Investigation of Waste Water Quality Parameters Discharged from Textile Manufacturing Industries of Bangladesh
M. Ahasanur Rabbi1
*
 , Jewel Hossen2
, Jewel Hossen2
 , Md. Mirja Sarwar3
, Pijush Kanti Roy4
, Sharmin Binte Shaheed5
and M. Mehedi Hasan6
, Md. Mirja Sarwar3
, Pijush Kanti Roy4
, Sharmin Binte Shaheed5
and M. Mehedi Hasan6

Corresponding author Email: rabbi_chem@yahoo.com
DOI: http://dx.doi.org/10.12944/CWE.13.2.05
Copy the following to cite this article:
Rabbi M. A, Hossen J, Sarwar M. M, Roy P. K, Shaheed S. B, Hasan M. M. Investigation of Waste Water Quality Parameters Discharged from Textile Manufacturing Industries of Bangladesh. Curr World Environ 2018;13(2). DOI:http://dx.doi.org/10.12944/CWE.13.2.05
Copy the following to cite this URL:
Rabbi M. A, Hossen J, Sarwar M. M, Roy P. K, Shaheed S. B, Hasan M. M. Investigation of Waste Water Quality Parameters Discharged from Textile Manufacturing Industries of Bangladesh. Curr World Environ 2018;13(2). Available from: http://www.cwejournal.org?p=1082/
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2018-03-19 |
|---|---|
| Accepted: | 2018-07-14 |
| Reviewed by: | 
 Dr. Jalil Jaafari (India)
Dr. Jalil Jaafari (India)
|
| Second Review by: |

 Dr. Prithvi Simha (India)
Dr. Prithvi Simha (India)
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Textile manufacturing sector of Bangladesh is the biggest earner of foreign currency and contributes significantly to the GDP of Bangladesh.1 With the rapid development of textile dyeing industry it has become the biggest sector in Bangladesh where large streams of waste effluents are produced. Textile manufacturing is a very complex and lengthy process. It is based on the three steps procedure like fiber into yarn, then yarn to fabric and finally to textiles. These are then fabricated into clothes or other artifacts. Slashing, bleaching, mercerizing, and dyeing are the major water consuming activities as well as waste water generating processes.2 During each stage different type of chemicals are used such as strong acids, strong alkalis, inorganic chlorinated compounds, hypochlorite of sodium, organic compounds such as dye stuff, bleaching agent, finishing chemicals, starch, thickening agent, surface active chemicals, wetting and dispensing agents and salts of metals.3 During the lengthy textile manufacturing process starch, waxes, Carboxymethyl Cellulose (CMC), Polyvinyl Alcohol (PVA), wetting agents and pectins are required for sizing and desizing processes which results in high Biochemical Oxygen demand (BOD) and Chemical Oxygen Demand (COD). Sodium hypochlorite, chlorine, alkali, peroxide, acids, surfactants, sodium phosphate are used in the bleaching process which increase the alkalinity and solid substances in the waste water. Various types of reducing agents, oxidizing agents, acetic acid, detergents, urea, starches, gums, oils, binders, thickeners, cross-linkers and different types of dyes are used in the dyeing and printing stages. The printing and dyeing process generate highly colored waste water with heavy metals and increase BOD.4 The waste water from the textile manufacturing operation may cause change of the physical, chemical and biological properties of surrounding aquatic ecosystem. Being land of rivers, Bangladesh is largely dependent on surface water. Extreme contamination of surface water may cause serious health hazard in the neighborhood, harm ripeness of the land, slaughter fishes and oceanic lives.3 The quality of surface water that people use has a great effect on their health, so effluent impact on water quality is a point of great concern.5-7 The term water quality is used to depict the state of the water, including its chemical, physical and natural attributes, normally concerning its appropriateness for a specific reason.8 Biochemical oxygen demand (BOD), chemical oxygen demand (COD), total dissolve solids (TDS), total suspended solids (TSS), pH, heavy metal content are often used to provide a measure of water quality.9-18
Kamal et al. investigated the effluents from five different industries in Dhaka export processing zone area and found the average BOD, COD and TS (dissolved and suspended both) values equal to 157 mg/L, 508.8 mg/L and 9140.8 mg/L respectively.19 Munnaf et al. investigated the water quality parameters discharged from seven textile dyeing industries at Konabari in Gazipur region of Bangladesh during winter season2 and found the pH value ranges from 9.2-10.5, TDS concentrations 4247-6700 mg/L, TSS values 100-390 mg/L, BOD values 390-515 mg/L, COD values ranged from 1120-1650 mg/L. Islam et al. investigated effluent quality discharged from the textile industry of Purbani group, Gazipur, Bangladesh and found pH values ranged from 6.8 to 9.4, TDS values from 1132 to 3072 ppm, BOD values from 23 to 155 ppm and COD values from 95-135 ppm.20 In 2015 Shahinur Kabir et al. invetigated the physico-chemical characteristic of waste water in savar region and according to the water quality index (WQI) he found some site were very poor and in most of the cases unfit for drinking, wildlife and fish culture.21
From the natural concern, these textile dyeing industries now saw as a noteworthy ecological danger in the modern territory of Bangladesh.22 In Bangladesh so many industries are running in some selective areas. In the Dhaka district and beside the capital city the industries are in a saturated level. Dhaka is Bangladesh’s most populous city and most densely industrialized region.23 Gazipur and Narayanganj are two major industrial areas around Dhaka where remarkable numbers of textile dyeing industries continuously produce textile products. A large portion of textile manufacturing industries in these zone produce huge amount of waste water all the time which are straightforwardly released into the surrounding lake, rural fields, irrigation channels, ponds and these at long lasting, go into the closest river.24 Consequently, a significant bit of the accessible water is being dirtied by the industrial effluents. In the developing countries a noteworthy quantities of research works have been done on industrial wastewater characterization. In the past years some investigative research works to evaluate the waste water quality have also been done in Bangladesh. But continuous monitoring of industrial waste water quality and effluent treatment plants (ETP) is necessary to prevent the risk of surface water pollution. The use of conventional waste water treatment process becomes increasingly challenged and advanced technologies such as anaerobic fluidized bed reactor, aerobic moving bed reactor can be used for the treatment of highly contaminated water.25-29 The objective of the present research was to characterize industrial effluents from six ready made garments industries of Gazipur, Savar and Narayanganj areas and assess the contamination potential.
Materials and Method
Sample Collection
The samples were collected in plastic bottles from six garments industries in Gazipur, Svar and Narayanganj areas in mid-July, 2017. All of the industries under consideration have the Effluent Treatment Plants (ETPs) and the samples were collected from ETP inlet water (before treatment) and ETP outlet water (after treatment) of each industry. The industries where waste water samples were collected located at different zones namely Kashimpur, Shreepur of Gazipur district, Nabinagar of Savar region and Fatullah of Narayanganj district. The six RMG industries are expressed as RMG- 1, RMG-2, RMG-3, RMG-4, RMG-5 and RMG-6. Among of these six industries RMG-1 and RMG-2 are at Kashimpur and Shreepur of Gazipur respectively, RMG-3 and RMG-4 are at Nabinagar of Svar, RMG-5 and RMG-6 are at Fatullah of Narayanganj. The collected samples were stored into separate plastic container and stored at ambient temperature prior to treatment.
pH Measurement
The pH of waste water before and after treatment was measured by potentiometric method. The measurements have been performed on same day of sample collection by using benchtop pH meter (PHS-25).
Biochemical Oxygen Demand Measurement
BOD of the collected samples was determined according to APHA 5210 B 22nd (2012) & BOD Track Instrument Manual of HACH. Most commonly BOD5 value has been used and reported for many applications to indicate the effects of sewage and other organic wastes on dissolved oxygen in surface waters. In this investigation the oxidation test period for BOD was 5 days at 20 degrees Celsius (°C) (BOD5).
Chemical Oxygen Demand (COD) Measurement
COD were determined according to APHA. 5220 B 22nd (2012).
Total Dissolve Solid (TDS) Measurement
TDS values of the samples were determined according to APHA-2540 C 22nd (2012). The collected samples were filtered by filter papers. Total filtrate and washing solution were transferred to evaporating dish and evaporated to dryness on a steam bath. Drying, cooling & weighing cycles were repeated until the constant weight was obtained.
Total Suspended Solid (TSS) Measurement
TSS values were measured according to APHA-2540 D 22nd (2012). GFC Whatman filter paper was dried at 105° C in oven for 1 hour and the fiter paper was cooled in a desicator for 15 minutes. After that the dry weight of the filter paper was taken and the filter paper was placed on filtration apparatus. 100 mL of sample was passed through the filter paper and wash with 10 mL of deionized water. The filter paper was carefully removed from the filtration apparatus and dried for 2 hours at 105° C in an oven. Finally the filter paper was cooled in a desicatior and constant weight was recored.
Results and Discussion
The quality parameters (pH, BOD5, COD, TDS and TSS) of waste water discharged from textile manufacturing industries in the Gazipur, Savar and Narayanganj area and standard values for Bangladesh according to the environment conservation rules.30 1997 are given in table 1.
Table 1: Quality of Waste Water before and after Treatment from the Six Industries
|
Water quality parameters |
RMG-1 |
RMG-2 |
RMG-3 |
RMG-4 |
RMG-5 |
RMG-6 |
ECR, 1997 Standard |
|
|
pH |
BT |
8.56 ± 0.03 |
7.94 ± 0.03 |
8.75 ± 0.03 |
8.05 ± 0.01 |
7.48 ± 0.03 |
7.84 ± 0.06 |
6.5-9.0 |
|
AT |
7.88 ± 0.02 |
7.56 ± 0.02 |
8.48 ± 0.03 |
7.85 ± 0.01 |
7.36 ± 0.02 |
7.52 ± 0.04 |
||
|
BOD5 |
BT |
340 ± 14.42 |
336 ± 6.65 |
425 ± 6.02 |
316 ± 9.07 |
298 ± 14.57 |
276 ± 19.85 |
≤ 150 |
|
AT |
188 ± 5.50 |
82 ± 4.16 |
210 ± 9.45 |
184 ± 7.57 |
168 ± 9.60 |
78 ± 6.08 |
||
|
COD |
BT |
1130 ± 26.65 |
1044 ± 17.78 |
1315 ± 18.14 |
1420 ± 20.29 |
972 ± 7.21 |
1200 ± 8.14 |
≤ 200 |
|
AT |
198 ± 11.93 |
135 ± 6.50 |
490 ± 7.63 |
276 ± 6.65 |
114 ± 3.22 |
178 ± 6.0 |
||
|
TDS |
BT |
3845 ± 30.41 |
4228 ± 26.68 |
3228 ± 36.85 |
3928 ± 32.14 |
2774 ± 24.33 |
3450 ± 31.62 |
≤ 2100 |
|
AT |
2480 ± 23.71 |
2608 ± 33.29 |
1980 ± 36.47 |
2295 ± 31.22 |
1175 ± 33.72 |
1954 ± 21.16 |
||
|
TSS |
BT |
244 ± 17.78 |
146 ± 5.50 |
158 ± 6.02 |
262 ± 11.71 |
115 ± 7.02 |
180 ± 9.71 |
≤ 100 |
|
AT |
164 ± 10.21 |
76 ± 7.23 |
103 ± 4.72 |
158 ± 4.04 |
85 ± 3.60 |
118 ± 4.72 |
||
The pH of waste water is an important parameter which reflects the acidity and alkalinity of the waste water. pH of water adversely affect the aquatic life, plants and human health. Most of the aquatic life such as fish can only survive in a narrow pH range roughly pH 6-9. Variation in pH values of effluent can affect the rate of biological reactions and survival of various microorganisms. The maximum pH value of the waste water before treatment was found for RMG-3 in the Gazipur region. The pH values of the waste water before and after treatment were found within the standard range. These values were ranged between 7.36-8.48 and 7.48-8.75 for treated and untreated waste water respectively (fig. 1).
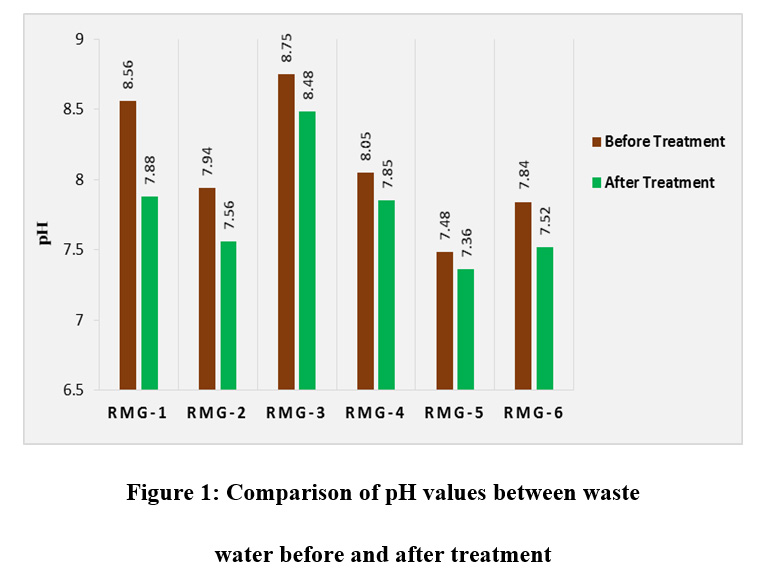 |
Figure 1: Comparison of pH values between Waste Water before and after Treatment |
The amount of oxygen required for micro-organisms to degrade the organic matter is calculated as Biochemcal Oxygen Demand (BOD) of waste water samples. The BOD values depend on the quantity of dissolved organic matter in the waste water samples and more the organic matter more the demand of oxygen by microbes to degrade it. BOD also measures the chemical oxidation of some inorganic matters. The BOD5 concentrations varied from 276-425 mg/L for untreated waste water and 78-210 mg/L for treated water. Befor treatment the recorded BOD5 concentrations of all samples from the all six garments industries were above the permissible limit for Bangladesh and maximum BOD5 concentration was found to be 425 mg/L for RMG-3. The waste water containing large amount of dissolve organic matter should not be discharged in any surface water body without treatment whereas the BOD5 concentrations for four industries were found above the standard limit even after treatment (fig.2). Water can hold only a limited supply of dissolved oxygen and it comes from diffusion from the atmosphere at the air-water interface, and as a byproduct of photosynthesis. The waste water with high BOD values can pollute the local water reservoir by reducing the dissolved oxygen which is a prime necessity for the survival of the living organism. If BOD becomes higher, aerobic bacteria will utilize the available dissolve oxygen of water to perform their metabolic activities. In case of excessive BOD there will be a drustic deficiency of DO and water will be in anaerobic condition resulting in mortality of living aquatic organisms.
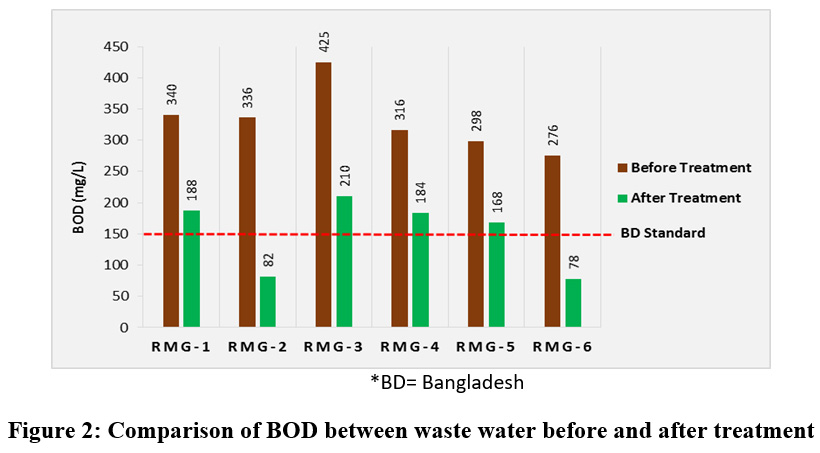 |
Figure 2: Comparison of BOD between Waste Water before and after Treatment Click here to view figure |
Chemical oxygen demand (COD) is a measure of all oxygen-demanding substances, including both biodegradable and non-biodegradable substances present in the waste water sample. So COD testing assesses the quantity of all chemically oxidizable substances and can be directly related to the true oxygen demand imposed by the effluent if released into the environment. COD values are always higher than the BOD values and more reliable parameter to qualify the water sample. So COD concentration must be reduced to below the standard limit through waste water treatment. Before treatment the COD values of all the water samples from six different garments industries were recorded and found to be in the range of 972 to 1420 mg/L and after treatment these values were found to be 114 to 490 mg/L. Before treatment COD concentrations of all the water samples were found to be much higher than the permissible limit and maximum COD value was found to be 1420 mg/L for RMG-4 in Savar region. Even after treatment COD concentrations for RMG-3 and RMG-4 were above the permissible limit (fig. 3). Higher COD concentration means a greater amount of oxidizable organic material are existing in the sample, which will reduce dissolved oxygen levels of the nearest water body. A reduction in dissolve oxygen in any surface water body leads it to anaerobic conditions, which is deleterious to higher aquatic life forms.
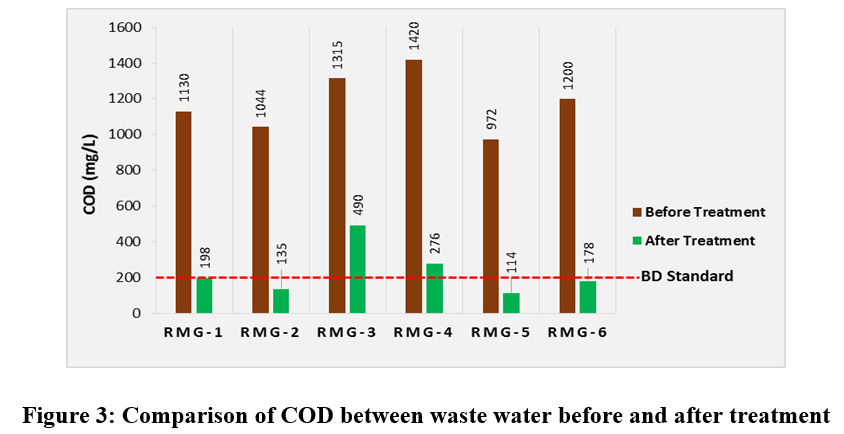 |
Figure 3: Comparison of COD between Waste Water before and after Treatment Click here to view figure |
Total Dissolved Solid (TDS) is a measurement of inorganic salts, organic matter and other dissolved materials in water.31 Before treatment the TDS concentrations of all the samples to be much higher than the permissible limit (fig. 4). Even after treatment the TDS concentrations for three industries were found to be higher than the standard value. However, for the other three industries these vules after treatment were slight lowered from the standard level. TDS concentrations before treatment varied from 2774 - 4228 mg/L and after treatment varied from 1175 - 2608 mg/L. A constant level of minerals in the water is necessary for aquatic life. Drinking water with moderate amounts of TDS is preferred over other kinds of water. But the high TDS concentration in a water body affects many different factors. Excess amount of dissolved solids cause toxicity through increasing salinity, changes the ionic composition of the water and toxicity of its individual ion.32 Specially the water containing chemicals like nitrates, sodium, chlorine, chloramines, cadmium, copper, sulfates, barium and fluoride may cause variety of health hazards. In the textile industries huge quantities of common salt are used in the dyeing process and a large portion of these salts are discharged through waste water. The higher levels of TDS are not favorable for the aquatic life and according to Chhatwal et al. for diverse fish production the permissible maximum TDS value is 400 mg/L.33 Whereas very low TDS concentration lower the density of water, causing the changes in the flow of water in and out of an organism’s cell and thereby interupt the physiological processes of the aquatic life. The TDS concentrations that are too high or too low may limit the growth and may affect the aquatic life. When TDS levels exceed 1000 mg/L, it is generally considered unfit for human consumption. The lowest TDS concentration after treatment was found to be 1175 mg/L for RMG-5.
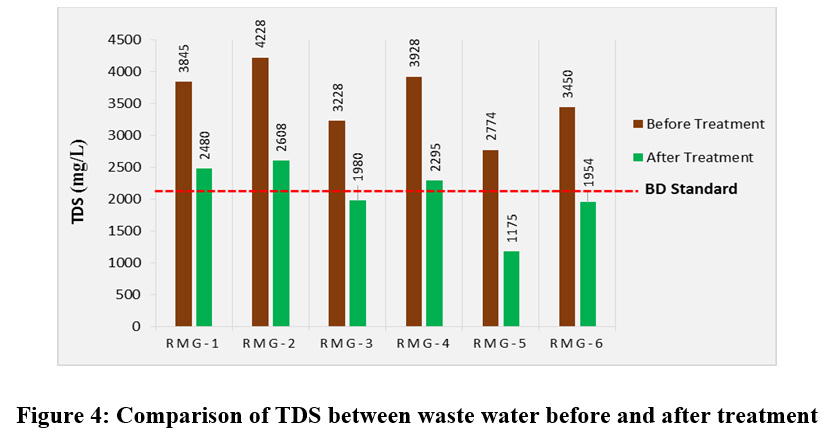 |
Figure 4: Comparison of TDS between Waste Water before and after Treatment Click here to view figure |
Before treatment Total Suspended Solid (TSS) concentrations of all the water samples from all the six garments industries were found to be much above the permissible level and varied from 115 mg/L to 262 mg/L. After treatment the values were reduced to the range of 75 to 164 mg/L. According to the Environment Conservation rules 1997 the TSS concentration in the textile effluent in Bangladesh should be less than 100 mg/L whereas the TSS concentrations in four industrial effluents after treatment were found to be higher than this standard level (fig. 5). If the waste water with high concentration of suspended solid is continuously discharged in the nearby reservoirs the water quality will be lowered in a number of ways. High levels of total suspended solids will increase water temperatures by absorbing more sunlight, lessen the ability of the water to hold oxygen necessary for aquatic life, increase the turbidity of water and thereby inhibit photosynthesis by blocking sunlight which also results in less oxygen production. As a result of deficiency of sunlight, the growth of plant life in watercourses will be inhibited. The combination of warmer water, less light and less oxygen makes it impossible for some forms of life to exist. Moreover sedimentation of some suspended solids during a long period of time at the bottom of a water body can impair lakes and other water bodies and increase flooding risks.
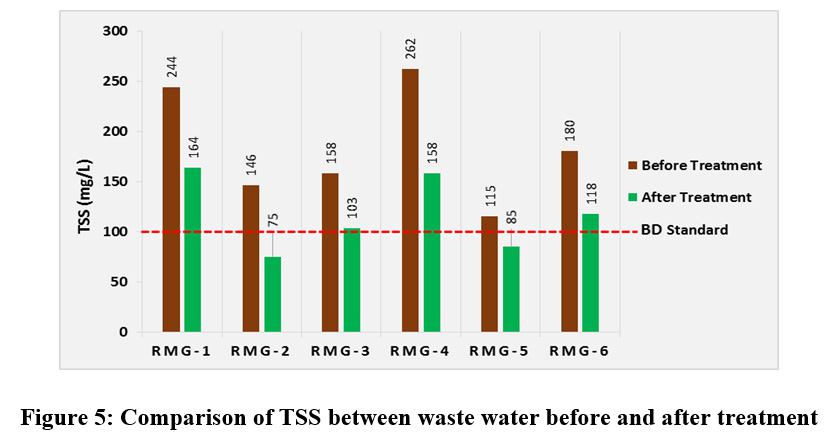 |
Figure 5: Comparison of TSS between waste water before and after treatment Click here to view figure |
Conclusion
In this investigation the BOD5, COD, TDS and TSS values for waste water before treatment were found much higher than the permissible limits for all the six garments industries. Most of the cases after treatment these parameters were found within the permissible level, although in a few cases the BOD5, COD, TDS and TSS values were found to lie above the standard limits even after treatments. This investigation suggests that the implementation of ETPs in all the dyeing and textile processing industries should be mandatory and all ETPs should follow the National Standards. The efficiency of the ETPs should be increased and simultaneously department of environment should strongly monitor the quality of industrial wastewater. Although ETP is one of the most important and prominent part of textile dyeing sector, a systematic approach to reduce the generation of waste at source is more important. To reduce the environmental pollution by industrial waste water, a number of waste minimization steps and techniques should be taken. Water consumption must be reduced with minimum leakages; faulty valves should be repaired to ensure maximized use of consumed water. For reducing chemical consumption, control of over dosing, recovery and reuse of chemical and improving scheduling may be highlighted. Moreover consumption of water and chemicals should be reduced drastically in the major textile processing steps including beaching, sizing, desizing scouring, dyeing, printing etc. Careful selection of synthetic dye for the dyeing process is important. Dyes with high fixation capacity, low toxicity, absence of heavy metals and low liquor ratios should be selected. The recipe of the dyeing bath should be optimized to ensure maximum fixation and minimum wash-off. New processes have to be developed to reuse the dye baths containing acid or basic dyes. Natural dye would be a good solution to protect the pollution.
Acknowledgments
The authors are grateful to the all six garments industries for their kind cooperation and Bangladesh Council of Scientific and Industrial Research (BCSIR) and University of Rajshahi for providing all research facilities.
References
- Rabbi, M. A., Hasan, M. M. and Akhter, A., Heavy metals content in inlet water, treated and untreated waste water of garments industries at Gazipur, Bangladesh.; Environmental Science: An Indian Journal. 2016;12(4):133-136.
- Munnaf, A., Islam, M. S., Tusher, T. R., Kabir, M. H. and Molla M. A. H., Investigation of Water Quality Parameters Discharged from Textile Dyeing Industries, Journal of Environmental Science & Natural Resources. 2014;7(1):257–263.
- Dey, S. and Islam, A., A Review on Textile Wastewater Characterization in Bangladesh, Resources and Environment.2015;5(1):15-44.
- Hannan, M. A., Rahman, M. A. and Haque, M. F., An Investigation on Quality Characterization and Magnitude of Pollution Implications with Textile Dyeing Industries’ Effluents using Bleaching Powder, DUET Journal. 2011;1(2):49-59.
- Chhikara, S., Rana, L. and Poonam, Physico-chemical Characterization of Textile Mill Effluent: A Case Study of Haryana, India, Environment & We An International Journal of Science & Technology. 2013;8:19-23.
- Yahaya, M. I., Mohammad, S. and Abdullahi, B. K., Seasonal variations of heavy Metals concentration in Abattoir dumping site soil in Nigeria. Journal of Applied Sciences for Environmental Management. 2009;'13(4):9-13.
- Alom, M. M., Effects on Environment and Health by Garments Factory Waste in Narayanganj City, Dhaka, American Journal of Civil Engineering. 2016;4(3):64-67.
- Diersing, N., Water Quality: Frequently Asked Questions, Florida Brooks National Marine Sanctuary, Department of Commerce, USA. 2009.
- Hangargekar, P. A. and Takpere, K. P., A Case Study on Waste Water Treatment Plant, CETP (Common Effluent Treatment Plant), International Journal of Innovative Research in Advanced Engineering. 2015;11(2):34-39.
- Aslam, M. M., Baig, M. A, Hassan, I., Qazi, I. A., Malik, M. and Saeed, H., Textile wastewater characterization and reduction of its COD & BOD by oxidation, Electronic Journal of Environmental, Agricultural and Food Chemistry. 2004;3(6):804-811.
- Maheshwari, R., Rani, B., Saxena, A., Prasad, M., Singh, U., Analysis of effluents released from recycled paper industry, Journal of Advanced Scientific Research. 2012;3(1):82-85.
- Islam, M. M., Mahmud, K., Faruk, O., and Billah, M. S., Textile Dyeing Industries in Bangladesh for Sustainable Development, International Journal of Environmental Science and Development. 2011;2(6):428-436.
- Sultana, Z., Ali, M. E., Uddin, M. S. and Haque, M. M., Implementation of Effluent Treatment Plants for WasteWater Treatment, Journal of Environmental Protection. 2013;4:301-308.
CrossRef - Singh, S. N., Srivastav, G. and Bhatt, A., Physicochemical Determination of Pollutants in Wastewater in Dheradun, Current World Environment. 2012;7(1):133-138.
- Popa, P., Timofti, M., Voiculescu, M., Dragan, S., Trif, C., and Georgescu, L. P., Study of Physico-Chemical Characteristics of Wastewater in an Urban Agglomeration in Romania, The ScientificWorld Journal. 2012:1-10.
CrossRef - Shivsharan, V. S., Wani, M. and Khetmalas, M. B., Characterization of Dairy Effluents by Physicochemical Parameters, British Biotechnology Journal. 2013;3(4):575-580.
CrossRef - Nagamani C., Devi C. S., Physico-chemical analysis of water samples, International Journal of Scientific & Engineering Research. 2015;6(1):2149-2155.
- Dizgah, S. H., Taghavi, K., Jaafari, J., Roohbakhsh, E., and Ashrafi, S.D., Data on pollutants content in the influent and effluent from wastewater treatment plant of Rasht in Guilan Province, Iran, Data in Brief. 2018;16:271–275.
CrossRef - Kamal, A.K.I., Ahmed, F., Hassan, M., Uddin, M. K. and Hossain, S. M., Characterization of Textile Effluents from Dhaka Export Processing Zone (DEPZ) Area in Dhaka, Bangladesh, Pollution. 2016;2(2):153-161.
- Islam, M. S., Chowdhury, M. A. H., Billah, M. M. S., Tusher, T. R. and Sultana, N., Investigation of effluent quality discharged from the textile industry of Purbani group, Gazipur, Bangladesh and its management. Bangladesh Journal of Environmental Science. 2012;23:123-130.
- Kabir, M. S., Rahman, M. M. and Ahmed, S. M. F., Physico-chemical and Bacteriological Attributes of Wastewater in Savar, Dhaka, Bangladesh, IOSR Journal of Environmental Science, Toxicology and Food Technology. 2015;9(7):06-10.
- Karim, M. M., Das, A. K., Lee, S. H., Treatment of colored effluent of the textile industry in Bangladesh using zinc chloride treated indigenous activated carbons. Analytica Chimica Acta. 2006;576(1):37-42.
CrossRef - Dhaka, Encyclopædia Britannica. 2017.
- Sultana, M. S., Islam, M. S., Saha, R. and Mansur, M. A. A., Impact of the effluents of textile dyeing industries on the surface water quality inside DND embankment, Narayanganj, Bangladesh Journal of Scientific & Industrial Research. 2009;44(1):65-80..
CrossRef - Jafari, J., Mesdaghinia, A., Nabizadeh, R., Farrokhi, M., Mahvi, A. H., Investigation of Anaerobic Fluidized Bed Reactor/ Aerobic Moving Bed Bio Reactor (AFBR/MMBR) System for Treatment of Currant Wastewater, Iranian Journal of Public Health. 2013;42(8): 860-867.
- Jaafari, J., Mesdaghinia, A., Nabizadeh, R., Hoseini, M., Kamani, H., and Mahvi, A. H., Influence of upflow velocity on performance and biofilm characteristics of Anaerobic Fluidized Bed Reactor (AFBR) in treating high-strength waste water, Journal of Environmental Health Science & Engineering. 2014;12(139):1-10.
- Naghipour, D., Taghavi, K., Jaafari, J., Mahdavi, Y., Ghozikali, M. G., Ameri, R., Jamshidi, A. and Mahvi, A.H., Statistical modeling and optimization of the phosphorus biosorption by modified Lemna minor from aqueous solution using response surface methodology (RSM), Desalination and Water Treatment. 2015;57(41):19431-19442.
CrossRef - Jaafari, J., Seyedsalehi, M., Arjestan, M. E., Barzanouni, H., Ghadimi, S., Kamani, H., Haratipour, P., Simultaneous biological organic matter and nutrient removal in an anaerobic/anoxic/oxic (A2O) moving bed biofilm reactor (MBBR) integrated system, International Journal of Environmental Science and Technology. 2017;14(2):291-304.
CrossRef - Seyedsalehi, M., Jaafari, J., Hélix- Nielsen, C., Hodaifa, G., Manshouri, M., Ghadimi, S., Hafizi, H., Barzanouni, H., Evaluation of moving-bed biofilm sequencing batch reactor (MBSBR) in operating A2O process with emphasis on biological removal of nutrients existing in wastewater, International Journal of Environmental Science and Technology. 2018;15(1):199-206.
CrossRef - Environment Conservation Rules (ECR), 1997, Schedule 10, Rules 13, Bangladesh Gazette. 1997;3132-3134.
- U.S. Environmental Protection Agency, Office of Water, Quality Criteria for Water, EPA 440/5-86-001. Washington D.C. 1986.
- Weber-Scannell, P. K. and Duffy, L. K., Effects of Total Dissolved Solids on Aquatic Organisms: A Review of Literature and Recommendation for Salmonid Species, American Journal of Environmental Sciences. 2007;3(1):1-6.
CrossRef - Chhatwal, G. R., Encyclopedia of Environmental Biology, Ammol Publication Private Ltd, New Delhi, India. 1998;287-301.







