Detecting the Level of Contaminations Caused by Heavy Metals in the Zayandeh Roud River and Clean up by Leaves of Beech Tree
Mohammad Karimi1 *
1
Department of Hydrology,
International University of Isfahan,
Isfahan,
Iran
DOI: http://dx.doi.org/10.12944/CWE.7.1.14
Zayandeh Roud is one of the most important rivers flowing in the central part of Iran. It originates from the Kouhrang Mountains. This river passes through Chahar Mahal & Bakhtiari and Esfahan Provinces and finally flows into the Gavakhouni pond. The water of this river is used for agriculture, industry and potable water. Determination of contamination level of heavy metals throughout the river is important and has a major role in controlling the ecological conditions of its environment. Clean up of the contaminations caused by heavy metals based on the natural methods including use of plants such as beech leaves will result in a healthy surrounding life while leaving no negative effects. The reason for selecting the leaves of beech tree is the abundance of this plant in different parts of Iran as well as its easy preparaation for use. In the spring of 2011, contamination level of the river to Cu, Zn, and Pb metals was measured in 6 stations and three stages and the effect of using the leaves of beech tree on the absorption of heavy metals was investigated.
Copy the following to cite this article:
Karimi M. Detecting the Level of Contaminations Caused by Heavy Metals in the Zayandeh Roud River and Clean up by Leaves of Beech Tree. Curr World Environ 2012;7(1):87-91 DOI:http://dx.doi.org/10.12944/CWE.7.1.14
Copy the following to cite this URL:
Karimi M. Detecting the Level of Contaminations Caused by Heavy Metals in the Zayandeh Roud River and Clean up by Leaves of Beech Tree. Curr World Environ 2012;7(1):87-91. Available from: http://www.cwejournal.org/?p=1871
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2012-04-25 |
|---|---|
| Accepted: | 2012-05-28 |
Introduction
Contamination of heavy metals causes a serious problem for health and generally for man’s life. Discharge of these metals is caused as the result of several activities such as production of chemicals, dyeing, plastering, mining activities, extractive metallurgy, nuclear activities and other industries. These metals have a destructive effect on the animals, lakes and rivers (Sayeri, Hamoudi, & Yang, 2005). Human activities such as urban and industrial sewages as well as atmospheric sediments and runoffs with unknown sources are the main source of the existing metals in the rivers. They are also one of the major environmental pollutants that are accumulated in the living organisms and cause serious hematological diseases, brain damage, anemia and bad performance of kidneys. Recently, the existing heavy metals in different rivers have been investigated (Wakida, Lara-Ruiz, Rodriguez-Ventura, Diaz, & Garcia-Flores, 2008). The toxicity caused by heavy metals is an issue for which there is much concern since it is very important for people’s health as well as for ecology. Furthermore, heavy metals may be accumulated in the soil in toxic levels due to long term use of untreated sewages. In the soils irrigated by using sewage, heavy metals are accumulated in the surface. When the soil capacity for keeping heavy metals is reduced due to frequent use of sewage, these metals flow into the underground water or into the existing soil solution for absorption by plants. Here is the beginning of the main risk and the transfer of contamination to the critical points should be avoided in a way (Sridhara, Kamalaa, & Samuel, 2008). Several methods have been used to clean up the contaminations caused by heavy metals and some troubles have also been observed after the application of the said methods (Qureshi & Memon, 2012). Efforts have been made in this study to investigate the acceptable reduction of heavy metals based on using the leaves of beech tree. One of the common techniques for measuring the concentration of heavy metals is Flame Atomic Absorption Spectrometry (FAAS). FAAS method is classified as a single-element method which requires a longer time to analyze several elements in a sample. Multi-element methods such as X-Ray Florescence (XRF) (Talebi, 1998), Neutron Activation Analysis (NAA) (Guéguen, Gilbin, Pardos, & Dominik, 2004), and Atomic Emission Spectrometry using Inductively Coupled Plasma (ICP-AES) (Bruder, Lgrade, Lerosy, Coughanower, & Enguehard, 2002), are used for synchronous and critical determination of heavy metals and provide reliable results. In this study, ICP-AES method was used as a multi-element technique to identify rare heavy metals in water.
In this study, the analysis of the selected metals (Zn, Pb, and Cu) in the Zayandeh Roud River at Esfahan Province (Central Part of Iran) and also clean up of the contamination of the aforesaid heavy metals by the leaves of beech tree were investigated. It should be noted that Esfahan province is one of the most important industrial centers of Iran. There are different industries in this province including steel (the largest industry of the country), power plants, aluminum, wood production factories, electronic, computer, petrochemical complexes and refineries. As a result, copper, zinc and lead may leak into the water ecosystem of Zayandeh Roud River.
Materials and Methods
Water samples were collected from 6 stations along Zayandeh Roud River at Esfahan, Iran. The list and specifications of sampling points are shown in table 1. Sampling was made in the spring of 2011 in three stages within one month. The tools for sampling and analyzing the rare metals were completely cleansed by acid before being used. Digestion of metals was performed based on USEPA method, 3010 (acidic digestion of extracts for analyzing dissolved or completely recoverable metals by FLAA or ICP spectroscopy) (US-EPA, 1991). All references were of analytic type. Hydrochloric, hydrofluoric and nitric of the acids were used for digestion of samples and preparation of standards. Standards solutions were used to determine the ICP of the analyzed elements. Glass and Teflon containers were treated in a 10% volumetric solution of nitric acid for 24 hours and were then washed by distilled and deionized water. The digested samples were kept in 15 ml polyethylene tubes in a cold room with a temperature of 4°C.
The samples were measured by using AA 220 Spectrophotometer Varian Spectra and Atomic Emission Spectrometer with Inductive Coupled Plasma (ICP-AES) and were analyzed for three times for the existence of Cu, Pb and Zn metals. The analytic qualitative control involved daily standard analysis as well as repeating the analysis of samples and blanks (Martin & Meybeck, 1979).
After preparing the samples and measuring the concentration of Cu, Pb and Zn metals, it was the turn for preparing the leaves of beech tree to be injected into the water samples.
The reason for selecting the leaves of beech tree is the abundance of this tree in different parts of Iran and its positive effect in similar studies for absorption of other pollutant elements. A sample leaf of beech tree is shown in figure 1.
In order to prepare the leaf of beech tree for injection into the water sample, 5 grams of the beech leaf were dried in a container in a temperature of 70°C. Then, one gram of the dried material was changed into ash by keeping in a furnace with a temperature of 450°C for 6 hours (Natarajan, et al., 2010). The ashes of the samples were poured into 100 ml polyethylene containers and 3 ml of nitric acid were added to them. They were then heated on a water bath with a temperature of 100°C to be completely digested. After digestion, the sample was removed from the water bath and was completely cooled. The obtained combination was measured by using AA 220 Spectrophotometer Varian Spectra and Atomic Emission Spectrometer with Inductive Coupled Plasma (ICP-AES).
In the next stage, 5 grams of beech leaf were injected into each of the water samples prepared from the river. Samples were then taken from the solutions within 30, 60, 90, 120, and 150-minute time intervals and were measured.
Ressults and Discussion
The results of measuring the samples of river water before injecting the leaf of beech tree are shown in tables 2 through 4. It should be noted that the concentrations of heavy metals including Cu, Pb and Zn are determined on ppm basis.
Basic concentrations of the three ions Cu, Pb and Zn in the beech leaf are shown in table 5.
Average final reduction percentages for concentration of each of the three ions of Cu, Pb and Zn as absorbers after the use of beech leaf are shown in table 6.
Average reduction percentages for the concentration of the three ions of Cu, Pb and Zn based on the time are shown in figures 2 through 4.
As it can be seen, the concentrations of the three ions of Cu, Pb and Zn in the river water have different values based on the station; however, as we get close to the downstream of the river, their concentrations increase. As a vital lifeline in the center of Iran, Zayandeh Roud River has several consumptions including supply of potable water and also necessary water for industries and agricultural activities (Nemati Varnosfaderany, Ebrahimi, Mirghaffary, & Safyanian, 2009). Rapid growth of population and agricultural and industrial activities has resulted in the entry of a considerable volume of toxic materials including heavy metals into the river. Absorption of heavy metals seems to have been reduced in the recent years due to saturation of pollution. This issue can be observed in the downstream points of the river (Zhao, Qiana, Huang, Li, Xuea, & Hua, 2012); (Laia, Yang, Hsieh, Wu, & Kao, 2011); (Chahinian, et al., 2011).
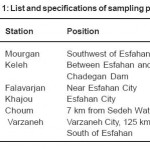 |
Table 1: List and specifications of sampling points Click here to View table |
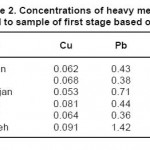 |
Table 2: Concentrations of heavy metals related to sample of first stage based on ppm Click here to View table |
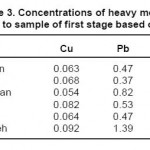 |
Table 3: Concentrations of heavy metals related to sample of first stage based on ppm Click here to View table |
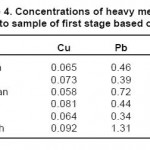 |
Table 4: Concentrations of heavy metals related to sample of first stage based on ppm Click here to View table |
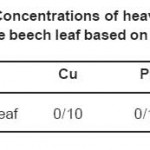 |
Table 5: Concentrations of heavy metals in the beech leaf based on ppm Click here to View table |
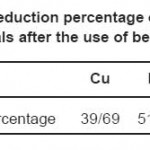 |
Table 6: Reduction percentage of each of the metals after the use of beech leaf Click here to View table |
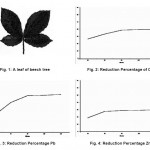 |
Figure 1: A leaf of beech tree Figure 2: Reduction Percentage of Cu Figure 3: Reduction Percentage Pb Figure 4: Reduction Percentage Zn Click here to View figure |
After sampling and injection of beech leaf into the samples, it can be seen that considerable percentages of the concentrations of heavy metals are absorbed. These percentages were almost 40% for copper, 52% for lead and 30% for zinc. Such reduction level seems acceptable considering the initial concentrations of the ions in the water samples (Liu, Jiang, Zhang, & Xu, 2011); (Kertész, Bakonyi, & Farkas, 2006); (Shikazono, Zakir, & Sudo, 2008).
Conclusion
Zayandeh Roud River, as the main source of water supply in the central plateau of Iran, has several consumptions including potable, industrial and agricultural waters. Population increase has been followed by increased consumption of river water and the contaminations caused by such consumptions have had negative effects on its quality. Considering the use of river water for irrigation of farming lands, high concentrations of heavy ions will cause irreparable damages in the near future. Therefore, one of the major concerns is to use a method for cleaning up the contaminations caused by heavy metals which is cost effective from economical and facilities viewpoints and above all, it lacks any secondary trouble. In this study, the effect of beech leaf on the absorption and reduction of concentrations of Cu, Pb and Zn ions was investigated. Considering the suitable reduction of concentrations of Cu, Pb and Zn ions after the use of beech leaf, it is recommended to use the leaf of beech tree as an almost free and quite natural solution in case of any need to the cleaning up of contaminations caused by heavy metals including Cu, Pb and Zn.
References
- Bruder, V., Lgrade, F., Lerosy, M., Coughanower, C., & Enguehard, F. (2002). Application of a sequential extraction procedure to study the release of elements from municipal solid waste incineration bottom ash. Anal, Chim, Acta , 258-295.
- Chahinian, N., Bancon-Montigny, C., Caro, A., Got, P., Perrin, J., Rosain, D., et al. (2011). The role of river sediments in contamination storage downstream of a waste water treatment plant in low flow conditions: Organotins, faecal indicator bacteria and nutrients. Estuarine, Coastal and Shelf Science , 4.
- Guéguen, C., Gilbin, R., Pardos, M., & Dominik, J. (2004). Water toxicity and metal contamination assessment of a polluted river: the Upper Vistula River (Poland). Applied Geochemistry .
- Kertész, V., Bakonyi, G., & Farkas, B. (2006). Water pollution by Cu and Pb can adversely affect mallard embryonic development. Ecotoxicology and Environmental Safety , 4.
- Kubiak, J. J., Khankhane, P. J., Kleingeld, P. J., & Lima, A. T. (2012). An attempt to electrically enhance phytoremediation of arsenic contaminated water. Chemosphere , 3.
- Laia, Y., Yang, C., Hsieh, C., Wu, C., & Kao, C. (2011). Evaluation of non-point source pollution and river water quality using a multimedia two-model system. Journal of Hydrology , 2-3.
- Liu, X., Jiang, S., Zhang, P., & Xu, L. (2011). Effect of recent climate change on Arctic Pb pollution: A comparative study of historical records in lake and peat sediments. Environmental Pollution , 2-4.
- Martin, J., & Meybeck, M. (1979). Elemental mass balance of material carried by major world rivers. Mar Chem .
- Natarajan, S., Stamps, R. H., Ma, L. Q., Saha, U. K., Hernandez, D., Cai, Y., et al. (2010). Phytoremediation of arsenic-contaminated groundwater using arsenichyperaccumulator Pteris vittata L.: Effects of frond harvesting regimes and arsenic levels in refill water. Journal of Hazardous Materials , 2-6.
- Nemati Varnosfaderany, M., Ebrahimi, E., Mirghaffary, N., & Safyanian, A. (2009). Biological assessment of the Zayandeh Rud River, Iran, using benthic macroinvertebrates. Biological assessment of the Zayandeh Rud River, Iran, using benthic macroinvertebrates , 3-4.
- Qureshi, I., & Memon, S. (2012). Synthesis and application of calixarene-based functional material for arsenic removal from water. Appl Water Sci , 1-2.
- Sayeri, A., Hamoudi, S., & Yang, Y. (2005). Applications of pore-expanded malodorous silica, removal of heavy metal captions and organic pollutants from waste water.
- Shikazono, N., Zakir, H., & Sudo, Y. (2008). Zinc contamination in river water and sediments at Taisyu Zn–Pb mine area, Tsushima Island, Japan. Journal of Geochemical Exploration , 3-6.
- Sridhara, C., Kamalaa, C., & Samuel, D. (2008). Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer Ecotoxicology and Environmental Safety. Environmental Contamination and Toxicology .
- Talebi, S. (1998). Determination of lead associated with air- borne particulate matter by flame atomic absorption and wave-length dispersive X-ray fluorescence spectrometry. Environ. Anal. Chem.
- US-EPA. (1991). Test Method for Evaluating of Solid Waste. Cincinnati. OH: US Environmental Protection Agency.
- Wakida, F., Lara-Ruiz, J., Rodriguez-Ventura, J., Diaz, C., & Garcia-Flores, E. (2008). Heavy metals in sediments of the Tecate River, Mexico. Environ Geol (54), 637-642.
- Zhao, L., Qiana, Y., Huang, R., Li, C., Xuea, J., & Hua, Y. (2012). Model of transfer tax on transboundary water pollution in China’s river basin. Operations Research Letters , 2.






