Use of p53 protein or its homologues isolated from freshwater bivalves as a pollution marker
Madhav V. Upadhye1 * , Sonal M. Manohar1 and Ujwala Jadhav1
DOI: http://dx.doi.org/10.12944/CWE.5.1.32
p53 is a tumor suppressor gene that is fundamental in cell cycle control and apoptosis. Bivalve Parreysia corrugata is an important component of freshwater ecosystem of Indian sub-continent. In the present study an attempt was made to check whether p53 protein and its homologues that are extracted and isolated from filter feeder mussels; can be used as pollution markers.
Copy the following to cite this article:
Upadhye M. V, Manohar S. M, Jadhav U. Use of p53 protein or its homologues isolated from freshwater bivalves as a pollution marker. Curr World Environ 2010;5(1):189-192 DOI:http://dx.doi.org/10.12944/CWE.5.1.32
Copy the following to cite this URL:
Upadhye M. V, Manohar S. M, Jadhav U. Use of p53 protein or its homologues isolated from freshwater bivalves as a pollution marker. Curr World Environ 2010;5(1):189-192. Available from: http://www.cwejournal.org/?p=1148
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-02-12 |
|---|---|
| Accepted: | 2010-03-19 |
Introduction
Bivalve mussels are important ecological, economical and cultural components of many aquatic communities. Being filter-feeders, they transfer energy to higher trophic levels and are used as monitors of water quality and contaminants.1
p53 protein is product of p53 gene which is a tumor suppressor gene that is fundamental in cell cycle control and apoptosis. Habitat pollution induced mutations in p53 have been shown to cause leukemia in soft-shelled clams Mya arenaria inhabiting polluted water. This has lead to indication that p53 protein and its homologues can also be used as pollution markers.
In the present study, the presence of p53 protein or its homologues was investigated in the tissues of bivalve mollusc Parreysia corrugata collected from two water bodies from the state of Maharashtra. The results obtained were compared with the limnological investigations in order to check the efficacy of these relatively new protein markers as pollution marker.
Material and Methods
Collection of Samples
Freshwater bivalve molluscs Parreysia corrugata were collected live from Vaitarana river in Thana district and Godavari river near Nanded city. Water samples from the occurring zone of these bivalve mussels were also collected for physico-chemical analysis as described by APHA.2 Water samples and live mussels were brought to the Aquaculture Biotechnology laboratory of Department of Life Sciences, University of Mumbai and processed within 24 hours of collection.
Preparation of Tissue Extract
P. corrugata were cleaned, opened and the internal organs were carefully dissected. Tissues were rinsed with Phosphate buffer saline and proteins were extracted by homogenizing 500 mg tissue in O’Farrell lysis buffer (9.5 M urea, 2% NP-40, 2% ampholytes pH 7 to 9, 100 mM Dithiothreitol) at 4°C to avoid proteolysis. The mixture was centrifuged at 12,000 rpm at 40C for 30 min; the supernatants were recovered and used immediately or stored at - 40°C till further use. Protein concentration was determined by Folin Lowry method3 using BSA as standard.
|
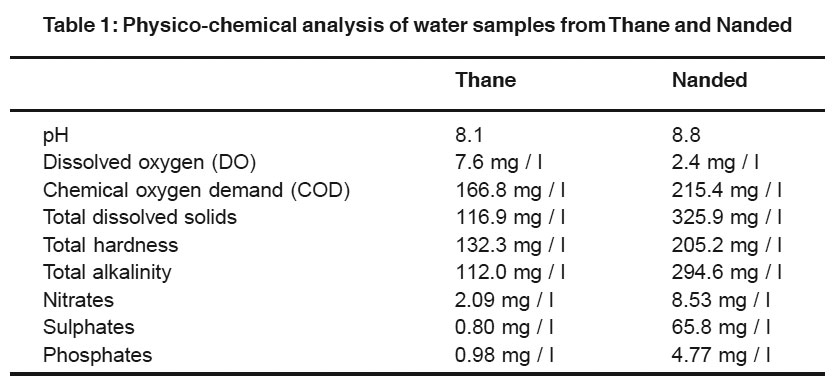
Table 1: Physico-chemical analysis of water samples from Thane and Nanded
Click here to view table |
Western Blotting
Western blot analysis was carried out to find out, if there are variants of p53 protein in the molluscan tissues that react with murine monoclonal anti-p53 antibody. Tissues analyzed were foot, gill, mantle and adductor muscles. Electrotransfer of proteins from polyacrylamide gel to PVDF membrane (Immobilon-P, Millipore, USA) was carried out using Mini-trans blot electrophoretic transfer-cell (BIO-RAD) at 400 mA/ 2 h using phosphate transfer buffer/ tris-glycine buffer with 20% methnol. After blocking for 1 h at room temperature with a solution composed of 20 mM Tris-HCl, (pH 7.4), 125 mM NaCl, 0.1% Tween-20, 5% non fat dry milk, immunodetection was carried out by probing with murine monoclonal anti-p53 (P 5813, Sigma) specific antibody at a dilution of 1:200 overnight at 4°C. Anti β-actin monoclonal antibody (Sigma) was used at a dilution of 1: 20,000 for confirming equal loading. The HRP coupled anti-mouse antibody (Santa Cruz Biotech, USA) was incubated at room temperature for 1 h at dilution of 1.5000. The protein detection was done by chemiluminescence (ECL, Sigma).
Results and Discussion
The pollution monitoring of Indian rivers and other aquatic ecosystems is generally carried out by analyzing various physico-chemcial and microbiological parameters. Mohanty4 carried out the pilot study to investigate the presence of p53 protein or its homologues in tissues of freshwater bivalve L. corrianus collected from the river Ganga at three sampling sites viz. Kanpur, Allahabad, and Varanasi.4 After SDS-PAGE, on immunoblot analysis, a 45 kDa protein (p45) was recognized by the monoclonal anti-p53 antibody in the molluscan tissues. Author concluded that the molluscan p45 is a p53-homolgue or p53-like protein can be used a biomarker for pollution monitoring.
|
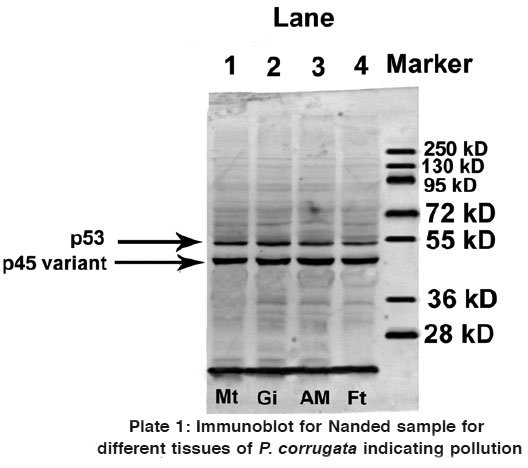
Plate 1: Immunoblot for Nanded sample for different tissues of P. corrugata indicating pollution
Click here to view plate |
In the present study, the expression of p53 protein or its homologues was checked in four different tissues taken from P. corrugata collected from Thane and Nanded of which physicochemical analysis was also carried out. That analysis indicated that samples from Nanded were heavily polluted whereas samples from Thane were comparatively better (Table 1).
Estimation of total extracted protein using Folin Lowry method indicated good protein yield. Gonads were observed to have highest protein concentration whereas least content was seen in mantle tissue. This was carried out in order to load equal amount of protein from all the four tissues so as to compare tissue specific expression of p53 and/ or its homologues.
|
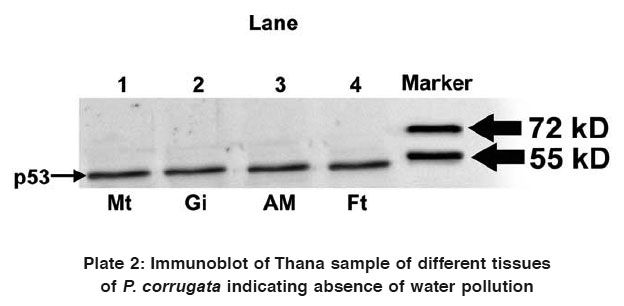
Plate 2: Immunoblot of Thana sample of different tissues of P. corrugata indicating absence of water pollution
Click here to view plate |
Western blot analysis using anti-p53 monoclonal antibody for samples collected from both Thane and Nanded indicated contradictory results. In addition to p53 protein, a 45 kDa protein (p45) was found to be recognized by the anti-p53 antibody in all the four tissues of study animals collected from Nanded (Plate 1). However, immunoblot analysis of all the four tissues collected from Thane did not exhibit presence of p45 variant protein, but a 53 kDa protein (p53) was found to be expressed in all the four tissues (Plate 2).
Thus it can be concluded from the present investigation that as reported by Mohanty,4 p45 or a p53 like protein is expressed in tissues of bivalve mussels collected from a site which was polluted and not in tissues of bivalve mussels collected from a site which was not polluted (or free from any water pollution). As the water sample collected from Nanded was also found to be heavily polluted, expression of p45 variant of p53 has served as pollution marker. More such studies in freshwater habitats rich in mussel populations are warranted to confirm these observations.
p53 is the most intensively studied gene in molecular oncology as it has the ability to act as a tumor suppressor. Some of the p53-homologues can activate p53- responsive genes such as p21, suppressing cell growth by inducing apoptosis in cultured cells,5 mediate apoptosis in response to DNA damage6 and promote thyroid cancer progression.7 In bivalves such studies are lacking. Therefore further structural and functional analyses are necessary to understand the role played by p45 protein in freshwater bivalve molluscs.
References
2.APHA, Standard Methods for the Examination of Water and Wastewater. Second Edition, American Public Health Association, Washington D.C, USA, pp. (1998) 1-1134.
3.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J., Journal of Biol Chem (1951) 193: 265-275.
4.Mohanty B.P., Indian Journal of Biochem and Biophys., (2006) 43: 247-250.
5.Cachot J., Cherel Y., Galgani F. and Vincent F., Mutat. Res., (2000) 464(2): 279-287.
6.Jost C. A., Marin M. C. and Kaelin W. G., Nature, (1997) 389: 191-194.
7.Malaguarnera, R., Mandarino, A., Mazzon, E., Vella, V., Gangemi, P., Vancheri, C.,Vigneri, P., Aloisi,. A., Vigneri, R. and Frasca F., Endocrine-Related Cancer, (2005) 12(4): 953 -971.