Acute and Chronic Toxicity of Endocrine Disruptive Heavy Metals and Pesticides Exposed to Freshwater Fish P. reticulata and P. sphenops
DOI: http://dx.doi.org/10.12944/CWE.16.2.09
Copy the following to cite this article:
Varghese V, Nagarani N, Balasubramani A. Acute and Chronic Toxicity of Endocrine Disruptive Heavy Metals and Pesticides Exposed to Freshwater Fish P. reticulata and P. sphenops. Curr World Environ 2021;16(2). DOI:http://dx.doi.org/10.12944/CWE.16.2.09
Copy the following to cite this URL:
Varghese V, Nagarani N, Balasubramani A. Acute and Chronic Toxicity of Endocrine Disruptive Heavy Metals and Pesticides Exposed to Freshwater Fish P. reticulata and P. sphenops. Curr World Environ 2021;16(2). Available From: https://bit.ly/3DL5XsX
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 20-04-2021 |
|---|---|
| Accepted: | 25-08-2021 |
| Reviewed by: | 
 Nidhal Mohammed
Nidhal Mohammed
|
| Second Review by: |

 Y. Vasudeva Rao
Y. Vasudeva Rao
|
| Final Approval by: | Dr. Marzia Ciampittiello |
Introduction
India is rich in diversity of organisms and plays a prominent role in international market in ornamental fish due to its rusticity and color patterns. Despite its potential, India ranks low in the Asian market, asmost of the ornamental fish is of wild variety1. Among the total 156 species in Tamil Nadu, 16% of them were under threat due to problem in maintaining the stock, seed production, environmental factors including contamination of water etc. Hence research in aquaculture is indeed important to maintain the potential resource of income generation through entrepreneurship in the rural areas of Tamil Nadu2.
Water quality is very important for survival and well-being of all the aquatic organisms. Toxicants from both point and non-point sources reach the aquatic ecosystem and pollute the environment through their receiving water bodies3. These toxicants cause adverse effects on the aquatic biota through food chains. Critical habitats are particularly vulnerable to pollution. The pollutants can directly or indirectly affect the metabolism, growth and development, and affect endocrine functions, reproduction, translation etc.,
The Endocrine Disrupting Chemicals (EDCs) affects the hormone receptors by competing with the natural hormones4. EDCs are widely distributed in various products such as plastic materials, pesticides, fungicides, metals, personal care products and even heavy metals5. When these chemicals are released into the water bodies it affects the metabolism of aquatic life. The mechanism of action is more complex which include direct actions on the receptors (mainly hormonal and/or neurotransmitter receptors), enzymes and hormone6,7. The interference in the hormones can alter the behavioural dimorphisms particularly when exposure occurs during critical developmental periods8,9. They are widespread throughout the aquatic ecosystems such as water and sediment disturbing the aquatic life through various routes, as runoff water from the agricultural fields, sewage/effluents of industries reaches the river and ocean.10,11.
Among the aquatic life, fishes are mainly vulnerable to toxicants and express behavioural, structural, chemical and molecular changes12. Therefore, fish are considered as indicator organisms to assess the EDCs13 which imitate the effects of natural oestrogens (xenoestrogens)14,15,16,17. Mostly heavy metals, pesticides and insecticides acts as EDCs individually or synergistically and become toxic to the organisms. This in turn affects survival, growth and reproduction of organisms18. When the exposure level to these chemicals exceed, it can induce cancer, damage the nuclear materials and ultimately lead to lethality7. The lethality depends on species and concentration of toxicants, which may lead to aquatic loss and disrupts the food chain19,20. Apart from lethality the toxicants may also affect the economy of the aquarist by affecting the behaviour, structure and health of the ornamental fishes.
Hence, regular monitoring helps in identifying the ill effects of the toxicants in organisms.Therefore the present study was aimed to derive safe levels on acute and chronic concentrations of EDCs such as heavy metals (Cadmium and Arsenic) and pesticides (Chlorpyrifos and Cypermethrin) in the fresh water fish.
Materials and Methods
Experimental Fish
Freshwater fish Poecilia reticulata and Poecilia sphenops were used to study the acute and chronic effects of the chosen chemicals. Healthy fish (1.0 ± 0.1 g) were procured from hatchery in Madurai. They were transported in the plastic bag separately with freshwater filled with oxygen to the laboratory at Madurai Kamaraj University, (9.?????? and 78.0105?E) in Madurai, Tamil Nadu, India. Before acclimation, fish were treated with KMnO4 solution (0.05%) for 2 min to protect from dermal infection. Healthy fish were transferred to tank in the ratio of 1 g of fish weight in 10 litre of chlorine free tape water and acclimated in the laboratory at ambient environmental conditions for 15 days. During acclimation period no mortality was observed. Local manufactured pellet fish feed was fed twice a day ad libitum and the excretory matters were frequently removed. The essential physico-chemical parameters such as temperature, pH, DO, total ammonia, phosphate, nitrate, and electrical conductivity were estimated during the test every 24 hrs, following the standard methods21. The photoperiod of 10 hrs light and 14 hrs dark was maintained.
Chemicals
Metals, i.e., Arsenic chloride (AsCl3) and Cadmium chloride (CdCl2) and commercially formulated pesticides, i.e., Chlorpyrifos 20% EC and Cypermethrin 25% EC were used for assessing the acute and chronic toxic effects. One percent stock solutions were prepared separately, for each metal and pesticide, in 1000 ml volumetric flasks. Whose concentration was 1 mg mL-1.The pesticides were dissolved in 0.5% acetone and also used in solvent control group.
Experimental Design for Acute and Chronic Toxicity Studies
Acute toxicity study was conducted to analyse the median lethal concentration (LC50) and safe level of the toxicants under static renewal bioassay method after 96 hrs of exposure. Before the definitive toxicity tests, the wide range of toxicant concentrations such as 0.0001, 0.001, 0.01, 0.1, 1.0, 10 & 100 mg L-1 were used to find out the concentrations for definitive toxicity tests21,22,23.
For acute and chronic toxicity studies, ten acclimated fish were introduced per toxicants in a separate tank. For pesticide toxicity, solvent control was maintained simultaneously. Based on the median lethal concentrations derived through definitive toxicity studies, the sub-lethal concentrations of each toxicant were selected for chronic toxicity studies by calculating 1/10th of the LC50 (96h) value of each toxicant24. During the chronic exposure studies the NOEC (No Observed Effect Concentration) and LOES (Lowest observed effect Concentration) were derived.
The test media were renewed every day to maintain the concentration of the toxicants. During the study period, the dead fish were removed immediately from the medium. No food was provided for test animals during the acute toxicity test. For acute toxicity testing, the mortality was observed up to 96h at an interval of 24 h.
The LC50 values of the toxicants were analysed at respective hours of exposure using Probit Analysis computer programme25,26. Safe concentrations of the toxicants were also calculated following the application factor, 1/100th of the 96h LC50 values27,28. The dose (concentration) vs response (mortality) curves were drawn using Excel programme to find out the effect of toxicants.
To find out the chronic toxicity endpoints a 28 day exposure was conducted. The artificial pellet fish feed was given ad libitum, during chronic studies29. The live animals in each media were counted every day. NOEC and LOEC were derived based on percentage survival of fishes on 28th day of the sub-lethal tests using Dunnet analysis computer program26,30. The statistical analysis was done using SPSS Version 23 to study the significance of the data.
Result and Discussion
Environment plays a significant role in the survival of an organism. When the fish were introduced to an induced environment, they exhibit behavioral changes to sustain in the new environment. Irrespective to the toxicants exposed both the fish species exhibited behavioral changes at higher concentration. On exposure the fish were alert and still in position. The fish exposed to metals and pesticides were found to be unsteady and showed erratic movements a sign of hyper excitability. Respiratory distresses with excess mucous secretion were observed in the toxicants exposed medium.
Their pectoral and pelvic fins were found to be hard may be due to muscle fatigue on pesticide exposure. On increasing the concentration of toxicants fish remained in a vertical position, exposing the mouth on to the surface of water indicating its effort to swallow air. The observed behavioural activity in the present study is due to increase in the level of ammonia and decrease in Na+, K+, Ca2+ in plasma which indirectly affects muscular contraction and impulse transmission31.
Acute Toxicity
The physicochemical parameters maintained throughout the experiments were listed in Table - 1. The toxicity endpoints are the primary criteria for the protection of fauna in the aquatic medium. Therefore, the present investigation aimed to elicit the acute and chronic endpoints of the chosen Endocrine Disrupting Chemicals such as heavy metals (Arsenic and Cadmium) and pesticides (Chlorpyrifos and Cyperimethrin) in freshwater fish viz., P. reticulata and P. sphenops.
Table 1: Water Quality Analysis.
|
Parameters |
Control |
Metal Exposure |
Pesticide Exposure |
|
Odour |
- |
- |
- |
|
Phosphate (mg/L) |
1 |
0.9 |
1.2 |
|
Hardness |
Nil |
Nil |
Nil |
|
Nitrate |
1.5 |
0.9 |
1.2 |
|
Total Ammonia (mg /L) |
2.00 |
2.42 |
2.54 |
|
Dissolved Oxygen (mg /L) |
5.79 |
5.06 |
4.84 |
|
pH |
7.2 |
7.4 |
7.4 |
|
Temperature (ºC) |
32 -33 |
31 – 32 |
33-35 |
|
Electrical conductivity (mScm-1) |
3.43 |
3.2 |
3.26 |
The dose dependent increase in the mortality of metals exposed fish were shown in Fig.1. There was no mortality during the experimental period in the control and solvent control (acetone 0.5%) groups.
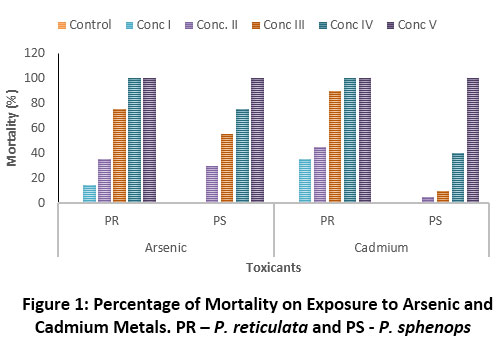 |
Figure 1: Percentage of mortality on exposure to Arsenic and Cadmium metals. PR – P. reticulata and PS - P. sphenops Click here to view Figure |
The LC50, 95% Confidence Interval and safe values of P. reticulata and P. sphenops calculated under acute static renewal test method were presented in Table 2. The LC50 values were higher during 24 hr exposure and declined on further increase in duration. The LC50 values of P. reticulata and P. sphenops exposure to metals and pesticides showed negative correlation with time. The calculated 96 h LC50 values for P. reticulata and P. sphenops exposed to As were 11.387 and 10.755 mg L-1 respectively and the LC50 values of respective fish exposed to Cd were 24.572 and 20.036 mg L-1 respectively (Table 2). The result showed that P. sphenops was more susceptible to heavy metals compared with P. reticulata irrespective of the metals. The results concluded that there was a significant effect of heavy metals in P. sphenops (R² = 0.9717 and 0.9476 for As and Cd respectively) hence, there exists a strong correlation between the dose and mortality compared with P. reticulata. Overall study inferred that the highest toxicity was reported in As exposed P. sphenops and the lowest toxicity in Cd exposed P. reticulata. On acute exposure, both fish were resistant to cadmium and sensitive to arsenic metal.
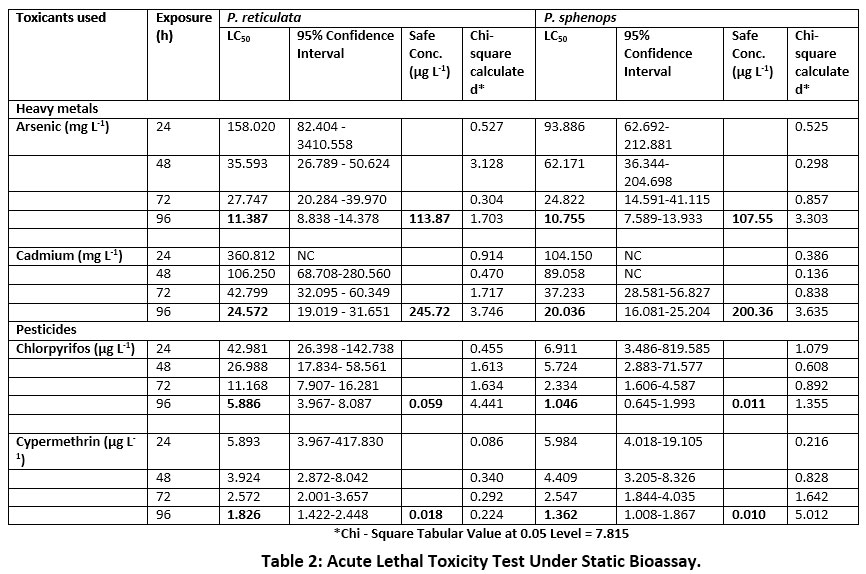 |
Table 2: Acute Lethal Toxicity Test Under Static Bioassay. Click here to view Table |
Similarly, in the acute toxicity test, P. reticulata and P. sphenops were shown to be sensitivity to Cypermethrin and resistant to Chlorpyrifos. The 96 h LC50 values for P. reticulata and P. sphenops exposed to Chlorpyrifos were 5.886 and 1.046 ppb and to Cypermethrin were 1.826 and 1.362 ppb respectively (Table-3; Fig 2). It was apparent that P. sphenops was highly sensitive to Chlorpyrifos under acute exposure. The present study on acute toxicity concluded that the pesticides were highly toxic to both fish species than heavy metals.
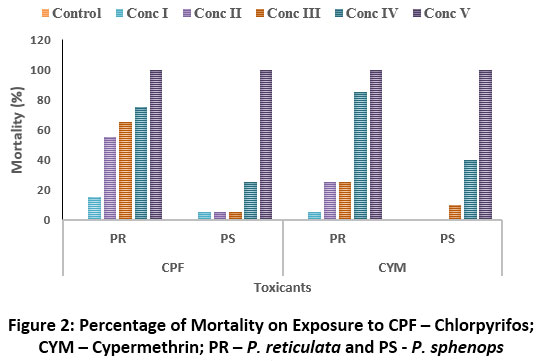 |
Figure 2: Percentage of mortality on exposure to CPF – Chlorpyrifos; CYM – Cypermethrin; PR – P. reticulata and PS - P. sphenops Click here to view Figure |
The mean survival of control animals and environmental factors was substantial to EPA/COE31. Thus confirmed the holding facilities of test animals and other environmental factors are under acceptable levels of the toxicity testing. Under acute toxicity studies, the mortality of test fish was positively correlated with increasing acute concentrations and exposure time, coincides with earlier reports for marine organisms32.
Among the chosen metals P. reticulata was more susceptible to cadmium and P. sphenops to arsenic. Hence, the study showed a species dependent response on exposure to heavy metals and also shown significant result to the previous investigation in freshwater fish exposed to heavy metal and pesticide under static bioassay method. The lethality depended on the test methodology, Shuhaimi et al.,33 reported less lethal concentration under static non-renewal test and Yilmaz34 reported higher lethal level for the same metals under static renewal test. The study confirmed that the test methods have influence on the toxic effect, due to continuous exposure to toxicants and bioaccumulation. In the present study the estimated LC50 values for As and Cd to test fish were higher than that of the previous studies35 invariable of test methods.
The calculated 96 LC50 values in the present study reveals that both P. reticulata and P. sphenops were more sensitive to As, compared to Heteropneustes fossilis (35.10 mg L-1; Kermiuet al.,36) and slightly resistant than Catla catla37 (10.16 mg L-1); and on exposure to Cd the test fish was resistance than reported in Ophiocephalus striatus (0.63 mg L-1) and sensitive than Gambusia holbrooki38 (37.3 mg L-1). Similarly for cadmium metal toxicity, present study reveals that the P. reticulata and P. shenops were sensitive than Heteropneustes fossilis39 (50.41 mg L-1) and resistant than Rasbora sumatrana33 (0.10 mg L-1).
The lethal concentration observed in the present study on exposure to Chlorpyrifos and Cypermethrin concentrations were less when compared to the earlier reported LC50 value for various organisms including P. reticulata under different experimental setup40,41,42,43; Danio rerio44 and rainbow trout45. The present study also reveals that P. shenops was more sensitive to Chlorpyrifos and the LC50 values observed in the study is less compared to earlier reports.
P. reticulata is also more sensitive to chlorpyrifos than other organic chemicals including pyrethroid46. Similarly, sensitive than other freshwater species i.e., Oreochromis niloticus47 (12.6 µg L-1); Heterobranchus bidorsalis48 (36 mg L-1) and Cyprinus carpio49 (3.31 µg L-1). Hence the present study concluded that there exists a species dependent response to the toxicant.
Chronic Toxicity
In the present study the NOEC, LOEC and chronic value for Arsenic exposed P. reticulata and P. sphenops were similar i.e., 0.07, 0.140 and 99.0 µg L-1 respectively. Nevertheless, there was variation in the value for Cadmium exposed P. reticulata (0.15, 0.31 and 216.0 µg L-1) and P. sphenops (0.13, 0.25 and 180.0 µg L-1) (Table 3). The pesticide exposed fish showed very less chronic endpoints (NOEC and LOEC) than that of metal exposed fish. The NOEC and LOEC value of P. reticulata and P. sphenops exposed to Chlorpyrifos were 0.038 and 0.007 ppb respectively and the Chronic value ranged between 0.53 and 0.010 ppb. At the same time the chronic value for cypermethrin was noted to be lower for P. reticulata.
Table 3: Chronic Toxicity Assay.
|
Test Species |
Toxicants |
NOEC |
LOEC |
Chronic value |
|
Heavy Metal |
|
|
|
|
|
P. reticulata |
Arsenic |
0.070 |
0.140 |
99.000 |
|
Cadmium |
0.150 |
0.310 |
216.000 |
|
|
P. sphenops |
Arsenic |
0.070 |
0.140 |
99.000 |
|
Cadmium |
0.130 |
0.250 |
180.000 |
|
|
Pesticide |
|
|
|
|
|
P. reticulata |
Chlorpyrifos |
0.038 |
0.075 |
0.053 |
|
Cypermethrin |
0.011 |
0.023 |
0.016 |
|
|
P. sphenops |
Chlorpyrifos |
0.007 |
0.014 |
0.010 |
|
Cypermethrin |
0.009 |
0.018 |
0.013 |
Similar to acute exposure, the fish reared in control media (freshwater control and solvent control) showed 100% survival during the period of chronic studies. The survival rate was observed to be 85% for P. reticulata on exposure to higher sub-lethal concentration of cadmium and chlorpyrifos. In the lowest sub-lethal concentrations of metals and pesticides, the fish survival was between 97.5% and 100%, hence these concentrations were found to be suitable for survival test50,51,52.
Acute and Chronic Safe Concentrations
Analogous to the acute safe level, the chronic safe values were also derived using the NOEC and LOEC values obtained through survival studies. The chronic values for Arsenic to P. reticulata and P. sphenops were same i.e., 99 µg L-1while the chronic values of Cd for respective fish were 216 and 180 µg L-1. The chronic value for Cd exposed P. reticulata was slightly higher. `Likewise, the chronic values of chlorpyrifos to respective fish were 0.053 and 0.010 µg L-1and for cypermethrin were 0.016 and 0.010 µg L-1.
No mortality in the control and solvent control groups were noticed in the present study ie., 100% survival rate observed in chronic test. Only 32.5% mortality was observed that too in the higher concentration of pesticide on the third week of exposure.
Conclusion
Maintenance of water quality is essential for survival, growth and physiology of aquatic organisms. The release of various pollutants including endocrine disruptive chemicals affects the quality of aquatic medium and leads to impairment of life in the aquatic environment. Therefore, the strict acute and chronic safe level criteria should be adopted to maintain water quality for the protection of most natural bio resources. Thus the present study on acute and chronic values of the chosen EDCs reveals that it was in good agreement with the criteria developed by USEPA26 under safe levels to protect the freshwater bodies. The data obtained in the present study can be used to assess and monitor the organic or inorganic contaminates in the aquarium fish culture. This in turn helps to improve the economy of the aquarist and growth of entrepreneurship in the ornamental fish trade of the world market.
Acknowledgment
The authors thank the Madurai Kamaraj University for providing necessary facility for the present study and Dr.C.M. Ramakritinan for his valuable suggestion in preparing the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- http://www.dof.gov.in/sites/default/files/2020-07/Ornamnetal_fisheries_development
- Mogalekar H. S., Canciyal J. Freshwater fish fauna of Tamil Nadu, India. Proceedings of the International Academy of Ecology and Environmental Sciences. 2018; 8 (4):213 – 230.
- Nagarani N., Anand M., Kumaraguru A.K. Environmental monitoring using biomarkers in relevance to heavy metal pollution in coastal areas of the Gulf of Mannar. Indian J Exp Biol. 2020; 58:794-802.
- Mary C Reiley. Science, policy, and trends of metals risk assessment at EPA: How understanding metals bioavailability has changed metals risk assessment at US EPA. Aquatic Toxicology. 2007; 84:292–298. https://doi.org/10.1016/j.aquatox.2007.05.014.
CrossRef - Segner H. Zebra fish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Phys C. 2009; 149:187–195.
CrossRef - Gore A.C., Patisaul H.B. Neuroendocrine Disruption: Historical Roots, Current Progress, Questions for the Future. Front Neuroendocrinol. 2010; 31(4): 395–399.
CrossRef - Janaki Devi V., Nagarani N., Yokesh Babu M., Kumaraguru A.K., Ramakritinan C.M. A study of proteotoxicity and genotoxicity induced by the pesticide and fungicide on marine invertebrate (Donaxfaba). Chemosphere, 2013; 90(3):1158-66.
CrossRef - Adeel M., Song X., Wang Y., Francis D., Yang Y. Environmental impact of estrogens on human, animal and plant life: A critical review.Environ Int. 2017; 99:107-119.
CrossRef - Sona S., Eva R., Alzbeta B.M. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer, Reprod Biol. 2016; 16(4):243-254.
CrossRef - Dan Liu., Wu S., Xu H., Zhang Q., Zhang S., Shi L., Yao C., Liu Y., Cheng J. Distribution and bioaccumulation of endocrine disrupting chemicals in water, sediment and fishes in a shallow Chinese freshwater lake: Implications for ecological and human health risks. Ecotoxicol Environ Saf. 2017; 140: 222-229.
CrossRef - Zhang C., Li Y., Wang, Niu L., Cai W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: a review. Crit. Rev. Environ. Sci. Technol. 2016; 46: 1–59.
CrossRef - Ayobahan S.U., Eilebrecht S., Baumann L., Teigeler M., Hollert H., Kalkhof S., Eilebrecht E., Schäfers C. Detection of biomarkers to differentiate endocrine disruption from hepatotoxicity in zebrafish (Danio rerio) using proteomics, Chemosphere. 2020; 240:124970.
CrossRef - Jin Y., Chen R., Liu W., Fu Z. Effect of endocrine disrupting chemicals on the transcription of genes related to the innate immune system in the early developmental stage of zebrafish (Danio rerio). Fish Shellfish Immunol. 2010; 28: 854–861.
CrossRef - Falconer I.R., Heather F.C., Michael R.M., Geetha R. Endocrine Distrupting Compounds: A review of their challenge to sustainable and safe water supply and water reuse. Inc. Environ Toxicol. 2006; 21: 181–191.
CrossRef - Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol, 2002; 36:1 202–11.
CrossRef - Metcalfe C.D., Tracy L.M., Yiannis K., Brenda G.K., Colin K., Richard J.H., Timothy R.C., Raymond E.M., Thomas P. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka(Oryzias latipes). Environ Toxicol Chem. 2001; 20(2): 297–308.
CrossRef - Piquer I.F., Silvia B., Fabiana P., Stefania S.D., Vincenzo D.M. , Hamid R.H., Francesca M., Oliana C. Effects of BPA on zebrafish gonads: Focus on the endocannabinoid system, Environ Pollut. 2020; 264:114710.
CrossRef - Balasubramani A., Pandian T. J. Endosulfan suppresses growth and reproduction in zebrafish, Curr. Sci. 2008; 883-890.
- Fu J., Hu X., Tao X., Yu H., Zhang X. Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze River and Taihu Lake, China. Chemosphere, 2013; 93 (9):1887-1895.
CrossRef - Afshan S., Ali S., Ameen U.S., Farid M., Bharwana S.A., Hannan F, Ahmad R. Effect of different heavy metal pollution on fish. Res. J. Chem. Environ. Sci. 2014; 2 (1): 74-79.
- APHA/AWWA/WEF, 1998. Standard Methods for the Examination of Water and Wastewater. 20th Ed. American Public Health Association, Washington DC, p. 1220.
- Sprague J.B. The ABCs of pollutant bioassay using fish. In: Cairns J, Dickson DL (eds) Biological methods for assessment of water quality. ASTM Special Technical. 1973; 528: 6–30.
- OECD. 1993. OECD guidelines for testing of chemicals. OECD, Paris.
- Palanikumar L., Kumaraguru A.K., Ramakritinan C.M., Anand M. Biochemical response of anthracene and benzo[a] pyrene in milkfish Chanos chanos. Ecotox Environ Saf. 2012; 75:187–197.
CrossRef - Finney, D.J. (1971) Probit Analysis. 3rd Edition, Cambridge University Press, Cambridge.
- USEPA. 1994. US EPA toxicity data analysis software. EMSL, Cincinnati
- Kameswara R. The Comparative Toxicities of Organophosphorus and Carbamate Pesticides. Mahasagar. 1974; 7(1&2).
- Ramakritinan C.M., Rathishri C., Kumaraguru A.K. Acute toxicity of metals: Cu, Pb, Cd, Hg and Zn on Marine Mollusc, Cerithidiac ingulata and Modiolus phillipinarum H. Indian J Geo- Mar Sci. 2012; 41(2): 141-145.
- Gundersen D.T., Kristanto S.W., Curtis L.R., A1-Yakoob S.N., Metwally M.M., A1-Ajmi D. Subacute Toxicity of the Water-Soluble Fractions of Kuwait Crude Oil and Partially Combusted Crude Oil on Menidia beryllina and Palaemonetes pugio. Arch Environ Con Tox. 1996; 31: 1-8.
CrossRef - USEPA (1985) Water quality standards. Environmental Protection Agency, Taipei, Taiwan, no. 547327 Meade, J. W. (1989) Aquaculture management. Van Nostrand Reinhold, New York.
- Mohan S. Behavioural responses in effect to chemical stress in fish: A review. Int. J. Fish. Aquat. 2019; 7(1): 01-05.
- USEPA and USACE. Evaluation of dredged material proposed for ocean disposal, Testing manual, (Washington, DC, EPA-503/8-91/001), 1991.
- Shuhaimi-Othman M., Nadzifah Y., Nur-Amalina R., Umirah N.S. Deriving freshwater quality criteria for copper, cadmium, aluminum and manganese for protection of aquatic life in Malaysia. Chemosphere. 2013; 90: 2631–2636.
CrossRef - Yilmaz M., Ali Gül., Erba?l K. Acute toxicity of alpha-cypermethrin to guppy (Poecilia reticulata, Pallas, 1859), Chemosphere. 2004; 56(4): 381-385.
CrossRef - Zakaria N.A., Kutty A.A., Mahazar M.A., Zainal Abidin M. Arsenic acute toxicity assessment on select freshwater organism species in Malaysia. AIMS Environ. Sci. 2016; 3(4): 804-814.
CrossRef - Kermi T., Sunkam N.R., Bidyadhar D. Effect of arsenic (As) and lead (Pb) on glycogen content and on the activities of selected enzymes involved in carbohydrate metabolism in freshwater catfish, Heteropneustes fossilis. Int Aquat Res. 2019; 11:253–266.
CrossRef - Kousar S., Javed M. Heavy metals toxicity and bioaccumulation patterns in the body organs of four fresh water fish species. Pak. Vet. J.2014; 34(2): 161?164.
- Bais U.E., Lokhande M.V. Toxicity evaluation of cadmium chloride in fresh water fish Ophiocephalus striatus. Int J Fish Aquat Stud. 2017; 5(1): 519-521
- Singh A., Jain D.K., Puneet Kumar. Determination of LC50 of cadmium chloride in Heteropneustes fossilis, GERF Bulletin of Biosciences. 2010; 1(1): 21-24.
- Sharbidre A.A., Vimal M., Priyanka P. Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish, Poecilia reticulata. Pestic Biochem Phys. 2011; 101: 132–141.
CrossRef - Abdolreza J., Fardin, S., Hedayati, A. Mortality Response of Silver Carp (Hypophthalmicthys molitrix), Gold Fish (Carassius auratus) and Roach (Rutilus rutilus) to Acute Exposure of Copper Sulphate. World Journal of Fish and Marine Sciences. 2012; 4 (4): 418-421.
- Shen M.F., Anupama K., Shu-Yan Ding., Sonia G. Comparative study on the toxicity of pyrethroids, α-cypermethrin and deltamethrin to Ceriodaphnia dubia. Ecotox Environ Safe. 2012; 78: 9-13.
CrossRef - Restrepo D.E., Jaramillo-Colorado B.E., Duarte-Jaramillo L. Effects of chlorpyrifos on the crustacean Litopenaeus vannamei. PLoS ONE,2020; 15(4): e0231310.
CrossRef - Ahmad S., Vineeta Y., Kaneez Z. Determination of LC50 of Chlorpyrifos with special reference to Behavioral and Morphological changes in zebra fish, Danio rerio. IJRAR 2019; 6(1): 655-668.
- Topal A., Atamanalp M., Oruc E., Demir Y., Beydemir S., Isik A. In vivo changes in carbonic anhydrase activity and histopathology of gill and liver tissues after acute exposure to chlorpyrifos in rainbow trout. Arh. Hig. RadaToksikol. 2014; 65: 377-385.
CrossRef - Mustafa C. Assessment of acute toxicity of cypermethrin alone and synergized with piperonylbutoxide to the male guppies, (Poecilia reticulata Peters, 1859). Fresenius Environmental Bulletin. 2017; 26(12): 7458-7462.
- Yaji A.J., Auta J., Oniye S.J., Adakole J.A., Usman J.I. Effects of cypermethrin on behaviour and biochemical indices of fresh water fish Oreochromis niloticus. Elec. J. Env.Agricult. Food Chem.2011; 10(2): 1927-1934.
- Olufayo M.O., Alade O.H. Acute toxicity and histological changes in gills, liver and kidney of catfish, Heterobranchus bidorsalis exposed to cypermethrin concentration. African Journal of Agricultural Research. 2012; 7(31): 4453-4459.
CrossRef - Neelima P., GovindaRao K., GopalaRao N., Chandra SekharaRao J. Acute toxicity of cypermethrin (25% EC) on nucleic acids (DNA and RNA) in Cyprinus carpio (linn.). Int J. Recent Sci. Res. 2015; 6(7):5219-5224.
- ASTM, Standard guide for conducting 10-day static sediment toxicity tests with marine and estuarine amphipods, (ASTM E 1367-90, American Society for Testing and Materials, Philadelphia, PA) 1990, pp. 1-24.
- Palanikumar L., Kumaraguru A.K., Ramakritinan C.M., Anand M. Toxicity, biochemical and clastogenic response of chlorpyrifos and carbendazim in milkfish Chanos chanos. Int. J. Environ. Sci. Technol. 2014; 11:765–774.
CrossRef - Shahbaa K., AL-Taee., Karam H., Hana Kh.Ismail. Review on Some Heavy Metals Toxicity on Freshwater Fishes, J. Appl.Vet. Sci. 2020; 5(3): 78 - 86. DOI: https://dx.doi.org/10.21608/javs.2020.100157.
CrossRef







