around an oversized vertical tube. For pumping system accomplished with airlift system or a variety of pumps in a series of parallel networks of tubes. Depending on the type of algae, the pump used will be a different model. One possibility is to incorporate a gas exchange system within the reactor. The ability to manually or automatically regulate the temperature is provided. Consistent mixing in the reactor ensures that algae cells do not stick to the tube walls 52.
Despite the development of several solutions, none of them can be considered cost-effective. While photobioreactors can be costly to operate and need significant input (lighting, carbon dioxide supply), other challenges are associated with their use, such as overgrowth and a lack of light penetration. However, on the technical scale, specific technological systems are put into practice that combines open and closed systems components in a single design. There are several advantages to using greenhouses for racetrack-type ponds, such as decreased evaporation and controlled access to predators, stability, temperature and the possibility to apply extra illumination. It is also possible to provide an additional source of CO2 to the greenhouse's interior, such as combustion emissions.
Closed Systems
Due to resource restrictions of open systems, closed system PBRs were created. Closed systems eliminate direct interactions between culture media and the environment, resulting in more consistent and reliable surroundings. Additional mass transfer, airtightness, and process parameter approach all add to the cost of these systems. Because proper lighting system necessitates a significant amount of energy, these systems are inefficient. Because of these restrictions, the commercial application of these systems is challenging. The considerable value products and fine chemicals produced in these closed systems can be used to make biopharmaceuticals and human health supplements, cosmetics, and biofuels.
Flat-Panel: A schematic example of a PBR with a flat panel is shown in Figure 4a. It may be constructed both indoors and outside. The flat panels are made of plastic bags, plexiglass, polycarbonates, glass, and other transparent or semi-transparent materials. Algae cells are mixed with a culture medium using air bubbles created by an air sparger and an air pump. The exhaust gas is emitted at the point where the gas and liquid collide. Outside reactors have a natural tendency to utilize as much light as possible.
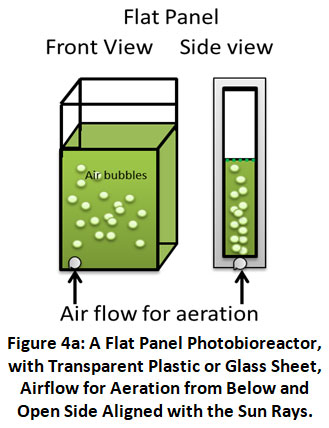 |
Figure 4a: A Flat Panel Photobioreactor, with Transparent Plastic or Glass Sheet, Airflow for Aeration from Below and Open Side Aligned with the Sun Rays. Click here to view Figure |
Vertical Tube: PBRs with vertical tubes can be set up in two different ways: a bubble column or an airlift (as shown in figure 4b, left and right, respectively). To ensure algal cell suspension, increased mass transfer, CO2 sequestration, and O2 release, a sparger on the bottom of both reactors release the spared gas into the culture fluid. The airlift reactor has changed the bubble column reactor.
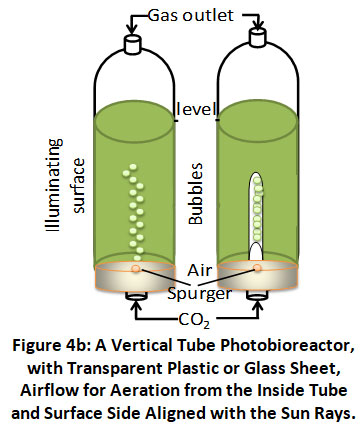 |
Figure 4b: A Vertical Tube Photobioreactor, with Transparent Plastic or Glass Sheet, Airflow for Aeration from the Inside Tube and Surface Side Aligned with the Sun Rays. Click here to view Figure |
Horizontal Tube: This type of PBR (shown in figure 4 c) has the highest surface-to-volume ratio, ensuring that algae cells get the most amount of light possible. Due to its uneven mass transfer, vast area requirements, and high maintenance and operational expenses, this type of PBR is deemed unsuitable for commercial-scale uses.
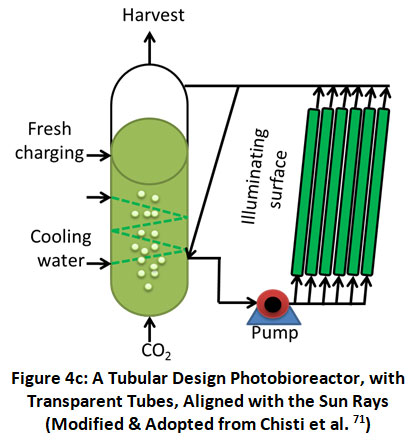 |
Figure 4c: A Tubular Design Photobioreactor, with Transparent Tubes, Aligned with the Sun Rays (Modified & Adopted from Chisti et al. 71) Click here to view Figure |
Stirred Tank: For this type of indoor reactor, an external source of light is required. The mixing is done with an electric motor. As a result, this reactor type has a high temperature, mass transfer, and mixing efficiency. A bottom-mounted air sparger supplies the CO2 for the reaction. The free headspace facilitates the flow of gases between the culture medium and the free headspace by providing 20%. On the other hand, a low surface-to-volume ratio limits light penetration, lowering microalgae activity.
Nano-Additives for Speedy Microalgae Culture
Recently, some researchers applied nano additives at different stages of microalgae culture to enhance biomass efficiency and byproducts development. Some enzymes are also immobilized with and support to enhance the catalytic activity because of high surface area and reactivity and stability. A different approach can be utilized to immobilize the enzyme with nanomaterials, enzyme covalently bound to the nanofibres through electrospinning and aggregation on the surface of nanotubes by coating. Different carbon structures like graphene oxide, muti-walled carbon nanotubes, oxidized carbon nanotubes, fullerene provide good stability and activity for the enzyme 17,19. The application of nanoparticles in different phases of microalgae cultivation provides an outstanding yield of biomass and biofuel. Some researchers claimed the nano-particle application enhances microalgae biomass up to 20-30% and makes it more cost-effective 60. Some nanoparticles were applied during the culturing of microalgae, where they improved the sunlit conversion efficiency in a photobioreactor. Due to the high growth of algal biomass, the light cannot reach the deep self-shading and biofilm establishment on the photobioreactor surface. The high biomass can be achieve by proper illumination and photoconversion in PBR equipped with the light-emitting diodes (LEDs). Recently, a scale-up algae culture has used Gallium aluminum arsenide (GAA) based on LEDs. When optical fibers are used during the algal culture, they can achieve high efficiency in growth and save additional light costs 60.
Additionally, the amendment of metallic nanoparticles can enhance the surface plasmon resonance and amplify the scattering light at a specific wavelength 61. For example, silver nanoparticles suspension strongly backscattered blue light in plasmonic mini-PBRs. The green microalgae have captured blue light significantly raised the photosynthetic efficiency and biomass. For example, Chlamydomonas reinhardtii, Cyanothece have obtained 30% higher biomass in blue light illumination 62. Carbon sequestration by microalgae cultivation is one of the best strategies to overcome the greenhouse gas effect on the environment. During the microalgae growth, CO2 absorption from the atmosphere boosts biomass growth. In a microalgae culture formation of nanobubbles and persisted for a longer time and made it more floatable biomass into the culture biomass density becomes improved by accumulating CO2, O2. The algal biomass with suspended nano-bubbles acts as an airlift-loop in bioreactor and provides much less energy than microbubbles formation 62,63.
The application of Nano-Al-droplet for biofuel mixtures has proven to be more efficient than other suspension. The nano-additives in liquid have a better action than n-decane-based fuels when used as a suspension in the fuel due to the high viscosity of ethanol inclined to generate a gel around the nanoparticles. In contrast, nano-Al suspension in ethanol has sufficient excellent for a more prolonged period than other nanoparticles when applied 63. For engine high efficiency, nano-droplets covered a monolayer on the mechanical essentials of the machine that were in contact with liquid fuel 60. The nanoparticles Al2O3, Al, CuO, MnO, ZnO and MgO,NPs combined with water–diesel–biodiesel emulsion and bioethanol were worked excellently. Because of mandatory disabling, super-high DTGmax value, more excellent heat of combustion, consistent torque boosting, lowest brake-specific fuel consumption, tiniest size of water droplets, and lowest values of Soot HC, CO, NOx, and Al2O3 performed the best among these NPs 64,65.
Significance of Biofuel
There is a great significance of the deployment of biofuels in the carriage sector. As the expansion in the economy and increment in population has been observed; thus, there is a huge demand for fossil fuels. The high needs of energy and biofuels are being produced as additional fossil fuels 66. Biofuels are more critical for a sustainable ecosystem than fossil fuels since they emit less carbon dioxide into the atmosphere. The use of biofuels instead of fossil fuels helps to reduce greenhouse gas emissions into the atmosphere, which causes climate change 67.
Furthermore, petroleum, which originated from old algae deposits, appears to be a limited resource and seems to become too expensive and ultimately run out 68. Due to these significant factors, there is a great significance in using biofuels instead of fossil fuels to produce energy. Therefore, several types of research have been conducted on different renewable resources to produce energy. The production of biofuels has efficiently replaced the use of fossil fuels for the production of energy 69.
The study of Pandey et al. In 2013 stated that microalgae are a valuable source of biofuels. Microalgae have been revealed to accumulate and synthesize a considerable amount of neutral lipids (i.e., 20 to 50 percent biomass’s dry weight), which is one of the significant advantages. It is further found that microalgae are grown at high rates, another considerable advantage of microalgae 66. The study has further suggested that microalgae can fulfill all-year-round production. It can ensure efficient oil yield for each area of the cultivated microalgae compared to oilseed crops.
In addition to this, less water is required for microalgae production compared to terrestrial crops. Thus, it reduces the need for a large amount of freshwater to cultivate microalgae. Furthermore, it has been observed that microalgae cultivation does not need pesticides or herbicides application 11. Microalgae eliminate CO2 gases and are released from flue gases emitted from fossil fuels. Thus, it can be said that the use of microalgae is playing a significant role in reducing substantial greenhouse gas emissions. It is further found that microalgae cultivation can be conveyed in seawater, saline water on non-arable land 70. Several technologies have been considered renewable energy sources.
Furthermore, no single strategy is being used to provide an effective solution. Thus, it appears that a combination of processes must be incorporated to ensure the dependence of biofuels for the production of energy 71. For instance, a study revealed that Brazil used sugarcane for ethanol production to fulfill their energy needs 72. In addition to this, oils originating from terrestrial plants are also being used for fulfilling the ever-increasing demand for energy. For instance, palm and soy plants are being deployed to be environmentally friendly and sustainable 73,74. However, it is found that these techniques work at a small scale, but these techniques have been increased 25. They require a massive amount of agricultural land that might be used to supplant a considerable proportion of petroleum being used by these strategies.
The use of hybrid technologies for energy generation is illustrated in the report. Two examples of these techniques are gasification of remaining biomass into syngas and cellulose conversion to sugars 75. These strategies can be deployed for the production of liquid fuels. Although these techniques are being used widely to produce fuels, these strategies do not accommodate the ever-increasing demand for liquid fuels 76.
Importance of Algae in Biofuel Production
Several studies have identified that microalgae can provide several advantages compared to the help of terrestrial plants. Jones & Mayfield 35 suggested using microalgae to produce different forms of biofuels. The best example is methane production grown by aerobic digestion originated from micro-algal oil made from bio-hydrogen 75. The Pienkos & Darzins 65 survey stated that microalgae are observed as single-celled organisms duplicated by high throughput technologies deployed for rapidly evolving strains. It has been revealed by the study that the use of microalgae has presented a reduced effect on the environment as compared to biomass’s terrestrial sources for efficient and sustainable production of biofuels 75. The study further highlighted that microalgae could be nurtured over the land, not utilized for agriculture. Additionally, microalgae cultivation does not require a large quantity of water. Hence, it can be said that the production of biofuels from microalgae also results in the reduction of the use of agricultural land and freshwater as compared to the production of biofuels from terrestrial plants 76. However, the study asserted that microalgae's cultivating process could reduce waste streams 77. Major waste streams include public wastewater to remove phosphates and nitrates before discharge. It also releases flue gas from combustible-based power plants or coal for capturing carbon dioxide gases and sulphates. Thus, effective bioengineering techniques can enable algal biofuels to compete against petroleum. It is because these techniques encompass a high potential for producing cost-competitive biofuels.
According to the study of Pittman et al. 22, it is found that the use of algae has received significant attention as the primary basis of biomass being used for the fabrication of renewable energy. The primary characteristic that has led to the adoption of algae for biofuel production is that it can provide the highest biomass yield per unit area and length 78. It also contains high starch and oil content, and it does not require agricultural land for its cultivation. Furthermore, the cultivation process of microalgae does not require freshwater. Therefore, carbon dioxide gases and wastewater can be used to cultivate microalgae. It is revealed that microalgae produce highly efficient energy through biomass conversion compared to other crops 79.
According to Patil et al. 10, microalgae are favored over macroalgae, complicating cultivation. Furthermore, macroalgae are less flexible and provide only one processing method; thus, anaerobic digestion is the best choice for biogas production. Microalgae, on the other hand, was thought to be a sunlight-driven technology that turns CO2 gas into possible plants, biofuels, high-value bioactivities, and feeds 80-81. Microalgae are also discovered to be phototrophic species that require potential growth sunlight and high light conversion efficiency. Furthermore, microalgae can absorb carbon dioxide gas emissions, resulting in less carbon dioxide waste in the atmosphere 82. Algae use carbon dioxide and account for more than 40% of global C- fixation, with marine microalgae accounting for most productivity 83. According to Singh & Olsen 8, microalgal biomass contains 50% carbon by dry weight.
It's also been discovered that algae produce a lot of biomass in a short amount of time. This is due to the fact that algae do not need cellulose for their roots, leaves, or branches. As a result, the growth rate of microalgae is much superior to that of terrestrial crops 84. Algae has a rapid growth rate, high lipid content, and the potential to breed fast, competitive strains and produce co-products, all of which make it a viable addition to environmental energy production techniques 85,86. On the other hand, Macroalgae contain several hydrocarbons, lipids, and other complex oils 87. For biodiesel production from the microalgae, simple steps involve selecting the collection of algae and extracting and converting biodiesel-required nanocatalysts for more advancement and high yield biodiesel production.
Conclusion
From the entire discussion, it is spotted that biofuels are being used extensively to produce energy to contribute to a friendly and sustainable environment. Biofuels as an energy source will play a substantial role in fulfilling the ever-increasing need for fuel in an eco-friendly and sustainable way. The most effective technique found for biofuel production is the use of microalgae. It is well known that microalgae are produced at a high rate of biomass in adverse conditions. Also, microalgae cultivation is not necessitating freshwater and fertile acreage. Hence, microalgae are found to be more effective as compared to other traditional terrestrial crops.
Acknowledgment
The authors greatly acknowledge to the Center of Excellence of Environmental Studies, King Abdulaziz University, Jeddah and Institutional.
Funding Source
Funding Program for the Research and a development grant number "IFPRP: 343-188-1442" under the umbrella of King Abdulaziz University Jeddah.
Conflict of Interest
The authors do not have any conflict of interest.
References
- Blanco?Canqui, H. (2010). Energy crops and their implications on soil and environment. Agronomy journal, 102(2), 403-419.
- Moustakas, K., Loizidou, M., Rehan, M. and Nizami, A.S., 2020. A review of recent developments in renewable and sustainable energy systems: Key challenges and future perspective (2020): 109418.
- Borowitzka, M.A. and Moheimani, N.R., 2013. Sustainable biofuels from algae. Mitigation and Adaptation Strategies for Global Change, 18(1), pp.13-25.
- Gaurav, N., Sivasankari, S., Kiran, G.S., Ninawe, A. and Selvin, J., 2017. Utilization of bioresources for sustainable biofuels: a review. Renewable and Sustainable Energy Reviews, 73, pp.205-214.
- Gouveia, L. and Oliveira, A.C., 2009. Microalgae as a raw material for biofuels production. Journal of industrial microbiology and biotechnology, 36(2), pp.269-274.
- McCollum, D.L., Zhou, W., Bertram, C., De Boer, H.S., Bosetti, V., Busch, S., Després, J., Drouet, L., Emmerling, J., Fay, M. and Fricko, O., 2018. Energy investment needs for fulfilling the Paris Agreement and achieving the Sustainable Development Goals. Nature Energy, 3(7), pp.589-599.
- Hannon, M., Gimpel, J., Tran, M., Rasala, B., and Mayfield, S., 2010. Biofuels from algae: challenges and potential. Biofuels, 1(5), pp.763-784.
- Singh, A. and Olsen, S.I., 2011. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Applied Energy, 88(10), pp.3548-3555.
- Liu, Y., Cruz-Morales, P., Zargar, A., Belcher, M.S., Pang, B., Englund, E., Dan, Q., Yin, K. and Keasling, J.D., 2021. Biofuels for a sustainable future. Cell.
- Patil, V., Tran, K.Q. and Giselrød, H.R., 2008. Towards sustainable production of biofuels from microalgae. International journal of molecular sciences, 9(7), pp.1188-1195.
- Diltz, R. & Pullammanappallil, P. (2013) Biofuels from Algae, I.N.T.E.C.H. Open Access Publisher
- Verma, M., Godbout, S., Brar, S.K., Solomatnikova, O., Lemay, S.P. and Larouche, J.P., 2012. Biofuels production from biomass by thermochemical conversion technologies. International Journal of Chemical Engineering, 2012.
- Trindade, S. C. (2011). Nanotech biofuels and fuel additives. Biofuel’s Engineering Process Technology. Rijeka, Croatia: InTech, 103-114.
- Nizami A, Rehan M. Towards nanotechnology-based biofuel industry.
Biofuel Res J. 2018;18:798–9.
- Palaniappan K. An overview of applications of nanotechnology in biofuel production. World Appl Sci J. 2017;35:1305–11.
- Hasannuddin AK, Yahya WJ, Sarah S, Ithnin AM, Syahrullail S, Sidik NAC, et al. Nano-additives incorporated water in diesel emulsion fuel: fuel properties, performance and emission characteristics assessment. Energy Convers Manag. 2018;169:291–314.
- Safarik I, Prochazkova G, Pospiskova K, Branyik T. Magnetically modified microalgae and their applications. Crit Rev Biotechnol. 2016;36:931–41
- Karthikeyan S, Prathima A. Microalgae biofuel with CeO2 nano additives as an eco-friendly fuel for CI engine. Energy Source Part A. 2017;39:1332–8.
- Kim J, Jia H, Wang P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol Adv. 2006;24:296–308
- Pattanaik, B.P. and Misra, R.D., 2017. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: A review. Renewable and Sustainable Energy Reviews, 73, pp.545-557.
- Hossain, A.B.M.S., A. Salleh, A., Nasrulhaq Boyce, A., Chowdhury, P. Naqiuddin M. (2008). Biodiesel fuel production from algae as renewable energy. American Journal of Biochemistry and Biotechnology 4 (3):250-254.
- Pittman, J.K., Dean, A.P. and Osundeko, O., 2011. The potential of sustainable algal biofuel production using wastewater resources. Bioresource technology, 102(1), pp.17-25.
- Antizar?Ladislao, B. and Turrion?Gomez, J.L., 2008. Second?generation biofuels and local bioenergy systems. Biofuels, Bioproducts and Biorefining: Innovation for a sustainable economy, 2(5), pp.455-469.
- Dragone, G., Fernandes, B.D., Vicente, A.A. and Teixeira, J.A., 2010. Third generation biofuels from microalgae.
- Charles, M.B., Ryan, R., Ryan, N. and Oloruntoba, R., 2007. Public policy and biofuels: The way forward?. Energy policy, 35(11), pp.5737-5746.
- Nigam, P.S. and Singh, A., 2011. Production of liquid biofuels from renewable resources. Progress in energy and combustion science, 37(1), pp.52-68.
- Von Blottnitz, H. and Curran, M.A., 2007. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. Journal of cleaner production, 15(7), pp.607-619.
- Naik, S.N., Goud, V.V., Rout, P.K. and Dalai, A.K., 2010. Production of first and second generation biofuels: a comprehensive review. Renewable and sustainable energy reviews, 14(2), pp.578-597.
- Antizar?Ladislao, B. and Turrion?Gomez, J.L., 2008. Second?generation biofuels and local bioenergy systems. Biofuels, Bioproducts and Biorefining: Innovation for a sustainable economy, 2(5), pp.455-469.
- Holladay, J.E., White, J.F., Bozell, J.J. and Johnson, D., 2007. Top value-added chemicals from biomass-Volume II—Results of screening for potential candidates from biorefinery lignin (No. PNNL-16983). Pacific Northwest National Lab.(PNNL), Richland, WA (United States).
- Hannon, M., Gimpel, J., Tran, M., Rasala, B. and Mayfield, S., 2010. Biofuels from algae: challenges and potential. Biofuels, 1(5), pp.763-784.
- Brennan, L. and Owende, P., 2010. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renewable and sustainable energy reviews, 14(2), pp.557-577.
- Adesanya, V.O., Davey, M.P., Scott, S.A. and Smith, A.G., 2014. Kinetic modelling of growth and storage molecule production in microalgae under mixotrophic and autotrophic conditions. Bioresource technology, 157, pp.293-304.
- Thomas, D.M., Mechery, J. and Paulose, S.V., 2016. Carbon dioxide capture strategies from flue gas using microalgae: a review. Environmental Science and Pollution Research, 23(17), pp.16926-16940.
- Jones, C. S. & Mayfield, S. P. (2012) Algae biofuels: versatility for the future of bioenergy, Current opinion in biotechnology, 23(3):346-351.
- Zhou, X., Jin, W., Wang, Q., Guo, S., Tu, R., Han, S. F., ... & Wang, Q. (2020). Enhancement of productivity of Chlorella pyrenoidosa lipids for biodiesel using co-culture with ammonia-oxidizing bacteria in municipal wastewater. Renewable Energy, 151, 598-603.
- Illman, A. M., Scragg, A. H., & Shales, S. W. (2000). Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and microbial technology, 27(8), 631-635.
- Scragg, A. H., Illman, A. M., Carden, A., & Shales, S. W. (2002). Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass and Bioenergy, 23(1), 67-73.
- Cheng, Y., Zhou, W., Gao, C., Lan, K., Gao, Y., & Wu, Q. (2009). Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology, 84(5), 777-781.
- Xiong, W., Li, X., Xiang, J., & Wu, Q. (2008). High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Applied microbiology and biotechnology, 78(1), 29-36.
- Xu, H., Miao, X., & Wu, Q. (2006). High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. Journal of biotechnology, 126(4), 499-507.
- Zhang, W., Hong, W. U., & Zong, M. H. (1992). Study on microbial oil production with Chlorella pyrenoidosa. Microbiology, (06). Microbiology/Weishengwuxue Tongbao, 35 (2008), pp. 855-860.
- Shen, Y., Yuan, W., Pei, Z., & Mao, E. (2010). Heterotrophic culture of Chlorella protothecoides in various nitrogen sources for lipid production. Applied Biochemistry and Biotechnology, 160(6), 1674-1684.
- Kalacheva, G. S., Zhila, N. O., & Volova, T. G. (2002). Lipid and hydrocarbon compositions of a collection strain and a wild sample of the green microalga Botryococcus. Aquatic Ecology, 36(2), 317-331.
- Chiu, S. Y., Kao, C. Y., Tsai, M. T., Ong, S. C., Chen, C. H., & Lin, C. S. (2009). Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresource technology, 100(2), 833-838.
- Rodolfi, L., Chini Zittelli, G., Bassi, N., Padovani, G., Biondi, N., Bonini, G., & Tredici, M. R. (2009). Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low?cost photobioreactor. Biotechnology and bioengineering, 102(1), 100-112.
- Pruvost, J., Van Vooren, G., Cogne, G., & Legrand, J. (2009). Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresource technology, 100(23), 5988-5995.
- Chin, H. J., Shen, T. F., Su, H. P., & Ding, S. T. (2006). Schizochytrium limacinum SR-21 as a source of docosahexaenoic acid: optimal growth and use as a dietary supplement for laying hens. Australian journal of agricultural research, 57(1), 13-20.
- Mandal, S., & Mallick, N. (2009). Microalga Scenedesmus obliquus as a potential source for biodiesel production. Applied microbiology and biotechnology, 84(2), 281-291.
- Demirbasa., Demirbasm.F. 2011. Importanceofalgae oilasa sourceofbiodiesel. Energy Convers.and Manage., 52: 163–170
- Molina-Grima E., Belarbi E.H., Acien Fernandez F.G., Robles-Medina A., chisti Y. 2003. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv., 20: 491–515.
- D?bowski, M., Zieli?ski, M., Krzemieniewski, M., Dudek, M. and Grala, A., 2012. Microalgae–cultivation methods. Polish Journal of Natural Sciences, 27(2), pp.151-164.
- Racault, Y. and Boutin, C., 2005. Waste stabilisation ponds in France: state of the art and recent trends. Water Science and Technology, 51(12), pp.1-9.
- Mara D., Pearson H. 1998. Design manual for waste stabilization ponds in mediterranean countries. Lagoon Technol. Int., Leeds, England.
- Tredici M.R., Zittelli G.C. 1998. Efficiency of sunlight utilization: tubular versus flat photobioreactor. Biotechnol. Bioeng., 57: 187–197.
- Borowitzka M.A. 1999. Commercial production of microalgae: ponds, tanks, tubes and fermenters. J. Biotechnol., 70: 313–321.
- Amin S. 2009. Review on biofuel oil and gas production processes from microalgae. Energy Convers. Manage., 50: 1834–1840.
- Baynes S.M., Emerson L., SCOTT A.P. 1979. Production of algae for use in the rearing of larval fish. Fish. Res. Tech. Report., 53: 13–18.
- Borowitzka M.A. 1999. Commercial production of microalgae: ponds, tanks, tubes and fermenters. J. Biotechnol., 70: 313–321.
- Pattarkine MV, Pattarkine VM. Nanotechnology for algal biofuels. In: Gordon R, Seckbach J, editors. The science of algal fuels. Berlin: Springer; 2012. p. 149–60.
- Steele JM, Grady NK, Nordlander P, Halas NJ. Plasmon hybridization in complex nanostructures. In: Brongersma ML, Kik PG, editors. Surface plasmon nanophotonics. Dordrecht: Springer; 2007. p. 183–96.
- Torkamani S, Wani SN, Tang YJ, Sureshkumar R. Plasmon-enhanced microalgal growth in mini photobioreactors. Appl Phys Lett. 2010;97:1–4.
- Zimmerman WB, Hewakandamby BN, Tesa? V, Bandulasena HCH, Omotowa OA. On the design and simulation of an airlift loop bioreactor with microbubble generation by fluidic oscillation. Food Bioprod Process. 2009;87:215–27.
- Zimmerman WB, Tesa? V, Bandulasena H. Towards energy efficient nanobubble generation with fluidic oscillation. Curr Opin Colloid Interface Sci. 2011;16:350–6.
- Gan Y, Qiao L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust Flame. 2011;158:354
- Pienkos, P.T. and Darzins, A.L., 2009. The promise and challenges of microalgal?derived biofuels. Biofuels, Bioproducts and Biorefining: Innovation for a sustainable economy, 3(4), pp.431-440.
- Pandey, A., Lee, D. J., Chang, J. S., Chisti, Y., & Soccol, C. R. (Eds.). (2018). Biomass, biofuels, biochemicals: biofuels from algae. Elsevier.
- Traverse, A., 1955. Occurrence of the oil-forming alga Botryococcus in lignites and other Tertiary sediments. Micropaleontology, 1(4), pp.343-348.
- Borowitzka, M.A. and Moheimani, N.R. eds., 2013. Algae for biofuels and energy (Vol. 5, pp. 133-152). Dordrecht: Springer.
- National Research Council. (2013). Sustainable development of algal biofuels in the United States. National Academies Press.
- Chisti, Y. & Yan, J. (2011) Energy from algae: Current status and future trends: Algal biofuels–A status report, Applied Energy, 88:3277-3279.
- Filoso, S., do Carmo, J.B., Mardegan, S.F., Lins, S.R.M., Gomes, T.F. and Martinelli, L.A., 2015. Reassessing the environmental impacts of sugarcane ethanol production in Brazil to help meet sustainability goals. Renewable and Sustainable Energy Reviews, 52, pp.1847-1856.
- Lam, M.K. and Lee, K.T., 2011. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): win–win strategies toward better environmental protection. Biotechnology Advances, 29(1), pp.124-141.
- Basiron, Y., 2007. Palm oil production through sustainable plantations. European Journal of Lipid Science and Technology, 109(4), pp.289-295.
- National Research Council. (2013). Sustainable development of algal biofuels in the United States. National Academies Press.
- Costa, J.A.V. and De Morais, M.G., 2011. The role of biochemical engineering in the production of biofuels from microalgae. Bioresource technology, 102(1), pp.2-9.
- Lam, M.K., Yusoff, M.I., Uemura, Y., Lim, J.W., Khoo, C.G., Lee, K.T. and Ong, H.C., 2017. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renewable energy, 103, pp.197-207.
- Scott, S.A., Davey, M.P., Dennis, J.S., Horst, I., Howe, C.J., Lea-Smith, D.J. and Smith, A.G., 2010. Biodiesel from algae: challenges and prospects. Current opinion in biotechnology, 21(3), pp.277-286.
- Klassen, V., Blifernez-Klassen, O., Wibberg, D., Winkler, A., Kalinowski, J., Posten, C. and Kruse, O., 2017. Highly efficient methane generation from untreated microalgae biomass. Biotechnology for biofuels, 10(1), pp.1-12.
- Li, X., Sun, H., Mao, X., Lao, Y. and Chen, F., 2020. Enhanced photosynthesis of carotenoids in microalgae driven by light-harvesting gold nanoparticles. A.C.S. Sustainable Chemistry & Engineering, 8(20), pp.7600-7608.
- Qari, H.A. and Oves, M., 2020. Fatty acid synthesis by Chlamydomonas reinhardtii in phosphorus limitation. Journal of bioenergetics and biomembranes, 52(1), pp.27-38.
- Moreira, D. and Pires, J.C., 2016. Atmospheric CO2 capture by algae: negative carbon dioxide emission path. Bioresource technology, 215, pp.371-379.
- Sayre, R., 2010. Microalgae: the potential for carbon capture. Bioscience, 60(9), pp.722-727.
- Dismukes, G.C., Carrieri, D., Bennette, N., Ananyev, G.M. and Posewitz, M.C., 2008. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Current opinion in biotechnology, 19(3), pp.235-240.
- Wahidin, S., Idris, A. and Shaleh, S.R.M., 2013. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresource technology, 129, pp.7-11.
- Georgianna, D.R. and Mayfield, S.P., 2012. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature, 488(7411), pp.329-335.
- Masri, M.A., Jurkowski, W., Shaigani, P., Haack, M., Mehlmer, N. and Brück, T., 2018. A waste-free, microbial oil centered cyclic bio-refinery approach based on flexible macroalgae biomass. Applied Energy, 224, pp.1-12.







