Exploration of Nano-material and Thin Film Technologies for Wastewater Analysis: An Overview
1
School of Engineering and Technology,
Jagran Lakecity University,
Bhopal,
Madhya Pradesh
India
2
Department of Mathematics,
Amity University,
Kolkata,
India
Corresponding author Email: drvandana@jlu.edu.in
DOI: http://dx.doi.org/10.12944/CWE.18.1.2
Copy the following to cite this article:
Rathore V, Bhardwaj R. Exploration of Nano-material and Thin Film Technologies for Wastewater Analysis: An Overview Curr World Environ 2023;18(1). DOI:http://dx.doi.org/10.12944/CWE.18.1.2
Copy the following to cite this URL:
Rathore V, Bhardwaj R. Exploration of Nano-material and Thin Film Technologies for Wastewater Analysis: An Overview Curr World Environ 2023;18(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-02-03 |
|---|---|
| Accepted: | 2023-03-27 |
| Reviewed by: | 
 Sina Aramyan
Sina Aramyan
|
| Second Review by: |

 Rawaa A. Faris Alsaady
Rawaa A. Faris Alsaady
|
| Final Approval by: | Dr. Mohammad Oves |
Introduction
The treatment of wastewater is critical for protecting public health and the environment, as untreated wastewater can lead to the spread of disease, pollution of waterways, and harm to aquatic life. Wastewater treatment is the process of removing contaminants from wastewater, including domestic sewage and industrial wastewater, before it is discharged into the environment. The sewerage processing generally has the following treatment stages:
Prefatory Process
This stage involves the removal of large objects and debris such as sticks, stones, and rags using screen and grit chambers.
Foremost Process
This juncture is mainly used for elimination of settle able and turbidity through sedimentation and skimming. The contaminants are endorsed to settle, and the solids that settle to the bottom are removed instantly.
Subordinate Process
This phenomenon is used for the removal microorganism and nutrients by using biological processes. The wastewater is mixed with microorganisms in aeration tanks, where the microorganisms consume the organic matter and convert it into carbon dioxide and water
Advance Treatment
This operation utilizes the removal of any remaining pollutants and disinfection of the wastewater using advance technologies (nano-materials and thin films). Various methods are used for tertiary treatment, including sand filtration, carbon adsorption, and chemical disinfection using chlorine or ultraviolet light.
Overall, wastewater treatment plays a crucial role in protecting public health and the environment by removing contaminants from wastewater before it is discharged into the environment. Nano-material and thin film technologies are emerging as powerful tools
for wastewater analysis, offering the potential for improved accuracy, sensitivity, and efficiency compared to traditional methods. This overview will explore the use of these technologies in wastewater analysis and their potential applications. Nano-materials exhibit exceptional characteristics specially surface-volume (SA:V) ratio, which make them attractive for use in wastewater analysis. For example, carbon nano-tubes (CNT), (SWCNTs), (MWCNTs) and activated carbon oxide have been used for the revelation of hard core rock, organic compounds, and other pollutants in wastewater. Thin film technologies involve the deposition of a thin layer of material onto a substrate. These films can be used for sensing applications, such as the detection of pollutants in wastewater. For example metal oxide thin films, including titanium dioxide (TiO2) and zinc oxide (ZnO), have been utilized in the identification of unprocessed compounds present in wastewater. Overall, the use of nano-material and thin film technologies for wastewater analysis shows great promise for improving the accuracy, sensitivity, and efficiency of wastewater analysis. With further development and refinement, these technologies are committed towards ensuring the safety of water resources and protecting public health in broad spectrum.
In a globalized world, commercial enterprise has been updated and highly developed. Our surroundings are packed with different types of hazardous waste discharged from human performance or industrial processes. Such venomous wastes include chlorofluorocarbons (CFCs), carbon monoxide (CO), significant metals (chromium, arsenic, cadmium, lead, zinc, and mercury), (N2O), organic compounds (dioxins and volatile organic compounds), and particulates. Anthropogenic activities, like coal, lubricating material, and ignition, have vast potential to change production from natural sources of water 1. There is also pollution caused by a variety of things, such as oil spills, waste disposal of insecticides, weed killer, triazine, pesticides, and fungicide, by-products of industrial practices, incineration, fertilization, and abundant use of natural gases. In the world, twenty nine percent of all water is not treed within the glaciers and merely eight percent of its healthful water2, an analogy of ladle of water against a five-cubic-decimetre container of drinkable liquids. In the current interval, obtaining purified water has become an imperative subject and is relatively complicated to solve the coupled issues 3,4.
Remediation is the procedure to take away, neutralize or minimize the aqueous adulteration which has harmful effects on the health of living organisms and simultaneously restrains the effect on ecosystems. Remediation automation can be put together mainly in three groups, explicitly (A) physicochemical (B) biological methods & (C) thermal techniques. Generally, conventional modes such as adsorption, extraction and oxidation are gradual, inefficient, and pricey, whereas the supplementary environment-friendly biological degradation is low-cost, but especially stagnant. Nanotechnology provides the facility to manage matter at the nano-scale and assemble materials in such a way that they have specific properties (size, temperature, resistance, etc.,) with a unambiguous function of nano materials6. Nano-materials are extremely tiny structures having dimensions (1-100nm), and the surface-volume ratio (SA:V) is very special and unique (107:1) that may be used to stimuli contaminants in aqueous pollutants7. The pace of advancement in nano-science could be utilized to avoid further adulteration simply by applying nano tools, advancement in industrialization processes, and importantly, by creating awareness. Therefore, the foremost requisitions of this technology in the given vicinity can be classified into three major parts as shown in the given (Figure 1). The first one is restoration: the ratio of surface area to volume is very high in nano-materials, hence they can be utilized to detect nano-contaminations as well as antibiotics in pure water. Secondly, sanctification of pollutants: the use of nanotechnology on specific structures at the nano-scale (1-100) nm for nano-materials and (100-200) nm for thin films. Lastly, detection by sensing techniques for pollution prevention techniques. By way of the speedy addition of adulterants group and their deliberation, the growth of the mechanism that is capable of indulgence and evasion when it is needed 8,9,10.
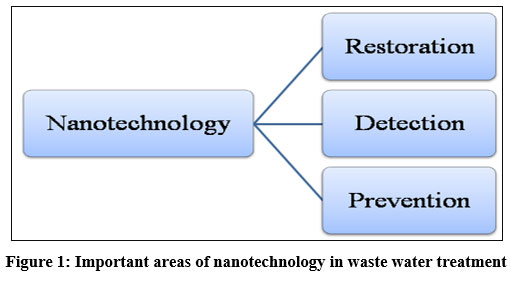 | Figure 1: Important areas of nanotechnology in waste water treatment
|
In this article authors are presenting comprehensive study regarding the role of nanotechnology in aqueous pollution remediation’s and focused on foremost categories of nano-materials and thin film technologies their relevance in wastewater treatment. Most importantly nano-adsorbents like metal based, carbon nano-tubes and polymerization, nano-photocatalyst, nano-membranes their scope and futuristic application are discussed as shown in (Figure 2).
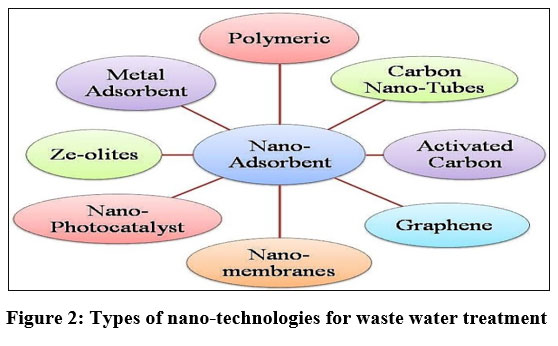 | Figure 2: Types of nano-technologies for waste water treatment
|
Materials and Methods
Nanotechnology for aqueous pollutants
The variation of tremendously advanced nanotechnology to standard system nanotechnologies gives new possibilities for improvement of superior water and wastewater technology techniques. Nanotechnology (1-100nm) simplifies the water cleansing methodologyas a result of size and area, enlarging underground water sources in a very economic way 11. The de-photo ionization techniques of exploitation nano-sized fibres as associate conductor don't seem to be solely low price, likewise further energy economical12. Ordinary hydrous filtering tactic use semi-permeable membranes for reverse electro-dialysis. Drop-off the pore size of the integument to the micro millimetre variation would enhance the property of the molecules allow to exceed from commencing to finish. The membranes that may still filter viruses square measure currently existing13. Engineering is what is more loosely employed in purification, separation, and removal processes square measure particle replace resins, that square measure untreated chemical compound membrane with nano-sized pores on the surface wherever ions square measure fascinated and changed for alternative ions14. Adsorption of Pollutants Sorbents square measure wide employed in aqueous adulteration and purification to get rid of organic and inorganic contaminants, for examples square measure carbon and ion-exchange resins. The utilization of nanoparticles could have the blessings over standard materials attributable to a lot of the larger expanse of nanoparticles on a mass basis. Additionally, the singularity of structural, electronic and optical properties of these nanoparticles will build them particularly powerful adsorbents. Many materials have properties that square measure captivated with size 15.
Nano-materials for wastewater treatments
Nano-materials have exceptional dimension-dependent characteristics, allied to their specific surface area (SSA) being elevated, which results in strong sorption, accelerated suspension, and inflated reactivity, as well as irregular properties. These properties include super-paramagnetism, restricted surface quasi-particle resonance, development of quantum confinement effect.
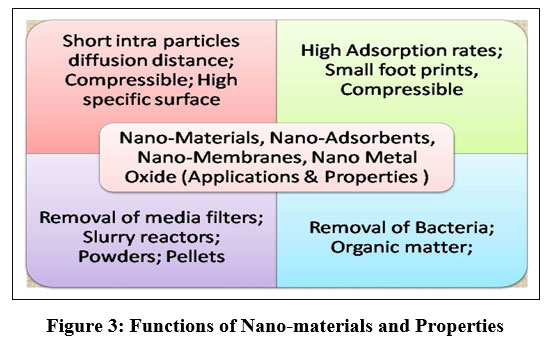 | Figure 3: Functions of Nano-materials and Properties.
|
The unique properties of nanostructure make them ideal for the creation of advanced high-tech substances which are utilized in more effective way for the treatment of water and contaminants. Such materials include pellicle, surface assimilation materials, functionalized surfaces, coatings, and reagents. Figure 3 illustrates the various functions of nano-materials and their applications in these areas.
Result and Discussions
Nano-materials as adsorbents
In the current scenario, nanoparticles are being considered for their potential application as adsorbents. Present research mechanism is to investigate the following types of nano-adsorbents: carbon activated nano-adsorbents, such as carbon nano-tubes (CNTs); metal-based nano-adsorbents; polymeric nano-adsorbents; and zeolites. Gubin and Kalfa et al. have found in their work that the smaller size of nanoparticles increases their surface area, which may enhance their chemical activity and ability to adsorb 16,17,18. Significant nano-materials used in this technology for transporting of trash metals in aqueous solutions include CNTs, activated carbon (AC), zinc oxide (ZnO), magnesium oxide (MgO), inorganic compounds such as manganese oxide (MnO), ferric oxide (Fe2O3), and titanium oxide (TiO2), and graphene oxide (C140H42O20) 19.
Inorganic nanoparticles are oxide-based nanoparticles that are generally prepared via metals and non-metals. These nanoparticles are ubiquitous used for the elimination of harmful adulterants from liquids. There include titanium oxides20, magnesium oxide21, manganese oxides22, ferric oxides 23, 24, and zinc oxides25. Graphene exists in many different forms and has unique characteristics that make it extremely favourable for numerous environmental and wastewater applications. The main characteristic of graphene that makes it extremely valuable and suitable as a surface assimilative for the subtraction of heavy metals is its high specific surface area (0.6 m2/g), light weight (? 0.77 mg), mechanical strength (?150 Gpa), chemical stability, and elasticity 26, 27. Furthermore, the occurrence of graphene oxide as a functional material also affects the adsorption quality, process, and their future scope 28. Table 1 represents the comparable values and percentage of removal of pollutants along with the types of metals, pH value, and adsorbent doses. Zinc sulphide29, graphene30, zeolite31, and magnetic Nanomaterials have been taken into consideration as nano-adsorbents for the comparison of the removal of pollutants32,33,34.
Table 1: Type of absorbents and amount of removal pollutants
S.N | Nano- Absorbents | Nano-Materials | Heavy Materials | Experimental Setting | Removal Pollutants (%) | Ref. | ||
| pH | Time (min) | Absorbent does (g/L) | ||||||
| 1 | Zinc Sulphide | Nano-Crystals | Hg (II) | 1-6 | 5 | 10 | 99.99 | [19] |
| 2 | Graphene (GNS)/d-MnO2 | Nano- Sheets | Ni (II) | NR | 20 | 5 | 77.04 | [20] |
| 3 | Magnet Coated Zeolite | Coated Nano-Particles | As (III) | 2.5 | 15 | 0.5 | 95.6 | [21] |
| 4 | Magnetic Nanomaterials | Nano- adsorbents | Pb2+ | 6 | 10 | 20 | 80 | [22] |
| 5 | Magnetic Zeolite | Cinders by products & sludge | Cadmium and Lead (II) Ion | 5.49-7.48 | 89 | 5.99 | >85.7 | [23] |
| 6 | Magnetic Nanomaterials | (MNPs) Resorbent | Fe2O3 ferrites & Zn+2 | 5.48 | 89.5 | 2.49 | ?95.6 | [24] |
The use of nano-materials as adsorbents has several advantages over traditional adsorbents. For example, nano-materials can remove pollutants at lower concentrations and in shorter contact times, reducing the overall treatment time and energy consumption. Additionally, the high surface area of nano-materials provides a greater number of adsorption sites, which can strength the chemisorptive capacity of the material. Advance carbon resources such as fullerenes, carbonise source, activated charcoal and hierarchical-carbon are widely used due to their excellent adsorption performance for numerous toxic waste, along with organic compounds, heavy metals, and dyes. Crystalline solids analogous to titanium dioxide and iron oxide have also shown promising results as adsorbents due to their broad area of surface and surface reactivity. These materials can remove pollutants through various mechanisms such as adsorption, precipitation, and photocatalysis
Despite the promising potential of nano-materials as adsorbents, there are still some challenges associated with their use. One of the main challenges is the cost of synthesis and functionalization of these materials additionally, the potential release of nano-materials into the environment is a concern that needs to be addressed. Overall, the use of nano-materials as adsorbents holds great promise for the elimination of contaminant from water and wastewater. With further development and optimization, these materials could become an essential component of water treatment processes, helping to ensure the safety and sustainability of water resources.
Nano-materials as catalyst
Nano catalyst plays a very important role in environmental protection and aqueous treatments. Important techniques which are used as a catalyst are: electro-catalysis and photo-catalyst. For organic compounds Fenton catalysis is used at large scale. Nano-materials which are highly considerable for purification and are gaining special attention in inorganic materials along with characteristics are shown in figure 2. Nano-catalysts are distinctive in nature and are used for wastewater treatment such as photo-catalysis, electro-catalysis 35 and catalysts typically consist of a solid support material, such as activated carbon or silica, that is functionalized with Fe2+ ions (Fenton catalysts) 36 for improved decomposition of organic contaminants 37 and germ destroying actions 38. In photocatalysis the extensively used Titanium (IV) oxide and Zinc oxide have experienced lot of inconvenience due to their high band gap of these materials (?3.39 eV) during ultraviolet radiation activity process, which need to be researched further.
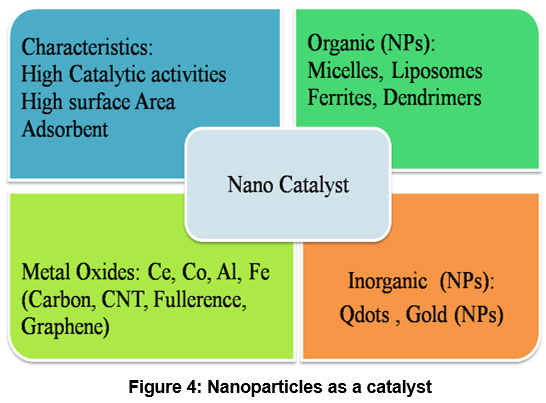 | Figure 4: Nanoparticles as a catalyst
|
Titanium dioxide (TiO2) (IV) is extensively used in photo-catalysis, anticipated its reactivity in response to imperceptible radiations (?k<400 nm), stability, substantial characteristics 39. Similarly, another biodegradable material, zinc oxide (ZnO), which is insoluble in water, has been extensively studied as a transducer and photo-catalytic agent because of its distinctive photoluminescence similar to titanium oxide 40. The separation of bands VB and CB describe energy level of considered materials and plays significant role at nano-scale atomic structures i.e. (forbidden energy gap) in cadmium sulfide (CdS) is ?2.42 eV, making it an appropriate semiconductor that can operate at a wavelength range of ?<495 nm 41. CdS nanoparticles have received intensive attention as a photo cathode for managing and purifying industrial pigments in sewage 42. Although titanium, zinc, and cadmium oxides are popularly acknowledged for their photocatalytic activity, they exhibit functional characteristics only under the ultraviolet range up to a wavelength range of [100-400 nm]. Importantly, due to the broad superlattice hetero-structured energy level ?[3.2-3.65] eV in titania, additional amendments are required for optimal catalytic analysis and to enhance their activities under visible light spectra stretching around [340-700 nm] for the degradation of natural aqueous pollutants. Figure 4 represents the scope of nano-catalysts in organic and inorganic nanoparticles. The effectiveness of various nano-catalysts for treating aqueous pollutants, their outcomes, conditions, and types of contaminants are represented in Table 2.
Table 2: Type of Nano-catalysts for finding aqueous impurity
| S.N. | Catalyst | Spectrum | Status | Pollutant | Outcome | Ref. |
1 | TiO2 (tri-titanate) | Ultraviolet spectrum region | quantity ?1 g/L, interval ?2 h | Chemical Compound and dye C4H12CIN | Dilapidation competence achieved up to 90% |
[33] |
2 | Silver Bromide/Zinc Oxide | ?410 nm Visible region | Dose 1 g/L, pH 6.85 Irradiation of sample 240 mins | Methylene blue (MB) | Confiscation of MB ?87%, decomposed by silver bromide/zinc oxide |
[34] |
3 | Semiconducting ZnO nano-rods | ?370 nm Ultraviolet spectrum region | Catalyst quantity 1 g/L, for 45 min | RhB
| Improved photo-catalytic degradation of RhB |
[35] |
4 | Zero-valent nano Copper | Visible light spectrum [380-700]nm | Catalyst dose = 0.16 g/L, for 80 min | C14H14N3NaO3S (MO) | Process of irradiation was used to degrade methyl orange ?82 minutes and efficiency up to ?36%. |
[36] |
5 | ZnO–FeO clino-ptilolite | Electromagnetic radiation solar rays | pH=8.3, mechanism dose = 0.1 g/L | Polluted Fish pond water | Approx 80-85% efficient |
[37] |
6 | Graphene- CoxZn1-xFe2O4 | Radiations from the zone of visibility
| Concentration of Methylene blue (60 min) = 5 mg/L, vehicle dose 7=100 mg/L | Methylthionine chloride
| Compared to cobalt doped zinc ferrite nanoparticles, the photo-catalytic efficiency of grapheme - cobalt doped zinc ferrite hetero-structures was found to be higher. |
[38] |
7 | 3D SnO2 | Ultra violet region ?[100-400] nm | Measured value =1.99 g/L, interval =2.52 h | spell =2 g/L, interval =2.5 h | Irradiation of sample up to 160 min, degradation of methyl orange may be achieved by 85% |
[39] |
TiO2 and silver bromide catalyst are effective in removing chemical compounds and dyes up to 90% 43,44. ZnO nano-roads have improved photo catalytic degradation of RhB compound 45. Catalyst used for removal of methyl orange (MO) 46,49, fish pollution 47, methylthionine chloride 48, catalyst used are respectively ZnO-FeO, graphene and tin Oxide.
Nano-materials have shown great potential in catalyzing reactions with high selectivity and efficiency, reducing reaction time and energy consumption. One of the most significant advantages of using nano-materials as catalysts are their surface area compared to volume is quite high, authorize for a greater number of active sites, enabling the catalytic reaction to occur more efficiently. Additionally, the tunable surface chemistry of nano-materials allows for the optimization of catalytic activity and selectivity. Due to their exceptional photo-catalytic properties, catalysts such as titanium dioxide, iron oxide, and zinc oxide have been studied extensively. These materials can catalyze reactions under visible or ultraviolet light, making them suitable for applications such as water treatment and air purification. The unique electronic properties and high surface area of carbon found materials, such as grapheme oxide and grapheme nanoplateles, have demonstrated encouraging outcomes as catalysts. These materials can catalyze reactions such as hydrogen evolution and oxygen reduction. Despite the significant advantages of nano-materials as catalysts, there are some challenges associated with their use. One of the primary challenges is the cost of synthesis and functionalization of these materials. Additionally, there may be concerns about the potential environmental impacts of these materials, especially if they are released into the environment. Overall, the use of nano-materials as catalysts shows great promise for improving the efficiency and selectivity of chemical reactions, reducing reaction time and energy consumption, and enabling the development of sustainable and environmentally friendly processes.
Nano-materials as membranes
Membranes play a very important role in waste water treatment. In addition, Nano filtration is a membrane filtration technique, synthetic structures where size of pores is approximately ?0.5-15nm, particularly used for wastewater treatment and usually known as nano-materials membranes.
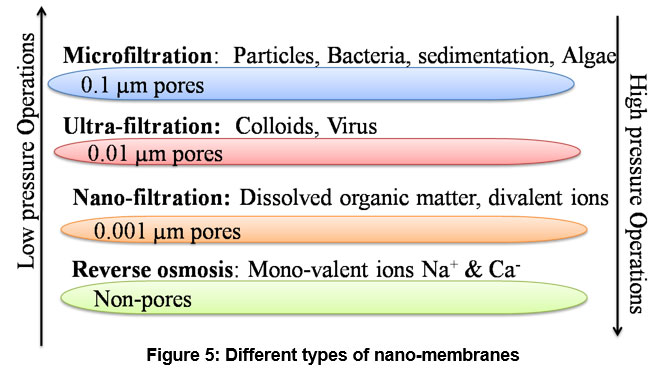 | Figure 5: Different types of nano-membranes
|
 | Figure 6: Process of filtration for Nano-materials as membranes.
|
Nano membranes are found very efficient for eradication of different types of contaminants mainly obtained from textile industry includes blue and red dyes. They have also found efficient for removing heavy metals like Co, As, Cr, Ni etc., 50. Figure (5, 6) represents different types of membranes and process of filtration. Along with the existing advance wastewater handling procedure, thin flexible tissues filtration technology made-up by Nano-materials known as membranes is one of the most scientific approaches due to its valuable properties 51. Nano membrane technology have many benefits due to its quality of treated water, highly disinfection ratio, acquiring less space for plants specially in for the use of irrigation and agricultural areas 52. Furthermore, it is very much economical, resourceful and comfy design compared to other techniques for water treatment 53. Particle filtration of liquid adulterants are novel membranes (1000 - 0.0001) microns and plays fundamental responsibility in the chemical disintegration of organic fouling materials 54. The One-dimensional structures of nano-materials encompass nano-tubes Carbon nano tubes (CNTs), Single wall carbon nano tubes (SWCNTs) like honeycomb structure, (NR) nano-ribbons (1D, 2D), and nano-fibres (synthetic polymers) are the compositions of these types of membranes made up from different nano-material have been found potential application in waste water treatments at very crucial stages 55. Under high pressure carbonaceous nano-fibres (CNFs) fabricated membrane showed exceptional selective filtration and removal efficiency in aqueous pollutants 56. The authors have reported the use of electro-spun membranes for the transportation of heavy metals contaminated with substances such as As, Ni, Cd, Cu, and Cr 57. Moreover, a review and competence of a variety of nano-membranes for treatment of adulterated water along with type of pollutants, nano-membrane efficiencies, prominent results and important remarks are presented in Table 3 by the author. With the help of nano-membranes microorganism58, lubricants59, Amputation60, metal oxides61, Total Suspended Solids62,63 and degradation of dairy effluents 64 can be removed up to 90-100%.
Table 3: Type of nano-membranes for investigation of adulterants in water
| S.N. | Nano-Membranes | Core Material & Major Pollutants | Efficacy | Result |
Ref. |
1 | Nano-membrane | c-alumina and titania nano-crystallites Pathogens and pigeons | Microorganism up to99% & pigeons 25% | Elimination of ions may be improved by pH corrections and pathogens can be removed 100%. |
[48] |
2 | Microstructures of nano scale
| (PBM) Polymer Membranes Propylene Oil removal | 99.75% of lubricants | Coupling of nano-filtration and floatation eliminates grease in aqueous purity, de-emulsification, augmented; weight absorption and adsorption capacity is high due to its porous structure. |
[49] |
3 | Polymer Composite Membrane with Polyvinyl Alcohol Layer, CNT | Macro-molecule membranes Treatment of adulterated water with lubricants | Amputation up to 94.9% | Incorporation of CNT with polymer composite augment suitability of membrane, definitive tensile strength & toughness, amplified CNT concentration increases membrane flux. |
[50] |
4 | Carbon nano-fiber membrane | nanoparticles and metal oxides | Removal up to 95% | Involuntarily well-built and malleable membrane, tolerate filtration with high pressure, knack to manufacture cost efficient nano-rods by electro spinning | 51 |
5 | Nano-porous membrane filtration | Total Suspended Solids, Total dissolved solids, Biological contaminants, lubricants, COD, BOD | Removal of adulterants up to TSS 99%, lubricants 80-90%, amount of oxygen dissolved 76% & TDS 44%, | Most favourable situation, feed temperature ?450C, velocity ?1.3 m/s, salt concentration ?11.2 g/L, trans-membrane pressure of 4 bar & pH 10, utilization of treated water for cultivation purposes. |
[52] |
6 | Micro-filtration membrane (ZrO2) | (CH3)2NC(O)H Pre treatment of dimethylformamide (DMF) | Opaqueness 99.6%, (TSS) Total suspended solids 99.98%. | The process of removing fine particles of TSS and recovering flux can be accomplished through a combination of ultrasonic cleaning, chemical cleaning, and flushing in DFM. |
[53] |
7 | Nano fiber membranes as photo-catalysts | Milk products vanished in technological cycle | Removal up to 75–95% | The use of nano-materials (AgTiO2) incorporated into nano-fiber membranes is crucial for the photo-catalytic degradation of dairy effluents. |
[54] |
A membrane is a thin layer of material that separates two or more substances, and it is commonly used to filter, purify, or separate different types of substances. Nano materials that are used as membranes include graphene oxide, carbon nano-tubes, nano-porous ceramics, and polymeric nano-fibres. These materials have been used in various applications, such as water filtration, gas separation and drug delivery. In conclusion, nano materials have the potential to be highly effective as membranes due to their small size, unique properties, high selectivity, and efficiency.
Nanotechnology and threats
Nanotechnology has a plethora of potential uses and has seen rapid advancements. However, it also has the potential to unintentionally harm human health and the environment. Materials that are non-toxic in their bulk form can become highly toxic at the nano-scale. This is especially concerning if these particles enter the food chain or drinking water supplies and do not biodegrade, as they can cause various diseases in humans upon intake. The Royal Commission on Environmental Pollution (RCEP) 65 and the European Union 66 acknowledge the potential risks associated with some nano-materials based on laboratory tests. However, research on the toxicity and health risks associated with nano-materials is currently limited and requires further exploration 67. This is a challenging task since monitoring the vast volume of diverse nanoparticles being produced and used and their subsequent impact is difficult. While the effectiveness of using nano-materials in water treatment has been demonstrated, the technique's drawbacks need to be examined, as nano-particles may release harmful gases or particles during preparation and treatment that are highly toxic and can accumulate for years, posing significant risks to health and the environment.
However, there are also some challenges associated with the use of nano-material and thin film technologies for wastewater analysis. For example, the synthesis and functionalization of these materials can be complex and time-consuming. In addition, there may be concerns about the potential environmental impacts of these materials, especially if they are released into the environment.
Health risks
The small size of nanoparticles makes them potentially hazardous to human health. They can easily cross the threshold and through oneself into different parts of the body, liver, lungs, absorption through the skin and accumulate in organs and tissues, potentially leading to health problems such as lung damage, cancer, and neurological disorders.
Ethical concerns
The use of nanotechnology raises ethical questions, such as the potential for human enhancement, surveillance, and privacy concerns.
Security risks
Nanotechnology can also be used for malicious purposes, such as developing new weapons or spy devices, which could pose a threat to national security.
To address these potential threats, researchers and policymakers must work together to develop appropriate safety guidelines and regulations for the development and use of nanotechnology. This will ensure that the benefits of nanotechnology are maximized while minimizing potential risks to living organism, the ecosystem and national security
Conclusion and Future aspects
There is a growing need for advanced water treatment technologies to ensure the provision of high-quality drinking water, mitigate micro-contamination, and enhance industrial production through the implementation of versatile water treatment systems. Nano-particle materials, such as nano-adsorbents/zeolites, nano-metals, photo-catalysts, and nano-membranes, hold promise for developing novel water technologies that can be easily customized to meet specific customer requirements. However, a major technical challenge associated with using nano-particle materials in water treatment is that they are often not adaptable to large-scale processes and are currently not cost-competitive with conventional treatment technologies. To overcome this limitation, further research is needed to develop nano-catalysts that are minimally toxic to the environment and reduce the health risks to living organisms.
Further work is required to reconsider the effect of toxic chemicals on biological organisms (eco-toxicity) prospective for all new adaptation in nano-catalyst and for existing nano-technology with zero toxicity. In spite of that nano-particles put forward huge potential for novelty in the upcoming decades in purification of adulterated water but in particular for distribution of functions and powers decentralized treatment systems, location based devices, size dimensions, cost and heavily degradable pollutants play a crucial role for further development. Novel findings of the present work may be concluded as follows:
Zn Sulphdie Nanomaterials acts as good absorbents may remove pollutant up to 99.99 % including heavy materials form the pollutants. Nano membranes are effective to investigate and remove microorganisms, pathogens and lubricants from the water pollutants up to 100%.
Nano catalyst are helpful in removal of chemical compound specially dyes up to range of 90% with the help of tri-titanate.
Acknowledgement
Authors would like to acknowledge Fraternity of Jagran Lakecity University for continuous support and motivation for the research work.
Conflict of interest
The author(s) don’t have any conflict of interest in article of research “Exploration of Nano-material and Thin Film Technologies for Wastewater Analysis: An Overview”
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Environmental Defence Fund, The health risks of burning coal for energy, Report, http://www.edf.org/climate/remaking-energy, (2006).
- Krantzberg G, Tanik A, do Carmo, JSA, Indarto A, and Ekda A. Advances in water quality control, 1st Ed Scientific Research Publishing, USA, 2010; 2:15-25.
- Zhang W, Nanotechnology for water purification and waste treatment, in Frontiers in Nanotechnology, U.S. EPA Millennium Lecture Series, Washington, DC, 2005.
- Yunus, I & H, Kurniawan, A, Adityawarman, D & I, Nanotechnologies in water and air pollution treatment. Environmental Technology Reviews, 2012; 1: 136-148.
- Sharmaa YC, Srivastava V, Singh VK, Kaul SN, Weng CH, Nano-adsorbents for the removal of metallic pollutants from water and wastewater, Environ. Technol. 2009; 30: 583-609.
- Roco MC, Williams S, Alivisatos P, (1999) Nanotechnology research directions: vision for nanotechnology in the next decade,IWGNWorkshop Report, U.S. National Science and Technology Council, Washington, DC.
- Lu GQ, Zhao XS, Nanoporous Materials Science and Engineering, in Nanoporous Materials-an Overview, World Scientific, Singapore 25:2025008, (2004).
- Liu, Mk., Liu, Yy., Bao, Dd. et al. Effective Removal of Tetracycline Antibiotics from Water using Hybrid Carbon Membranes. Sci Rep 2017; 7: 43717.
- Niela P, Sorina M, Florica M, Chapter 9 - Carbon nano-materials-based sensors for water treatment, Elsevier. 2022: 125-148.
- Indarto A, Choi JW, Lee H, Song HK, Decomposition of greenhouse gases by plasma, Environ. Chem. Lett. 2008; 6: 215-222.
- Nutt MO, Hughes JB, Wong MS, Designing Pd-on-Au bimetallic nanoparticle catalysts for trichloroethene hydrodechlorination Environmental Science & Technology. 2005; 39: 1346-1353.
- Hairom NH, Soon CF, Mohamed R, Morsin M, Zainal N, Nayan N, Zulkifli CZ, Harun NH, A review of nanotechnological applications to detect and control surface water pollution, Environmental Technology & Innovation. 2021; 24: 102032
- Tepper F, Kaledin L, Virus and Protein Separation Using Nano Alumina Fiber Media. 2009.
- Alchin D, Ion Exchange Resins. Environmental Protection report. 2008.
- Hochella MF Jr, There's plenty of room at the bottom: Nanoscience in geochemistry, Geochim. Cosmochim. Acta 2002; 66: 735-743.
- Gehrke I, Geiser A, Somborn-Schulz A, Innovations in nanotechnology for water treatment, Nanotechnol Sci Appl. 2015; 8:1-17.
- Gubin, SP, Koksharov YA, Khomutov GB, Yurkov GYE, Magnetic nanoparticles: preparation, structure and properties. Russ. Chem. Rev. 2005; 74: 489-520.
- Kalfa OM, Yalc ?nkaya, O, Turker AR, Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to pre concentration and separation of cadmium. J. Hazard. Mater. 2009; 166: 455-461.
- Gupta VK, Tyagi I, Sadegh H, Shahryari-Ghoshekand, R, Makhlouf ASH, Maazinejad B, Nanoparticles as adsorbent; a positive approach for removal of noxious metal ions, A review. Sci. Technol. Dev.2015; 34:195-214
- Gao C, Zhang W, Li H, Lang L, Xu Z, Controllable fabrication of mesoporous MgO with various morphologies and their absorption performance for toxic pollutants in water. Cryst. Growth Des. 2008; 8: 3785-3790.
- Gupta VK, Agarwal S, Saleh, TA, Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011; 185: 17-23.
- Feng L, Cao M, Ma X, Zhu Y, Hu C, Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012; 217: 433-446.
- Xu D, Tan X, Chen C, Wang X, Removal of Pb (II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008;154:407-416.
- Xiuping F, Ruixin Y, Qinggang Z, Qun W, Takeshi H, Ryoichi I, Long K, Xinde C, Liang Li, Nano ferric oxide adsorbents with self-acidification effect for efficient adsorption of Sb(V), Chemosphere 2021; 272:129933, ISSN 0045-6535.
- Xu Z, Gu Q, Hu H, Li F, A novel electrospun polysulfone fiber membrane application to advanced treatment of secondary bio-treatment sewage. Environ. Technol. 2008; 29:13-21
- Gopalakrishnan A, Krishnan R, Thangavel S, Venugopal G, Kim SJ, Removal of heavy metal ions from pharmaeffluents using graphene-oxide nanosorbents and study of their adsorption kinetics. J. Ind. Eng. Chem. 2015; 30:14-19
- Taherian F, Marcon V, van der Vegt NF, Leroy F, What is the contact angle of water on grapheme, Langmuir. 2013; 29: 1457-1465
- Zare-Dorabei R, Ferdowsi SM, Barzin A, Tadjarodi A, Highly efficient simultaneous ultrasonic-assisted adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) ions from aqueous solutions by graphene oxide modified with 2, 20-dipyridylamine: central composite design optimization. Ultrason. Sonochem. 2016; 32:265-276
- Qu Z, Yan L, Li L, Xu J, Liu M, Li Z, Yan N, Ultraeffective ZnS nanocrystals sorbent for mercury (II) removal based on size-dependent cation exchange. ACS App. Mater. Int. 2014; 6(20):18026-18032.
- Varma S, Sarode D, Wakale S, Bhanvase BA, Deosarkar MP, Removal of nickel from waste water using graphene nanocomposite. Int. J. Chem. Phys. Sci. 2013; 2:132-139.
- Attia STM, Hu XL, Yin DQ, Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J. Experiment. Nanosci. 2014; 9(6): 551-560.
- Khani R, Sobhani S, Beyki MH, Highly selective and efficient removal of lead with magnetic nano-adsorbent: multivariate optimization, isotherm and thermodynamic studies. J. Colloid Inter. Sci. 2016; 466:198-205.
- Visa M, Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016; 294:338-347.
- Shirsath DS, Shirivastava VS, Adsorptive removal of heavy metals by magnetic nanoadsorbent: an equilibrium and thermodynamic study. Appl. Nanosci. 2015; 5(8):927-935.
- Dutta AK, Maji SK, Adhikary B, C-Fe2O3 nanoparticles: an easily recoverable effective photo-catalyst for the degradation of rose bengal and methylene blue dyes in the waste-water treatment plant. Mater. Res. Bull. 2014; 49: 28-34.
- Kurian M, Nair DS, Heterogeneous Fenton behavior of nano nickel zinc ferrite catalysts in the degradation of 4-chlorophenol from water under neutral conditions. J. Water Process Eng. 2015; 8: 37-49.
- Ma H, Wang H, Na C, Microwave-assisted optimization of platinum-nickel nanoalloys for catalytic water treatment. Appl. Catal. B: Environ. 2015; 163:198-204.
- Chaturvedi S, Dave PN, Shah NK, Applications of nanocatalyst in new era. J. Saudi Chem. Soc. 2012; 16:307-325
- Akhavan O, Lasting antibacterial activities of Ag–TiO2/Ag/a- TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009; 336:117-124.
- Lin ST, Thirumavalavan M, Jiang TY, Lee JF, Synthesis of ZnO/Zn nano photocatalyst using modified polysaccharides for photodegradation of dyes. Carbohyd. Polym. 2014; 105:1-9.
- Khallaf H, Oladeji IO, Chow L, Optimization of chemical bath deposited CdS thin films using nitrilotriacetic acid as a complexing agent. Thin Solid Films 2008; 516: 5967-5973
- Zhu H, Jiang R, Xiao L, Chang Y, Guan Y, Li X, Zeng G, Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J. Hazard. Mater. 2009; 169:933-940.
- Chen CC, Lin CL, Chen LC, Functionalized carbon nano material supported palladium nano-catalysts for electro catalytic glucose oxidation reaction. Electrochim. Acta 2015;152: 408-416.
- Dai K, Lv J, Lu L, Liu Q, Zhu G, Li D, Synthesis of micro-nano heterostructure AgBr/ZnO composite for advancedvisible light photocatalysis. Mater. Lett. 2014; 130: 5-8.
- Fang J, Fan H, Ma Y, Wang Z, Chang Q, Surface defects control for ZnO nanorods synthesized by quenching and their antirecombination in photocatalysis. Appl. Surface Sci. 2015; 332: 47-54.
- Liu H, Gong C, Wang J, Liu X, Liu H, Cheng F, Wang G, Zheng G, Qin C, Wen S, Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications, Carbohyd. Polym. 2016;136:1379-1385.
- Bahrami M, Nezamzadeh-Ejhieh A, Effect of supporting and hybridizing of FeO and ZnO semiconductors onto an Iranian clinoptilolite nano-particles and the effect of ZnO/FeO ratio in the solar photodegradation of fish ponds waste water. Mater. Sci. Semiconduct. Process. 2014;27: 833-840
- Nazim S, Kousar T, Shahid M, Khan MA, Nasar G, Sher M, Warsi MF, New graphene-CoxZn1_xFe2O4 nanoheterostructures: magnetically separable visible light photocatalytic materials. Ceram. Int. 2016; 42:7647-7654.
- Cui Y, Wang F, Iqbal MZ, Wang Z, Li Y, Tu J, Synthesis of novel 3D SnO flower-like hierarchical architectures self-assembled by nano-leaves and its photocatalysis. Mater. Res. Bull. 2015; 70:784-788.
- Jie G, Kongyin Z, Xinxin Z, Zhijiang C, Min C, Tian C, Junfu W, Preparation and characterization of carboxyl multi-walled carbon nanotubes/calcium alginate composite hydrogel nano-filtration membrane. Mater. Lett. 2015;157:112-115.
- Ho HL, Chan WK, Blondy A, Yeung KL, Schrotter JC, Experiment and modeling of advanced ozone membrane reactor for treatment of organic endocrine disrupting pollutants in water. Catal. Today 2012;193:120-127.
- Jang JH, Lee J, Jung SY, Choi DC, Won YJ, Ahn KH, Park PK and Lee CH, Correlation between particle deposition and the size ratio of particles to patterns in nano-and micropatterned membrane filtration systems. Sep. Purific. Technol. 2015; 156: 608–616
- Guo J, Zhang Q, Cai Z, Zhao K, Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Separ. Purific. Technol. 2016; 161:69-79.
- Yang B, Geng P, Chen G, One-dimensional structured IrO2 nanorods modified membrane for electrochemical anti-fouling infiltration of oily wastewater. Separ. Purific. Technol. 2015; 156:931-941
- Liu T, Li B, Hao Y, Yao Z, MoO3-nanowire membrane and Bi2Mo3O12/MoO3 nano-heterostructural photocatalyst for wastewater treatment. Chem. Eng. J. 2014; 244:382-390.
- Liang HW, Wang L, Chen PY, Lin HT, Chen LF, He D, Yu SH, Carbonaceous nanofiber membranes for selective filtration and separation of nanoparticles. Adv. Mater. 2010; 22: 4691- 4695.
- Nasreen SAAN, Sundarrajan S, Nizar SAS, Balamurugan R, Ramakrishna S, Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes. 2013; 3(4): 266-284.
- Shayesteh M, Samimi A, Shafiee Afarani M, Khorram M, Synthesis of titania–c-alumina multilayer nanomembranes on performance-improved alumina supports for wastewater treatment. Desalin Water Treat. 2016; 57(20):9115-9122.
- Ahmed FE, Lalia BS, Hashaikeh R, A review on electrospinning for membrane fabrication: challenges and applications. Desalin 2015; 356:15-30.
- Maphutha S, Moothi K, Meyyappan M, Iyuke SE, A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing waste water. Sci. Rep. 3 (2013).
- Faccini M, Borja G, Boerrigter M, Martin DM, Crespiera S M, Vazquez-Campos S, Amantia D, Electrospun carbon nanofiber membranes for filtration of nanoparticles from water. J. Nanomater. 2015; 2:1-9
- Salahi A, Mohammadi T, Behbahani RM, Hemmati M, Asymmetric polyethersulfone ultrafiltration membranes for oily wastewater treatment: synthesis, characterization, ANFIS modeling, and performance. J. Environ. Chem. Eng. 2018; 3(1):170-178.
- Zhang Q, Xu R, Xu P, Chen R, He Q, Zhong J, Gu X, Performance study of ZrO2 ceramic micro-filtration membranes used in pre-treatment of DMF wastewater. Desalination. 2014; 346:1-8.
- Kanjwal MA, Barakat NA, Sheikh FA, Khil MS, Kim HY, Functionalization of electrospun titanium oxide nanofibers with silver nanoparticles: strongly effective photocatalyst. Int. J. App. Ceramic Technol. 2010; 7 (s1).
- Aaron D, Tsouris D, Separation of CO2 from flue gases: a review, Separat. Sci. Technol. 2005; 40:321-348
- European Commission, Communicating nanotechnology. Why, to whom, saying what and how? 2010.
- Wickson F, Nielsen KN, Quist D, Nano and the environment: potential risks, real uncertainties and urgent issues, 2011.







