Groundwater Chemistry at Deep Aquifer in Koyra: Khulna, Bangladesh
DOI: http://dx.doi.org/10.12944/CWE.16.2.12
Copy the following to cite this article:
Das T. K, Shaibur M. R, Rahman M. M. Groundwater Chemistry at Deep Aquifer in Koyra: Khulna, Bangladesh. Curr World Environ 2021;16(2). DOI:http://dx.doi.org/10.12944/CWE.16.2.12
Copy the following to cite this URL:
Das T. K, Shaibur M. R, Rahman M. M. Groundwater Chemistry at Deep Aquifer in Koyra: Khulna, Bangladesh. Curr World Environ 2021;16(2). Available From : https://bit.ly/3uiJYo3
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 17-09-2020 |
|---|---|
| Accepted: | 24-04-2021 |
| Reviewed by: | 
 Chadetrik Rout
Chadetrik Rout
|
| Second Review by: |

 Babak Vaheddost
Babak Vaheddost
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Water is the foundation of life on globe. The water quality is the key to all of the roles that water plays in human body or their natural surroundings. Water is vital for human life, ecological functioning, socio-economic evolution, food supply and also poverty lessening.1 The superiority of accessible water varies spatially. The varying geological and climatic factors determine the provincial differences in water individuality.2 Depending on weather, the quality of accessible water varies to a great extent whether it may comes from aquifers or exterior water bodies (ponds and rivers).3 Both the excellence and extent of water in Bangladesh is of great apprehension due to its natural settings and human population. Bangladesh is a low-lying deltaic country with large residents, exposed to a variety of environmental pressures and usual disasters. These pressures are exacerbating the complexity of obtaining drinking water.4 However; the quality of available water may amplify the degree of water uncertainty.
Twenty out of 64 Districts in Bangladeshi are characterized as coastal District (covers approximately 47,201 km2).5 The coastal people of Bangladesh essentially employ exterior and groundwater for drinking, irrigation, bathing and household purposes.6, 7 In coastal area of Bangladesh, usual water sources such as rivers and groundwater are habitually polluted by salts and other metal ions due to salinity intrusion from the Bay of Bengal.8, 9 Exterior water in the coastal part is subjected to seawater intrusion due to unbroken pressure of high and low tides and the salinity is mounting with time due to effects of climate alteration.9 Throughout the monsoon, salinity of exterior water decreases but in other times salinity remains high depending on the geology of the area. In the past two decades, shrimp culture has been practiced to the highest degree which tainted the local landscape and pessimistically affecting exterior and groundwater resources.8
Groundwater is the central resource and must meet growing household, agricultural and industrial requirements. The weakening of water quality is directly interrelated to human health has exacerbated the global groundwater crises.9 Groundwater quality is unevenly distributed and it is extensively depended on position, lithology, renew water quality and environmental factors. The factors determine the quality of groundwater are the geological setting, characteristics of source rock, composition of invigorated water, soil formation and extent of water been trapped in underground.7 Throughout Bangladesh, groundwater is far and wide used as drinking water. In coastal areas, the largest part of the groundwater used for water supply is pumped from a depth of 150 m, but nearly all of it is brine.9, 10 In coastal Bangladesh, lots of shallow aquifers have high salinity due to seawater intrution.9 The profound aquifer of coastal area of Bangladesh has been tainted by salinization due to entrapped seawater. The half of the profound aquifer of the Northern side of Bangladesh is also secure for irrigation as well as for drinking purpose in respect of SAR, SP, EC, RSC, arsenic and physico-chemical characteristics of water.11
Due to diversified geographic locations of Bangladesh, the groundwater quality of all aquifers is not appropriate for drinking and irrigation purpose. Since each and every location of groundwater are not tainted with saline water, it is therefore necessary to make out likely aquifers which can supply water as much as needed to meet up the demand of local community. Some parts of Bangladesh are contaminated with arsenic and some parts with salinity. The Southern parts of Bangladesh (Khulna and Satkhira Districts) are contaminated with saline water which ultimately responsible for the scarcity of drinking water (Abedin et al., 2014).4 The pond water of Gabura and DTW water of Gabura and Burigualini Unions was saline and was not appropriate for drinking. However, pond water of Buri Goalinin Union was not saline (Shaibur et al., 2019).9 Salinity causes due to intrusion of saline water both in surface and groundwater. The groundwater of the South Central costal region (Barguna and Patuakhli Districts) of Bangladesh was affected by seawater intrusion. The higher EC (>5,000 µS cm-1) and TDS (>4,500 ppm) clearly showed that the groundwater was not suitable for drinking, irrigation and domestic purposes (Islam et al., 2016).7 The literature showed that the salinity of groundwater in the Southern part of Bangladesh was determined, but there was hardly or almost none about the water quality of Koyra (Khulna). Therefore, the present research was conducted. The aims of the investigation were to uncover the physico-chemical properties of deep aquifer water and to identify the convenient water sources for drinking and irrigational use. Additionally, the governing species of the water quality and the sources of the mineral elements were also calculated.
Materials and Methods
Study Area
The study was regulated at Dakshin Bedkashi Union of Koyra Upazila, Khulna, Bangladesh. Total population in Dakshin Bedkashi was 16,755 with 3,881 households.12 The Union is encircled by Shamnagar Upazila on the West, Sundarban Mangrove forest on the North, East and South. The natives are predominantly involved with agriculture and aquaculture and rice is cultivated in a single season.13 There are mammoth numbers of planted trees in this village.13 This region receives a total of 2,500 mm rainfall in monsoon (June to October). The winter season (October to March) was very cold and dry, followed by a hot summer from March to June. At the end of summer (April and May), the tidal river showed high salinity.14 People in this area drink DTW water.12
Water Sample Collection
About 30 water samples were collected randomly in 1.0 L potable cleaned plastic bottles from DTW in December 2016. The bottles were prewashed with acidic water, rinsed with distilled water and were oven dried for 24 h. The bottles were again rinsed properly with sample water to avoid probable contamination. The depth of all tube well varied from 550 to 700 feet and the tube well were pumped exact times with respect to the depth of the tube well in feet (i.e. a 500 feet tube well was primarily pumped for 500 times) and thereafter water samples were collected. The physiographic positions of the sampling points were marked in Fig.1.
 |
Figure 1: Sample Location of Sampling Point along the Study Area. Click here to view Figure |
Water Sample Analysis
The temperature, EC, pH, TDS and salinity of the samples were measured at the source point. The temperature and pH were determined by using a Microprocessor pH meter (model- HANNA instrument pH 211). The EC and TDS were deliberated by using an EC/TDS/Temperature Tester (HANNA; HI 98312 model; IP57 waterproof; Mauritius). The salinity was measured by using multimeter analyzer (Sense Ion 156, HACH, USA). The Na+ and K+ were measured by using flame photometer (flame photometer-PEP7; London, UK). The Ca2+, Mg2+and HCO3- were analyzed by using titration colorimetric method. The NO3-, PO4-, and SO42-were analyzed by using Turbidimetric method with Spectrophotometer (model- UV-visible spectrophotometer, helios 949923045811)15 and Cl- by using Argenometric method.
Data Analysis
The data were evaluated by MS Excel 2017, SPSS version 20 and AquaCham Version 4. Hydro-chemical arrangement and groundwater appraisal were discussed by using carbonate Vs silicate weathering, PCA, Gibbs diagram, Piper diagram and Wilcox diagram. The obtained results were compared with WHO, USEPA and BBS drinking water quality standards to evaluate drinking water quality. Gibbs diagrams are widely used to establish the rapport between water content and lithological uniqueness of aquifers.16 Gibbs ratio I (for anions) = Cl-/(Cl- + HCO3); Gibbs ratio II (for cations) = Na+ / (Na++ Ca+2), where all ion concentrations were expressed in meq L-1. The Wilcox chart represents the suitability of water for irrigation and domestic use.17 Samples were collected with 3 replications.
Results and Discussion
General Hydro-Geochemistry
The temperature variation of groundwater samples ranged from 29 ºC to 31 ºC (results were not shown). Most of the samples were monochrome, but few samples exhibited brownish color.18, 19 The brownish color was possibly due to dissolve silicate contents.19, 20 The collected samples were turbid in nature. The turbidity of groundwater (results were not shown) was possibly due to dissolve solids and silicates.20
The EC value varied from 383.3 to 3,317 µS cm-1 with the middling value of 1,400.91 µS cm-1 (Table 1). The WHO suggested value of drinking water is 750.0 µS cm-1 and BBS suggested value is 300 to 1,500 µS cm-1 (Table 1). Considering the middling standard value the DTW water could be consumed. But the true fact was that the EC values of some samples were to a large extent which needs to be clogged without delay for drinking. Again, the DoE suggested value of EC for irrigation is 2,250.0 µS cm-1 and the FAO suggested value is 700.0 to 3,000.0 µS cm-1. 9 Bearing in mind the above facts, the deep tube well water could be applied for irrigation purpose. But care must be taken as continuous application of this type of water may develop soil EC which is damaging for crop production. The EC value in DTW water was reported to be 1,330.0 to 7,790.0 µS cm-1 in coastal Unions of Buri Goalini and Gabura, Shyamnagar, Satkhira, Bangladesh.9
Table 1: Comparison of Drinking Water Quality with the Recommended Values of WHO (1984)45, USEPA (1992)46 and BSS (2009)47. The table contained minimum, maximum and mean value of determined parameter.
|
Parameters |
Range |
Mean (± SD) |
Standards |
||||||
|
WHO |
USEPA |
BBS |
|||||||
|
General hydro-geochemistry |
|||||||||
|
EC (µS/cm) |
383.3 – 3,317.0 |
1,400.91 (±904.18) |
750 |
* |
300-1,500 |
||||
|
pH |
6.73 - 8.03 |
7.33 (± 0.35) |
6.5-8.5 |
6.5-8.5 |
6.5-8.5 |
||||
|
TDS (ppm) |
230.5 – 2,052.0 |
841.23 (±546.51) |
1,000 |
1,000 |
1,000 |
||||
|
Salinity (ppt) |
0.20 - 1.60 |
0.65 (± 0.43) |
* |
* |
* |
||||
|
Major cation chemistry |
|||||||||
|
Na+ (ppm) |
130.29 - 930.78 |
308.37 (±192.42) |
200 |
* |
200 |
||||
|
K+(ppm) |
6.15 - 27.59 |
10.41 (± 4.92) |
30 |
* |
* |
||||
|
Ca2+(ppm) |
8.0 – 146.0 |
60 (± 45.03) |
100 |
* |
75 |
||||
|
Mg2+(ppm) |
1.20 - 69.60 |
22.24 (± 20.68) |
150 |
* |
30-35 |
||||
|
Major anion chemistry |
|||||||||
|
HCO3- (ppm) |
280.6 - 841.8 |
429.64 (±115.24) |
* |
* |
* |
||||
|
NO3- (ppm) |
0.32- 4.64 |
1.43 (± 1.12) |
50 |
* |
10 |
||||
|
PO43- (ppm) |
0.006 - 3.69 |
0.61 (± 1.01) |
* |
10 |
6 |
||||
|
SO42- (ppm) |
2.24 - 85.16 |
9.62 (± 14.40) |
400 |
250 |
400 |
||||
|
Cl- (ppm) |
20.21 - 939.43 |
331.06 (±282.03) |
250 |
250 |
600 |
||||
The pH of the water samples were in the tolerable boundary of WHO and USEPA drinking water quality standards, which ranged from 6.73 to 8.03 where the typical value was 7.33 (± 0.35; Table 1). Thus, the water could be used for drinking purpose and without doubt for irrigation. The conclusion of this study is comparable with the study of Buri Goalini and Gabura Unions of Shyamnagar Upazila, Bangladesh, where the groundwater is acidic to minor alkaline in nature.9 The pH ranged from 7.07 to 8.01 in the DTW water of those samples. 9 A comparable discovery was also reported for Shyamnagar Upazila. The pH ranged from 7.23 to 8.01 in the DTW water samples with the represent value of 7.69. 21 The other reports demonstrated that the pH of tube well water was from 6.53 to 6.66 in the salinity affected South-west coastal region of Bangladesh.22 The pH in soil samples in coastal Gabura Union wide-ranging from 6.5 to 7.20, but in Buri Goalini Union the values varied from 6.70 to 7.50. 23 The pH of groundwater in South-central part ranged from 6.38 to 7.35, which was comparable to current study.24
Salinity of the samples varied from 0.20 to 1.60 ppt with the middling value of 0.60 ppt (Table 1). The salinity level ~ 0.50 ppt is considered as fresh water. The DoE and FAO suggested value for irrigation water is ~ 2.00 ppt.9 For that reason, the sampled water could be applied for irrigation. Again, long term impacts of using brackish water needs to be considered gravely.
The TDS in groundwater samples ranged from 230.5 to 2,052.0 ppm with a representative value of 841.23 (±546.51; Table 1). Most of the groundwater samples were suitable for drinking, because TDS concentration of nearly every one of the samples did not exceed the WHO, USEPA and BBS drinking water excellence standards. The TDS < 1,000.0 ppm is believed to be as fresh and the TDS 1,000.0 to 3,000.0 ppm is fresh to brackish, 3,000.0 to 5,000.0 ppm is brackish, 5,000.0 to 35,000 ppm is saline water and ˃ 35,000 ppm is hyper saline in nature.25, 26 About 21 samples out of 30 samples were in the class of fresh water and 9 samples were in the class of fresh to brackish water. But the brackish water could be used for irrigation purpose. The farmers need to be alert, because salt ions could be accumulated in soil after long period use of fresh to brackish water. The TDS must be considered in the result of water excellence, because many toxic solid substances may be embedded in the water, which may cause harm. The TDS in DTW water in coastal Union Gabura and Buri Goalini Unions in the coastal Shyamnagar Upazila wide-ranging from 665.0 to 3,900.0 ppm. 9 A lately published paper demonstrated that the TDS of DTW water samples of 6 Unions of Shymnagar Upazil (Satkhira, Khulna Division, Bangladesh) varied from 161.0 to 2,929.0 ppm with the middling value of 905.88 ppm.21 On the contrary, the TDS of beel water connected with tidal river in Keshabpur Upazil (Jashore) is moderately higher and the values ranged from 1,780.0 to 9,390.0 ppm.26 The TDS of water samples of Nolamara Beel (Narail, Bangladesh) was reported to be 2,105.6 to 2,924.9.27
Major Cation Chemistry
The combination of Na+, K+, Ca2+ and Mg2+ ranged from 130.29 to 930.78 ppm, 6.15 to 27.59 ppm, 8.0 to 146.0 ppm and 1.2 to 69.6 ppm, respectively with mean values of 308.37 (±192.42) ppm, 10.41 (± 4.92) ppm, 60.0 (± 45.03) ppm and 22.24 (± 20.68) ppm, respectively. The results were just about comparable to a different study conducted at adjacent coastal Upazila, Shyamnagar (Satkhira, Bangladesh).9, 21 The elevated concentration of Na+, Ca2+ and Mg2+ in groundwater was mainly caused by clay minerals such as montmorillonite, illite and chlorite.28 In most cases, the core cations (Na+, K+, Ca2+ and Mg2+) go beyond the WHO drinking water quality standard. Among the 30 samples, 21 exceeded the standards limit of Na+, 5 K+, 7 Ca2+ and 9 Mg2+. Sodium is one of the indispensable ions for human strength but elevated intake may cause congenial heart disease e.g. - hypertension, kidney diseases and nervous disorder. High salt content in drinking water can make the water turbid and taste less.7, 9 It was establish that nearly all of the water samples contained elevated concentration of Na+ ions. For that reason the water should not be used for drinking purpose considering Na+. However, the DTW water could be used for irrigation but care should be taken as extended time application of groundwater may increase Na+ concentration in the farming soils.
The Ca2+ and Mg2+ ions present in groundwater might enter from the leaching of limestone, dolomite, gypsum and anhydrous gypsum. Furthermore, Ca2+ ion might come from cation substitute process.28 Compared with Ca2+, the concentration of Mg2+ in groundwater samples is somewhat far above the ground, and both minerals are caused by weathering and leaching of dolomite (Ca, Mg)CO3 + CO2 + H2O = 2HCO3- + Ca+2 + Mg+ .
Again, in HCO3-Vs Na+ disperse diagram29, the largest part of the samples were below the contour, which indicated that silicate weathering was the foremost process of releasing Na + ions in groundwater (Fig. 2a). In addition to silicate weathering, carbonate weathering might be a supplier to those ions in groundwater. The probable origin of Na+ in groundwater was a good number probably caused by dissolution of rock salt and weathering of Na+ bearing minerals. The (Ca+2+ Mg+2) Vs (SO4- + HCO3-) dispersed diagram30 represented that nearly all of the samples were below contour, which proved that most of the Ca2+ and Mg2+ in groundwater originated from silicate weathering (Fig. 2b). Compared with the Na+ ion in groundwater, the increase in HCO3- concentration indicated that the silicate weathering progression was governing. The lofty concentration of HCO3- can maintain it well.
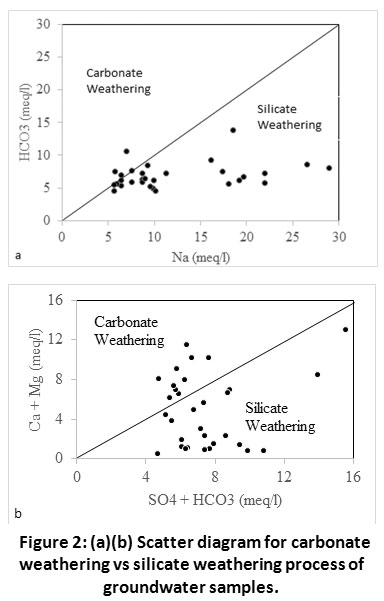 |
Figure 2: (a)(b) Scatter Diagram for Carbonate Weathering vs Silicate Weathering Process of Groundwater Samples. Click here to view Figure |
Major Anion Chemistry
The concentration of HCO3- in groundwater sample varied from 280.6 to 841.8 ppm (Table 1). The HCO3- was one of the dominating anions in the study area. The concentration of CO32- and HCO3- in groundwater might come from carbonate weathering and the dissolution of carbonic acid in aquifers.31 The dissolution process could be offered by the equation of CaCO3 + CO2 + H2O = Ca2+ + 2HCO3- and CO2 + H2O = H+ + HCO3-. 31 The accessibility of CO32-minerals in the aquifer and silicate weathering might be liable for mounting HCO3- in the groundwater.
The Cl- concentration in groundwater ranged from 20.20 to 939.42 ppm (Table 1). About 17 samples out of 30 exceeded the principles limit for Cl- (WHO and USEPA). Natural processes such as weathering, salt dissolution and irrigation drainage backflow may be the cause of the Cl- content in groundwater, which is supported by Cl-/HCO3- ratio of 0.4 to 3.0.32 The continuation of Cl- in drinking water is advised as one of the central causes of salinity. The lofty concentration of Cl- in drinking water can make it inapt for drinking.
The concentration of SO42- ranged from 2.24 to 85.16 ppm (Table 1). The SO42- in groundwater might come from weathering of sulfate minerals and sedimentary rocks containing gypsum.31 The SO42- concentration in all water samples were within WHO, USEPA and BBS drinking water excellence values. The PO43- concentration along study area ranged from 0.0059 to 3.69 ppm with the common concentration of 0.61 ppm (± 1.01). The domino effect indicated that the stage did not go beyond the permissible limit prescribed by WHO, USEPA and BSS. The pretty low concentration of PO43- was reported in the different sources e.g. profound tube well water, pond water and pond sand filter water.9
The NO3- consolidation in groundwater samples varied from 0.32 to 4.64 ppm (Table 1). The WHO approved concentration of NO3- in drinking water is 50.0 ppm and the BBS permissible concentration of NO3- is 10.0 ppm.9, 19 Considering the both values mentioned, the investigational samples were incredibly fine for drinking purpose. The NO3- concentration in DTW water varied from 2.50 to 4.60 ppm in Gabura and Buri Goalini Unions of Shyamnagar Upazila of the coastal District Satkhira.9 It is also reported that the NO3- concentration in the water of Beel Khuksia varied from 9.50 to 10.21 ppm.26 It is believed that the existence of lower combination of NO3- in the drinking water is good. Conversely, consumption of lofty NO3- water can cause blue babies or methemoglobinemia, stomach cancer, abnormal pain, central nervous system, birth defects and diabetes.34 The juvenile coal deposit is universally found in all the coastal zone of Bangladesh, which might be the apparent source of NO3- in groundwater samples. The lower concentration of NO3- in groundwater water samples used for drinking purpose were recently reported for Jashore University of Science and Technology Campus, Bangladesh.35
Correlation Matrix and Factor Analysis
The correlation matrix of physico-chemical parameters of groundwater samples was shown in Table 2. The PCA results showed that three foremost element have eigen value >1 and it explained about 82.718% of total variance of the data. The PC1 explain about 46.99% of entire variance, while PC2 and PC3 explained about 26.88% and 8.84%, respectively. The component loading >.6 is considered as momentous and used for progress interpolation.36 The PC1 explained about 46.99% of total variance and have a encouraging loading of salinity, EC, TDS, K+, Ca2+, Mg2+, Cl-, HCO3-. The lofty loading factors of TDS and EC were due to occurrence of dissolve ions in groundwater. The foremost ions like K+, Ca2+, Mg2+ and Cl- might be linked with hydro-chemical variables from mineral dissolution and water-rock interface in the aquifer, which indicates PC1 habitually reflects geological effects.37 The PC2 explained about 26.88 % of total variance and high positive loading of PO43-, NO3- and HCO3-. The loading of NO3- and HCO3- represents the presence of organic matter in the aquifer.38 The PC3 explained about 8.84% of total variance and positive loading of SO42-. Weathering process and human input are the two main factors that change the geochemical composition of groundwater.36
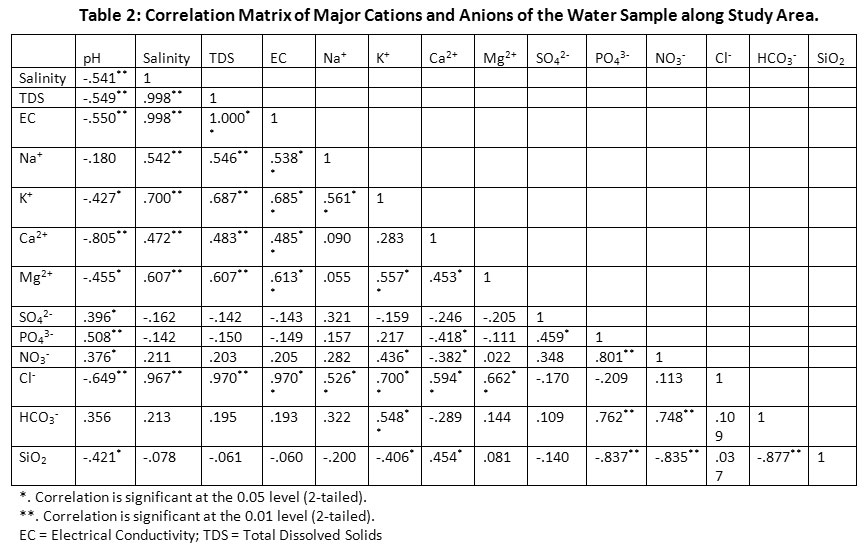 |
Table 2: Correlation Matrix of Major Cations and Anions of the Water Sample along Study Area. Click here to view Table |
Table 3: Factor Analysis of Major Cations and Anion of the Groundwater Sample.
|
Water quality variables |
PC1 |
PC2 |
PC3 |
|
pH |
-0.691 |
0.548 |
-.010 |
|
Salinity |
.963 |
.093 |
.059 |
|
TDS |
.963 |
.083 |
.083 |
|
EC |
.963 |
.082 |
.077 |
|
Na+ |
.534 |
.412 |
.575 |
|
K+ |
.780 |
.386 |
-.212 |
|
Ca2+ |
.616 |
-.519 |
.042 |
|
Mg2+ |
.697 |
-.062 |
-.372 |
|
SO42- |
-.223 |
.485 |
.685 |
|
PO43- |
-.183 |
.889 |
-.123 |
|
NO3- |
.122 |
.906 |
-.112 |
|
Cl- |
.981 |
-.017 |
.078 |
|
HCO3- |
.174 |
.857 |
-.338 |
|
Eigenvalue |
6.109 |
3.495 |
1.150 |
|
% of Variance explained |
46.992 |
26.882 |
8.844 |
|
Cumulative % |
46.992 |
73.875 |
82.718 |
|
Extraction Method: Principal Component Analysis. |
|||
Piper and Gibbs Diagram
The hydro-chemical facies is fundamental to identifying the chemical background of water, and these phases can be explained by portrayal Piper diagrams.39 It is commonly used to make out the foremost ionic composition of the groundwater sample.40 This diagram also represents the corollary of chemical reaction occurred between the minerals and groundwater.41 The concentration of main cations (Na+, K+, Ca2+, and Mg2+), anions (CO32-, HCO3-, SO42- and Cl-) and TDS in meq L-1 were plotted in Piper trilinear diagram to evaluate the hydro-chemistry of the groundwater of the study area (Fig. 4). The figure indicated that the mixing composition of Na-Ca-HCO3, Na-Ca-Cl were NaCl type domination in hydro-geochemical facies in groundwater. The lofty concentrations of Na and Cl were the sign of their derivation from identical sources.
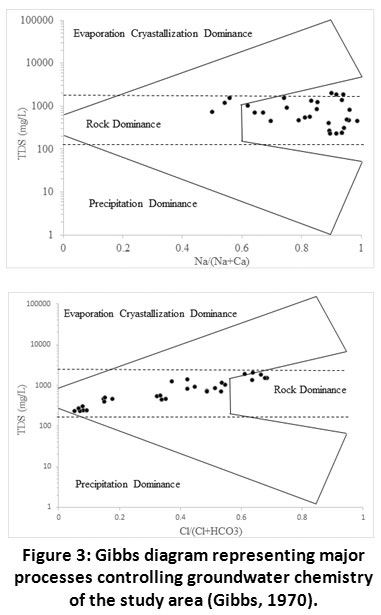 |
Figure 3: Gibbs Diagram Representing Major Processes Controlling Groundwater Chemistry of the Study Area (Gibbs, 1970). Click here to view Figure |
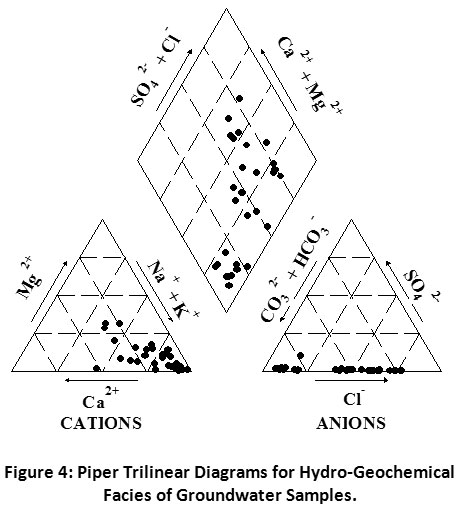 |
Figure 4: Piper Trilinear Diagrams for Hydro-Geochemical Facies of Groundwater Samples. Click here to view Figure |
The Gibbs diagram was universally used to evaluate hydro-chemistry of water in the study area. This diagram assess the functional sources of dissolve chemicals in water, such as precipitation dominance, rock dominance and evaporation dominance.16, 42 The ratios of Na+/(Na+ + Ca2+) and Cl-/(Cl- + HCO3-) is the representation of a function of TDS. The distribution of sampling points in Gibbs diagram was shown in Fig. 3. The diagram showed that most of the water samples were in rock domain area towards the zone of evaporation. This suggested that the evolution of chemical composition along study area was habitually related with chemical weathering of rock forming minerals. A small number of samples were in evaporation dominance zone which suggested the salt precipitation and anthropogenic influence.43
Wilcox Classification of Water Samples
Sodium percent is plotted alongside EC and the illustration was termed as Wilcox diagram.17 The chemical breakdown of 30 water samples were plotted in Wilcox diagram (Fig. 5). About 12 samples were in tolerable to distrustful, 8 samples were distrustful to inappropriate, 3 samples were inappropriate, 5 samples were good to tolerable and 2 samples were admirable to fine. Based on Wilcox diagram larger part of water samples were apposite for irrigation in every type of soil. The EC and Na+ played a central role in aptness of irrigation water.44 The TDS and EC are the indicators of salinity even in absence of non-ionic dissolved constituents. The TDS values <450 mg L-1 represents as “none saline”. Similarly, 450 to 2,000 mg L-1 represents “slight to moderate salinity” and the value >2,000 mg L-1represents ‘severe’ salinity for irrigation water.45
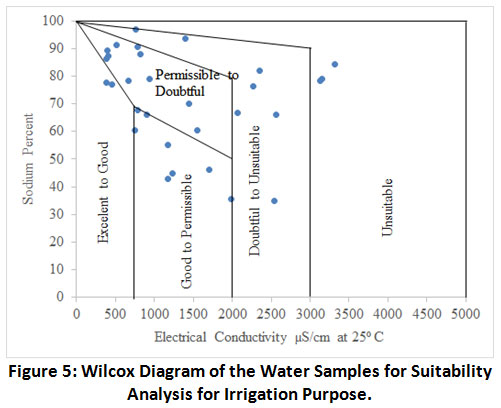 |
Figure 5: Wilcox Diagram of the Water Samples for Suitability Analysis for Irrigation Purpose. Click here to view Figure |
Conclusions
The collected samples were slightly alkaline in nature and high TDS value was mainly due to excessive dissolved salts. A total of three foremost components were found to explain 82.718% of data set when subjected to principal component analysis. The PCA outcome showed that mineral dissolution, rock weathering, ion exchange and sea water intrusion were the overriding factors of overall geochemistry of the study area. The groundwater was habitually Na-Ca-HCO3- type, which indicated that the organic deposits in aquifer. Rock weathering was the principal process along the study area. Silicate weathering was essentially at fault for dissolved ions in groundwater. Irrigation with these types of saline water can boost up the salinity of soil and exterior water. The development of management strategies and using of different water sources will be the most excellent solution to meet the increasing demand of drinking and irrigation water. The hydro-geochemical composition of groundwater in the study area was largely prejudiced by geologic factors, while anthropogenic factors have very insignificant effects.
Compliance with Ethical Standards
Acknowledgements
The research team is thankful to the people of Dakshin Bedkashi Union of Koyra Upazil of Khulna District for providing indispensable information concerning the research objectives. Thanks are also to the all kinds of Officials (Government and Nongovernment) of the Koyra Upazila and Khulna District for helping us. The team is also gratifying to their friends and colleagues for helping the team during the research.
Funding Source
We the authors did not receive any financial support for publication of this research or for conducting the research.
Conflict of Interest
We are fully agreed with the sequence of authorship.
Authors Contribution
Tusar Kumar Das formulated the research questions, collected and analyzed the samples and prepared the draft manuscript. Mohammad Mahfuzur Rahman helped to prepare the manuscript. Molla Rahman Shaibur collected the information from different sources and he is the lead researcher of this group. He edited and finalized the manuscript for submission and production in Current World Environment.
Declaration
Please note that all the errors present in the manuscript are ours.
References
- Sajeev S, Sekar S, Kumar B, Senapathi V, Chung SY, Gopalakrishnan G. 2020. Variations of water quality deterioration based on GIS techniques in surface and groundwater resources in and around Vembanad Lake, Kerala, India. Geochemistry125: 626.
CrossRef - Shirazi SM, Ismail Z, Akib S, Sholichin M, Islam MN. 2011. Climatic parameters and net irrigation requirement of crops. International Journal of Physical Sciences 6(1): 15-26.
- Rowe DR, Abdel-Magid IM. 1995. Handbook of wastewater reclamation and reuse. CRC press.
- Abedin MA, Habiba U, Shaw R. 2014. Community perception and adaptation to safe drinking water scarcity: salinity, arsenic, and drought risks in coastal Bangladesh. International Journal of Disaster Risk Science 5(2): 110-124.
CrossRef - Sarwar MGM. 2005. Impacts of sea level rise on the coastal zone of Bangladesh. See http://static. weadapt. org/placemarks/files/225/golam_sarwar. pdf.
- Shaibur MR, Anzum, HMN, Rana MS, Sarwar S. 2019. Water supply and sanitation status in Jashore Municipality, Bangladesh. Environmental and Biological Research, 1(1): 12-21.
- Islam MA, Zahid A, Rahman MM, Rahman MS, Islam MJ, Akter Y, Roy B. 2017. Investigation of groundwater quality and its suitability for drinking and agricultural use in the south central part of the coastal region in Bangladesh. Exposure and Health 9(1): 27-41.
CrossRef - Khan AE, Ireson A, Kovats S, Mojumder S. K, Khusru A, Rahman A, Vineis P. 2011. Drinking water salinity and maternal health in coastal Bangladesh: implications of climate change. Environmental Health Perspectives 119(9): 1328-1332.
CrossRef - Shaibur MR, Shamim AHM, Khan MH. 2019. Water quality of different sources at Buri Goalini and Gabura Unions of Shyamnagar Upazila, Bangladesh. Environmental and Biological Research 1(1): 32-43.
- Chowdhury NT. 2010. Water management in Bangladesh: an analytical review. Water Policy 2010: 12(1): 32-51.
CrossRef - Shirazi SM, Islam MA, Ismail Z, Jameel M, Alengaram UJ, Mahrez A. 2011b. Arsenic contamination of aquifers: A detailed investigation on irrigation and portability. Scientific Research and Essays 6(5): 1089-1100.
- BBS. 2011. Statistical Year book, Khulna Zila. Bangladesh Bureau of Statistics, Ministry of Planning, Government of Bangladesh, 7-12
- Naus FL, Schot P, Ahmed KM, Griffioen J. 2019. Influence of landscape features on the large variation of shallow groundwater salinity in southwestern Bangladesh. Journal of Hydrology X. 5: 100043.
CrossRef - Bhuiyan MJAN, Dutta D. 2012. Assessing impacts of sea level rise on river salinity in the Gorai river network, Bangladesh. Estuarine, Coastal and Shelf Science 96: 219-227.
CrossRef - APHA. 1915. American Public Health Association, American Water Works Association, Water Pollution Control Federation, & Water Environment Federation. (1915). Standard Methods for the Examination of Water and Wastewater 2.
- Gibbs RJ. 1970. Mechanisms controlling world water chemistry. Science 170(3962): 1088-1090.
CrossRef - Wilcox L. 1995. Classification and use of irrigation waters (No. 969). US Department of Agriculture.
- Shaibur MR, Anzum HMN, Rana MS, Khan MAS. 2012. Assessment of supplied water quality at Jashore Municipality (Pourashava), Bangladesh. Bangladesh Journal of Environmental Research 10: 69- 87.
- Shaibur MR, Hossain MS, Sony SJ. 2019. Drinking water quality of hand tube well water at Suburban areas of Jashore Municipality, Bangladesh. Journal of Jessore University of Science and Technology 4(1): 11-22.
- Tong H, Zhao P, Huang C, Zhang H, Tian Y, Li Z. 2016. Development of iron release, turbidity, and dissolved silica integrated models for desalinated water in drinking water distribution systems. Desalination and Water Treatment 57(1): 398-407.
CrossRef - Das TK, Mamun MA, Shaibur MR. 2019. Hydro-geochemistry and quality assessment of deep groundwater in Shyamnagur Upazila, South-western Bangladesh. International Journal of Experimental Agriculture 9(1): 14-21.
CrossRef - Das TK, Choudhury M, Sultana M. 2017. Determination of drinking water quality: a case study on saline prone south-west coastal belt of Bangladesh. Journal of Environmental Science and Natural Resources 10(1): 101-108.
CrossRef - Shaibur MR, Shamim AHM, Khan MH, Tanzia FKS. 2017. Exploration of soil quality in agricultural perspective at Gabura and BuriGoalini Union: Shyamnagar, Satkhira, Bangladesh. Bangladesh Journal of Environmental Science 32: 89-96.
- Bodrud-Doza M, Islam AT, Ahmed F, Das S, Saha N, Rahman MS. 2016. Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Science 30(1): 19-40.
CrossRef - Freeze RA, Cherry JA. 1979. Groundwater. Englewood Cliffs, NJ: Prentice-Hall.
- Shaibur MR, Rizvi MM, Islam MK, Shamsunnahar 2019. Salinity causes ecosystem disruption and biodiversity losses in Beel Khuksia: Keshabpur, Jashore, Bangladesh. Environmental and Biological Research 1(1): 1-11.
- Shaibur MR, Tanzia FKS, Karim MM, Rahman MH. 2017. Aspects of Bangladesh water development board on the ecosystems and water quality of Nolamara Beel in Narail District of Bangladesh. Bangladesh Journal of Environmental Science32: 144-151.
- Garrels RM. 1976. A survey of low temperature water-mineral relations. In Interpretation of environmental isotope and hydrochemical data in groundwater hydrology.
- Meybeck M. 1987. Global chemical weathering of surficial rocks estimated from river dissolved loads. American Journal of Science, 287(5): 401-428.
CrossRef - Datta PS, Tyagi SK. 1996. Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. Journal-Geological Society of India47: 179-188.
- Jeevanandam M, Kannan R, Srinivasalu S, Rammohan V. 2007. Hydro-geochemistry and groundwater quality assessment of lower part of the Ponnaiyar river Bbasin, Cuddalore district, South India. Environmental Monitoring and Assessment 132(1-3): 263-274.
CrossRef - Lusczynski NJ, Swarzenski WV. 1996. Saltwater encroachment in Southern Nassu and SE Queen Countries, Long Island, New York. USGS Paper, 1613.
- Ahmed A, Ghosh PK, Hasan M, Rahman A. 2020. Surface and groundwater quality assessment and identification of hydrochemical characteristics of a south-western coastal area of Bangladesh. Environmental Monitoring and Assessment 192: 1-15.
CrossRef - Samatya S, Kabay N, Yüksel Ü, Arda M, Yüksel M. 2006. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. Reactive and Functional Polymers 66(11): 1206-1214.
CrossRef - Shaibur MR, Ahmmed I, Rumpa SS, Parvin S, Hossain MS, Rahman MM, Sarwar S (2019): Physico-chemical parameters of groundwater at Jashore University of Science and Technology campus and its surrounding villages. Journal of Jessore University of Science and Technology, 4(1): 34-45.
- Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J. 2017. Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. Journal of Geochemical Exploration 175: 59-71.
CrossRef - Al-Farraj AS, Al-Wabel MI, El-Saeid MH, El-Naggar AH, Ahmed Z. 2013. Evaluation of groundwater for arsenic contamination using hydrogeochemical properties and multivariate statistical methods in Saudi Arabia. Journal of Chemistry 812365.
CrossRef - Jia Y, Guo H, Jiang Y, Wu Y, Zhou Y. 2014. Hydrogeochemical zonation and its implication for arsenic mobilization in deep groundwater’s near alluvial fans in the Hetao Basin, Inner Mongolia. Journal of Hydrology 518: 410-420.
CrossRef - Piper WW, Roth WL. 1953. Perfect crystals of zinc sulfide. Physical Review 92(2): 503.
CrossRef - Sivasubramanian P, Balasubramanian N, Soundranayagam JP, Chandrasekar N. 2013.Hydrochemical characteristics of coastal aquifers of Kadaladi, Ramanathapuram District, Tamilnadu, India. Applied Water Science 3(3): 603-612.
CrossRef - Selvam S, Singaraja C, Venkatramanan S, Chung SY. 2018. Geochemical appraisal of groundwater quality in OttapidaramTaluk, Thoothukudi District, Tamil Nadu using graphical and numerical method. Journal of the Geological Society of India 92(3): 313-320.
CrossRef - Gupta SK, Gupta RC, Chhabra SK, Eskiocak S, Gupta AB, Gupta R. 2008. Health issues related to N pollution in water and air. Current Science 94(11):1469-1477.
- Marghade D, Malpe DB, Zade AB. 2012. Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environmental Monitoring and Assessment 184(4): 2405-2418.
CrossRef - Rao NS. 2006. Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environmental Geology 49(3): 413-429.
CrossRef - Islam MS, Shamsad SZKM. 2009. Assessment of irrigation water quality of Bogra district in Bangladesh. Bangladesh Journal of Agricultural Research 34(4): 507-608.
CrossRef - WHO. 1984. Guidelines for drinking water quality recommendations.
- USEPA. 1992. Guidelines for water use. USEPA, Washington, Tech.; Report 81, pp 252.
- BBS. 2009. Bangladesh Bureau of Statistics. Planning Division, Ministry of Planning, Government People’s Republic of Bangladesh. Bangladesh National Drinking Water Quality Survey 2009.







