Impact of Parasitic Diseases on Fishes of North West Himalayan Streams
Feroz A. Shah1 , Imtiyaz Qayoom1 * , Masood H. Balkhi1 and Ashwani Kumar1
1
Faculty of Fisheries SKUAST-K Rangil,
Ganderbal,
Jammu and Kashmir
India
DOI: http://dx.doi.org/10.12944/CWE.10.3.22
Pathological disorders caused due to metazoan parasitic infestation were studied in the hill stream fishes of northwest Himalayan region. Host specificity was found to be one of the fundamental features of metazoan parasites which belonged to the class Cestoda, Nematoda, Trematoda and phylum Acanthocephala. The study indicates that a successful co-evolution of the host and its parasite has caused the adaptation of the later by developing evading mechanisms in order to avoid extinction. Besides this it was also observed that some parasites have even understood to benefit from the well developed antiparasitic armament in fish intestinal epithelia. Thus, parasites are exploiting the antiparasitic response mechanism of the host to optimize, host finding, invasion and survival in the host. Such interaction between host and parasites are considered phylogenetically old. Some monogeneans, cestodes, digeneans and acanthocephalans were found to resist pronounced cellular and host reactions which even improved the attachment of parasite into the host predilection site. Scanning Electron Microscopy and hitstopathological examination was conducted on parasites recovered from fishes in order to understand the host parasite interaction and the damage inflicted by parasite on hill stream fish species.
Copy the following to cite this article:
Shah F. A, Qayoom I, Balkhi M. H, Kumar A. Impact of Parasitic Diseases on Fishes of North West Himalayan Streams. Curr World Environ 2015;10(3) DOI:http://dx.doi.org/10.12944/CWE.10.3.22
Copy the following to cite this URL:
Shah F. A, Qayoom I, Balkhi M. H, Kumar A. Impact of Parasitic Diseases on Fishes of North West Himalayan Streams. Available from: http://www.cwejournal.org/?p=13130
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-09-15 |
|---|---|
| Accepted: | 2015-10-11 |
Introduction
Parasites are specialists in host environment with close co-evolutionary ties. Convolution embodies two components, togetherness (Co-) and history (Evolution). Co-evolution encompasses both microevolutionary and macroevolutionary associations. Host specificity (by the parasite) and defense mechanism (by the host) play important role in the microevolutionary studies. It is important to integrate micro and macroevolutionary patterns and processes in order to generate a more robust and unified evolutionary biology in host-parasite evolution. Co-evolutionary patterns in parasite-host association indicate that both classes of organisms show a phylogenetic retention of evolved traits that is sufficient to allow reconstruction of their historical relationship.
Number of investigations has shown beyond any doubt that both innate and acquired immune effector mechanisms against parasites are working in a range of teleosts (Woo, 1992). However, if these mechanisms were highly effective and able to offer total protection to the host it would result in extinction of parasites. Evidently this is not the case a number of investigations, including this one, have documented the existence of rich parasitofauna in every habitat supporting teleosts. We know that every fish species have one or several often very specific parasites in addition to a number of less specific parasites. This will include both protozoans and metazoans and we know that the total number of described fish helminth parasites alone at present exceed far more than 30,000 (Williams and Jones, 1994) therefore it is evident that a parasite must possess an evading mechanism in order to survive as species.
Parasite infections in teleost fish species are limited by constitutive innate defense mechanism that render the host refractory or reduce the severity of infection. Acanthocephalan, the spiny headed worm penetrates the intestinal wall of the host, the inflated bulb behind the spines improves the attachment. However, the most effective anchoring of the worm was actually accomplished by the host itself due to inflammatory reactions and tissue reaction around the proboscis and the bulb secures the firm attachment making it almost impossible to remove this acanthocephalan from intestinal tissue. All helminth parasites are circumventing the response of the host by utilizing the tissue reaction for improved attachment and survival in the host fish. Three broad areas of such host parasite interaction are demonstrated, (I). mechanism by which parasite invades, transmit and colonize variety of niches within the host. (II) optimization of the conditions of host micro-environment for parasites own survival by inducing physical or biochemical changes. (III) evasion of defense mechanism of the host in a variety of ways.
Methodology
Histopathology
Fishes were sacrificed and tissue infested sites were collected for histopathological examination. Fishes were well euthanized before killing them (Nickum, et al., 2005). Sections of tissues were stained with Hematoxylin and Eosin stain and examined microscopically. Histopathology methods described by John Morison & Sonia Momford (2007) were employed.
Scanning Electron Microscopy (SEM) studies
samples were fixed in 2.5% gluteraldehyde, buffered in 0.1 M Sorensen’s phosphate buffer, post fixation in Caulifield’s 1% buffer Osmium tetraoxide (OsO4) for 1-2 hour. Afterwards the sample was dehydrated by passing through an ascending series of graded alcohol started from 30%, 50%, 70%, 90% and finally absolute alcohol for 15-20 minutes. Fixed dehydration of the sample was done in critical point dryer (Balzers Union Critical Point Dryer). The material was placed each in a metal basket which was then lowered into a bath of absolute alcohol and after about an hour the material was placed in the bumb of the dryer filled with absolute alcohol, the alcohol was gradually replaced by liquid carbon dioxide (CO2). Specimens were dehydrated by achieving critical point of carbon dioxide (31°C, pressure 72.9 atm) the gaseous carbon dioxide was released and the dried material was taken out of the bumb.
Dried specimens were then affixed properly on aluminum stubby silver deg (collidal silver). It was then sputter coated by a thin coating (100-150 Ao) of the gold palladium alloy in a sputter-coater (Polaron SEM coating unit E500) using argon gas. The processed samples were then loaded in a JEOL JSM-T330 Scanning Electron Microscope.
Results
Acanthocephalans
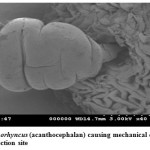
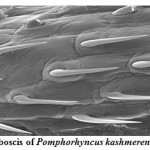
Pomphorhynchus is a metazoan parasite of freshwater fishes, parasitizing the gastrointestinal tract of the hill stream fishes. This spiny headed worm was seen to insert its proboscis into the submucosa and an inflated bulb behind the spines improves the attachment. However, the most effective anchoring of the worm is actually accomplished by the host itself. The presence of these parasites in the intestinal tract was identified by the appearance of yellowish white granules on the outer surface of the intestine; these represent connective tissue capsules developing around the proboscis while the parasite is piercing around the intestinal wall. In some cases the Pomphorhynchus infection was found to cause immunopatological situations in the fishes by inviting the immunocompetant cells to the spot of larval infestation, these cells sometime initiated a granulomatous type of reaction against the larval stages of the parasite thus giving rise to the nodules, the abundance of the parasite was sometimes causing blockade of the gut of the fish. In parasitized Schizothorax niger, four main types of reaction against the body of the acanthocephalan were recognized. Pomphorhynchus kashmirensis caused local damage to the intestinal wall, eliciting catarrhal-erosive enteritis in the lumen and a fibroblastic-collagenous and fibro-epithelioid encapsulation in its thickness with tissue zonation according to the depth of parasite penetration, the parasite circumvented all these reactions for its firm anchoring in the predilection site of host tissue.
The principal effect on the host by the thorny headed worms consisted of mechanical damage to the intestinal wall and in deprival of nourishment, especially when the infection was severe. In such case number of worms in the intestine varied from 20 to 120. Very often there were a few worms in the intestine, and the Schizothorax (Endemic fish) was founded more susceptible to the infection of Acanthocephala. Very extensive tissue proliferations were found which were damaging the neighboring tissue of the liver and pancreas. The appearance of a large number of nodules is based on the fact that the duration of the pomphorhynchoids remaining in the intestine is not long, coming to an end of spring, and that after the worm proper has fallen off, and the proboscis itself perishes in the intestinal wall. In this way more yellow nodules and eruptive spots appear in the intestinal wall. It was also found that P. kashmirensis attack the entire intestine, sometimes close to the anus, so much so that one end can protrude. Pomphorhynchus was also found to penetrate the body cavity, whereby the liver very often was pierced which resulted in necrosis of the liver. This type of damage was associated with the clinical signs like inflammation and infectious ascites. Results of this study indicated that a severe attack by this worm can kill the fish or deprive them of their power of resistance against other injuries.
 |
|
 |
|
 |
|
 |
|
Neoechinorhynchus
In view of short proboscis with few hooks was found to penetrate less deeply in the intestine. Even if it bores through the epithelium and destroys the mucosa at some places with its hooks. However, it was found that if proboscis remains in the submucosa, the stratum compactum was not reached. Only the tissue lacerated by the proboscis, become necrotic, and even when the worms establish themselves along the length of the intestine, the intestinal tissue under it remains unaffected. The body of the parasite in most cases was surrounded by mucus, no leucocyte accumulated even around the proboscis which can be easily withdrawn from the intestine, because the Neoechinorhynchus often changes its position in the intestine. The older females and males parasites die soon after copulation and are to be found predominantly in the posterior part of the intestine. Neoechinorhynchosis was accompanied by protrusion of eyes of hosts, the reason for this is that gut in these cases was so infested with Neoechinorhynchus that the entire intestine from the stomach onward was occupied by parasites. In the case of Cyprinus carpio the intestinal wall was not pierced, but the intestinal epithelium was severely destroyed in the vicinity of the proboscis and the connective tissue was found to develop proliferations. Some fishes were found completely emaciated due to the damage of the intestine by Pomphorhynchus infection; in such conditions the fishes were not marketable. The additional observations on the account of this disease were presence of yellowish white fibro-epithelioma on the lip, skin and fins. The acanthocephalosis gradually abated only with the disappearance of the worms at the end of summer.
 |
|
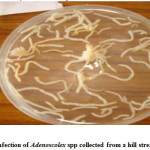
Cestoda Trematoda and Nematoda
The attachment of these parasites was considerably improved due to the inflammatory reaction of the host fish, which encapsulate the scolex in a spherical tissue structure comprising a constriction below the cup-shaped scolex. In case of Adenoscolex and Bothriocephalus infection, due to unarmored scolex they do not attach onto the surface of intestinal villi, their attachment was characterized by the secretions of the gland cells present in the scolex and the anterior body wall, however in heavy infection the intestine which is normally opaque, becomes transparent. The parasite produces shallow lesions within the intestinal tissue, causing destruction of mucosa and submucosa. The pathological changes manifested in the form of shortening of intestinal villi or compression of the muscosal folds due to heavy infection which probably is a consequence of necrosis. Furthermore the cestode parasites deprive the host from some essential nutrients especially vitamins. The monogenean trematode Diplozoon (ectoparasite) attach themselves between the series of gill filaments and impair respiration of host, they destroyed brachial tissues leading to Inflammation and gill lamilar oedema. The Camallanus (nematode) was found to cause mild damage to the host. The histopathological study of the infected organs showed that the surface epithelium, lamina proporia, muscle tissue and submucosa were disintegrated at the site of entry of the nematodes into the wall; adjacent cells became flattened with displaced nuclei. Worm portions present in the submucosa were surrounded by fibroblasts, histiocytes, eosinophils and lymphocytes but no capsule was present. Blood vessels were congested and dilated, and in heavy infections there was a wide spread haemorrhage and complete loss of muscle layers. The host tissue response of fish infected with Bothriocephalus was some time pronounced as intact pleurocercoids were found encapsulated with connective tissue that was infiltrated with lymphocytes and macrophages, granulomatous tissue that was fibrotic was also observed. Spleen demonstrated a reduction in cellularity and increased connective tissue.
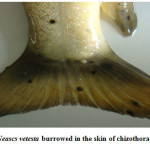
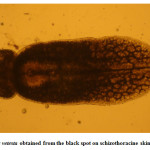
Moderate incidence of the black grub infection occurred in the adult and fingerlings of schizothoracine fishes, the infected fingerlings showed behavioural changes like laziness and less feed uptake. On examining the black spot, a cyst was recovered which was identified as metacercarial cyst of Neascs vetesta which is known to have many intermediate hosts and fish is one of them. The metacercaria of N. vetesta was found to burrow into the skin of fish fingerling causing the formation of a cyst approximately 0.5 mm in diameter. This parasite is known to have a complex life cycle that requires fish eating birds or mammals, snails, and fish at different stages in order to survive. In general, even heavy infestations of these parasites do relatively little damage to the fish. But in the case of juvenile fish the infestation can be dangerous. The black grub is actually white and not black; the black spots are the result of pigment from the fish that surrounded the encysted grub. The black grub was found only in the flesh and the abdominal cavity. The histopathological investigations showed the fibrous capsule formation around the cyst which was putting mechanical pressure to the surrounding tissues, hence reducing the peritoneal space of the fingerling sometimes resulting in abdominal distension. The table size fish infested by black grub did not cause any serious health problem to fish, but infected fish was not marketable.
 |
|
 |
|
 |
|
Discussion
The research activity in the field of fish parasitology has expanded significantly during the latest decades and we are gaining more information about the intricate reactions of teleosts against various pathogens. It is known from the mammalian immunology that some parasites utilize central molecules of the immune system to invade the host the classic example is that of Leishmania infecting macrophages, one of the most important cells of the host. The binding between the parasite and the macrophage is crucial for the infection. This is accomplished by interaction between complement receptors on the host cell membrane and deposited complement factors on the parasites (Wakelin, 1996). Entry into parasitophorus vacuole of the macrophage the Leishmania parasite survives by modulating the oxidative burst of the cell and changing expression of MHC II and cytokines of the host cell (Bogdon and Rollinghoff, 1999). Also the immunologically important M cells (Membranous epithelial cells) in mucosal epithelia (which transport antigen from the surface of the underlying lymphoid tissue) are exploited by pathogens as a gateway into the host. In this study, we have found the evasive mechanisms by the parasites which are little different from the mammalian system. The parasites which were inhabiting the alimentary canal of fishes were found to cause severe damage to the intestinal lumen and to the surrounding tissue by penetrating through the intestinal wall and finally leading to necrosis of the infested tissue. The parasites were sometimes found to damage the nervous system of the host, interfering with their nutrition and metabolism and disturbing the movement and secretary functions of the alimentary canal. Early works in the field of fish histopathology are supporting our observations, noteworthy is the work of Bell et al. (1990) who on the basis of his exhaustive work on intestinal histopathology of fishes reported many histopathological and histomorphological manifestations in fishes. The presence of helminth parasite in intestinal lumen was found to invite various immunocompetent cells (mast cells and eosinophils) which were degranulating and releasing some helminthotoxic substances hence causing destruction of the larval helminth parasites in the alimentary canal of the fish but on the other hand these helminthotoxic histamines were found to cause local inflammatory reaction in the intestinal tissue thus damaging the intestinal lumen, also the movement of the parasite was putting mechanical pressure on to the surrounding tissue leading to the necrosis of tissue. The change of the predilection site by the parasite was leaving ulcer caused by its proboscis/scolex, which was found to be a suitable site for the secondary infection by the microorganisms. The juvenile parasites were causing severe granulomatous reaction in the tissue, which they were inhibiting hence altering the normal histological setup of the tissue by accumulation of eosinophils and fibrous tissue around the infestation site. These results are in accordance with the findings of Crompton (1973) and Chishti et al. (2002) who published a complete review on the sites occupied by some parasitic helminths, and the extent of damage thereby caused. They laid emphasis on the histopathology caused by the Acanthocephala spiny headed gastrointestinal parasite on the freshwater fishes. They were of the same opinion that juvenile Pomphorhynchus parasites were causing severe granulomatous reaction in host tissue.
The mucosal epithelium of the gut adjacent to the metasoma of the worm suffered compression and abrasion. The prosoma of the P. kashmirensis penetrated the mucosal epithelium, lamina proporia, stratum compactum, stratum granulosum, muscularis and serosa of the gut wall, and was invested by fibrous capsule of inflammatory tissue. This was found to be composed of 4 principal cell types. Eosinophilic granulate cells (EGCs); type A cell, type B cell; fibroblasts, some melanocytes and lymphocytes were also present. The role of EGCs in the inflammation response is not known, but may not be an active one. The type A cells resembled the neutrophils of other fish species, and were tentatively placed in this category. The type B cells resembled the plasma cells and may have been involved in a humoral response to the parasitic infection, the integument of P. kashmirensis did not appear to be damaged by the cells of the inflammatory tissue, and it seems likely that this host response was incapable of rejecting the parasite. Same conclusion has been drawn by Cohen and Sadam (1976) working on P. levis infecting rainbow trout. Our results are in accordance with the results of Martins et al, (2001) who, while working on Pomphorhynchus histopathology drew almost the same.
In cestode infections caused either by Bothriocephalus or Adenoscolex there was excessive mucus production and lymphocyte infiltration associated with focal pressure necrosis, localized haemorrhages were sometimes found. Some fishes were found to have distended intestines due to blockage by aggregates of worms. Same observations were made by Scott and Grizzle (1979) on pathology of cyprinid fishes caused by Bothriocephalus idella and reported the disorders like focal pressure necrosis and excessive mucous production in fish. Furthermore hyperplasia due to mechanical irritation was found to be the reaction of connective tissue, by contrast the unarmed scoleces does not cause tissue reactions and inflammation. Almost same results were found by Korting (1977) who presented a brief review on hyperplasia and mechanical irritation caused due to cestode parasites, and concluded that this association does not cause any serious tissue inflammation. In the current study, inflammatory foci of mononuclear cells and haemorrhages in the intestine was predominant in the Acanthocephala infection these observations corroborate with the findings of Ferraz et al, (1990) who had drawn the same conclusion while studying the histopathology caused by P. mesopotamicus. Further, Esch and Huffins (1973) studied the tissue reaction of M. dolomieui infected with acanthocephalans and reported fibrosis and eosinophilic granulocytosis as observed in the present work. According to Hedrick, (1998) the presence of great number of helminths may cause inapettence and susceptibility to secondary infection of fungus and bacteria. These findings are in accordance with our observations in which the lesions caused by the helminth parasites were attacked by the secondary invaders like bacteria and fungi. Intestinal obstruction, compression of villi, haemorrhages, presence of macrophages and fibroblasts were tissue changes reported by Amin & Heckmann (1992) from C. columbianus infected with N. idahoensis. This confirmed the present observations in which the proboscis attachment provoked the host tissue response in both common carp and schizothoracine fishes. The same conclusion was drawn by Martins et al, (2001) who reported severe histopathological manifestations as a result of Acanthocephala infection in fishes. Longshan, et al, (2004) reported the same results while working on the greater pipe fish, but did not mention about the presence of immunocompetant cells in the predilection site of the parasite. The nematode parasites were found to cause localized damage in the predilection sites, by boring through the tissue. In the present study was coincide with Mathen et al, (2004) who working on the effect of parasitism on Europeaneels by nematode parasites.
Acknowledgement
Corresponding author is highly thankful to Department of Science and Technology (DST) for funding the research work through a sponsored Fast Tract Young Scientist project. Thanks are also due to Dr. Kurt Buckmann of Royal Veterinary Faculty, Denmark, for providing research literature and valuable suggestions.
References
- Amin, O.M and Heckmann. 1992. Histopathological study of GI helminth parasites in fish. Journal Fish Disease. 13: 2, 220-230.
- Bell, G.R., Hoffman, R.W. and Bronen, L.L. 1990. Pathology of experimental infections of the sable fish, Anoplana fimbria (Pallas) with Renibacterium salmoninarum the agent of bacterial kidney disease in salmonids. Journal of Fish Disease, 13 (5): 355-368.
- Bogdon, C. and Rollinghoff, M. 1999. How do protozoan parasites survive inside macrophages. Parasitology Today. 15,22-28.
- Chishti, M. Z., Feroz, A. S. and Fayaz, A. 2002. Histopathological study of Pomphorhynchosis in the fishes of In: Proceedings of the 16th National Congress of Parasitology. Barelli. India., pp176-180.June 2003.
- Cohen, S. and Sadam, E. H. 1976. Immunology of parasitic infection. Blackwell Scientific Publication: Oxford UK 266pp.
- Crompton, D. W. T. 1973. 'The sites occupied by some parasitic helminths in the alimentary tract of vertebrates. Biological Reviews of the Cambridge Philosophical Society, 48, 27-83.
- Esch, G. W. and Huffines, W. J. 1973. Histopathology associated with endoparasitic helminths in Bass. Journal . 59 (2): 306-313.
- Ferraz, D. Lima, C. L. B., Ferraz, J. A., and Ceccarelli, P. S. 1990. Ocorrencecia de acantocefalos parasitando pacu, Piaractus mesopotamics Holmberg, Em. Piscicultra. Bol. Tec. CEPTA, 2: 43-51.
- Hedrick, R. P. 1998. Relationship of the host, pathogen and environment: implication for disease of cultured and wild fish populations. Journal of Aquature and Animal Health. 10: 107-111.
- John, M. and Sonia, M. 2007. Fish Histology and Histopathology. Laboratory Manual. USFWS-NCTC Publishing, Chapter 1. 237-250pp.
- Korting, W. 1977. The host reaction against some parasites. Und. Umwelt., 4, 37-48.
- Longshan, M., Green, M.J., Feist, S.W. 2004. Histopathology of parasitic infections in greater pipe fishes Syngnathus acus from an estuary in UK. Journal of Fish Disease. 27:(4) 245-248.
- Mathen, J. G., Clive, R., Kenedy, E., Susanne, Q. J and B. 2004. The effect of parasitism of European eels with nematodes, Angullicola crassus on the impact of netting and aerial exposure. Aquaculture. 233 (1-4) 45-54.
- Martins, M. L., Moraaes, F. R., Fujimota, R. Y., Onaka, E. M and Quintana, C. I. F. Prevalence and histopathology of Neoechinorhynchus curemai Noronha, 1973 (Acanthocephala) in Prochilodus lineatus, Valenciennes, 1836 from volta grade reservoir, MG. Brazil. Aquaculture., 3:(1), 88-92.
- Nickum, J. G.; Chair, H. L.; Bart, Jr., Bowser, P. R.; Greer, I. E. ; Hubbs, C.; Jenkins, J. A.; MacMillan, R. ; Rachlin, J. W.; Rose, J. D.; Sorensen, P. W. and Tomasso, J. R. (2004). Guidelines for the Use of fishes in research. Aaron Lerner, Publications Manager. American Fisheries Society, 5410 Grosvenor Lane, Bethesda, M.D. 20814.
- Scott, A. L and Grizzle, J. M. 1979. Pathology of cyprinoid fishes caused by Bothriocephalus qowkongensis 1955 (Cestode; Pseudophyllidia) Journal of Fish Disease., 2:(1), 69-73.
- Wakelin, D. 1996. Immunity to parasites. Cambridge University Press, 2nd Cambridge UK.
- Williams, H & Jones, A.1994. Parasitic worms of fish. Taylor & Francis publishers, London UK, 422pp.
- Woo, P. T. K.1992. Immunological response of fish to parasitic organisms. In: Annual. Review of Fish Diseases. 2, 339-366.







