Status of ground water quality in Bhopal city
Sanjay Telang1 * and Anoop Chaturvedi2
1
Department of Zoology,
Government Science and Commerce College,
Benazeer,
Bhopal,
Madhya Pradesh
India
2
Central Pollution Control Board,
Bhopal,
Madhya Pradesh
India
DOI: http://dx.doi.org/10.12944/CWE.3.2.19
The concentration of cations and anaions in ground water of Bhopal city was studied during March 2008. The concentration of various cations like Sodium (9.7 mg/L to 79 mg/L). Sulphates (12 mg/L to 372 mg/L) and Potassium (5 mg/L to 45 mg/L) was found. At 50% of the location the totalhardness values of ground water exceeded the values prescribed for drinkding water (IS 10500). The concentration of the anions like Fluoride (0.76 mg/L to 1.24 mg/L), total alkalinity (120 mg/L to 360 mg/L) were observed whereas the Nitate (5.2 mg/L to 42 mg/L) Chloride (24 mg/l to 306 mg/L) and Ammonical nitrogen (0.03 mg/L to 0.62 mg/L) were found
Copy the following to cite this article:
Telang S, Chaturvedi A. Status of ground water quality in Bhopal city. Curr World Environ 2008;3(2):313-316 DOI:http://dx.doi.org/10.12944/CWE.3.2.19
Copy the following to cite this URL:
Telang S, Chaturvedi A. Status of ground water quality in Bhopal city. Curr World Environ 2008;3(2):313-316. Available from:http://www.cwejournal.org?p=142/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-06-18 |
|---|---|
| Accepted: | 2008-09-07 |
Introduction
Population is increasing many fold progressively and there is great demand of water in all major sectors namely irrigation, industrial and domestic purpose. Under the prevailing hydrological condition the availability of fresh water resources is more or less constant. Ground water for construction activities/improper drainage system, the need of ground water quality assessment isessential.
To evaluate the water quality with references to its use the ground water samples were collected during March 2008. Selection of these locations was based on the use of ground water and then vicinity to the population sources. Sampling location and other relevant details are furnished in table 1. Analysis result of collected water samples and the data is presented in table 2.
Material and Methods
Bhopal city is situated between 23°35' N latitude and 77°23' E longitude. Topography of the city is generally flat but there are several hills in the city. The area of the city is 285 Sqkm. with maximum and minimum altitude 625 m and 490m above sea level. The urban population of the city is about 20 lakhs.
Ground water samples were collected in LDPE carbuoy. The primary preservation of the samples were carried at the site followed by analysis in the laboratory. pH was recorded with the help of handy digital pH meter on the site. The standard methods as prescribed in APHA were adopted for analysis of ground water during study.1-2
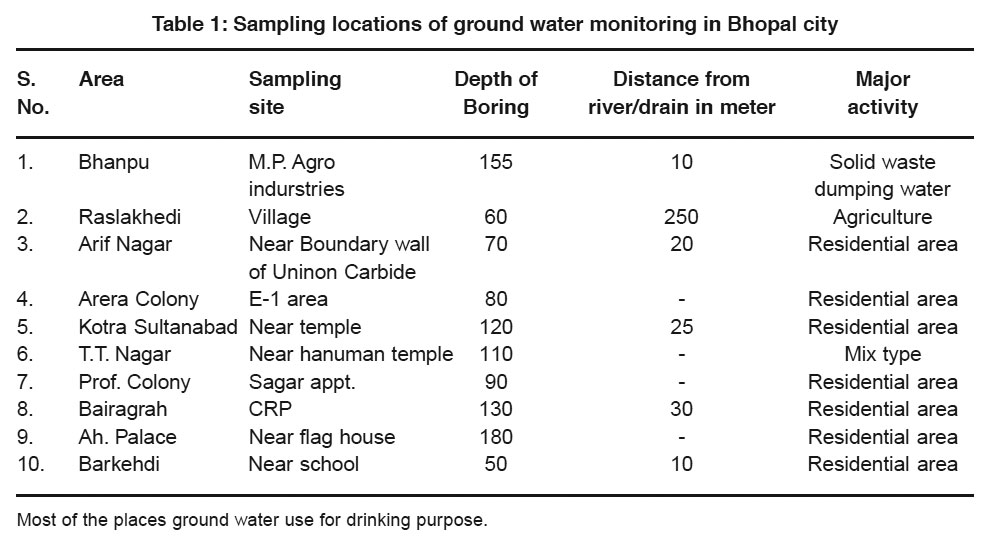 |
Table 1: Sampling locations of ground water monitoring in Bhopal city Click here to view table |
Results and Discussion
The minimum and maximum pH were recorded as 6.91 & 9.41 at new Arif Nagar and Kotra respectively. Kotra Sultanabad is the only station where the pH value exceeded the drinking water standard (6.5 to 8.5). The conductivity of the monitored samples in the city ranged from 210µS to 132µS at Arera Colony and Bhanpur respectively. It is and indication of the availability of various ions.
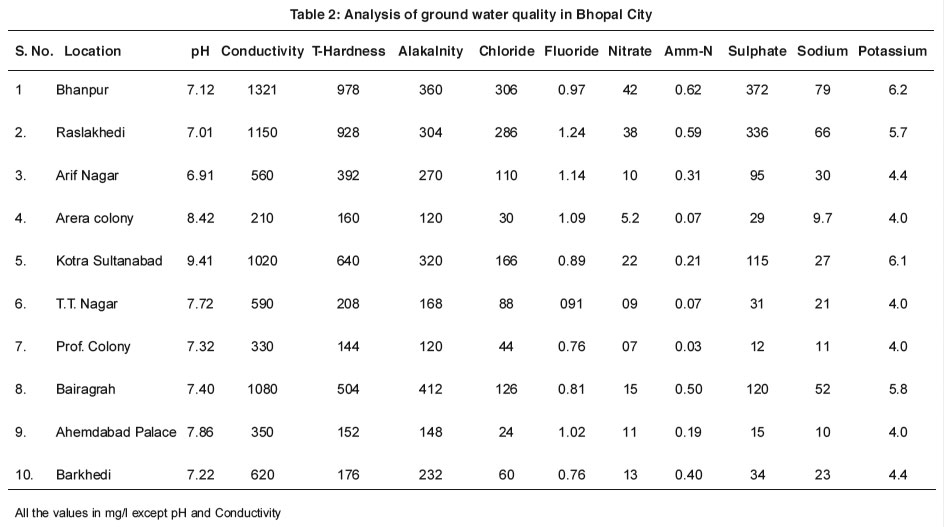 |
Table 2: Analysis of ground water quality in Bhopal City Click here to view table |
The concentration of various cations like Sodium (9.7 mg/l to 79 mg/l), Sulphates (12 mg/l to 372 mg/l) and Potassium (5 mg/l to 46 mg/l). The values of total hardness was found in the range of 144mg/l to 978 mg/l at professors colony and Bhanpur respectively. At 50% of the locations the total hardness values of ground water exceeded the values prescribed for drinking water (IS 10500).
The concentration of the anion in the ground water that is Fluoride (0.76 mg/L to 1.24 mg/L), total alkalinity (120 mg/L to 360 mg/L) were also found in higher range where as the Nitrate (5.2 mg.l to 42 mg/L) Chloride (24 mg/L to 306 mg/L) and Ammoniacal nitrogen (0.03 mg/L to 0.62 mg/L) were found marginally high at Bhanpur, Rasalkhedi and Bairagarh.
Findings
The alkalinity of the ground water was found on higher side at 06 locations whereas pH was found well within the prescribed limit except at one location i.e. Kotra Sultanabad. The tube well where the higher pH was dug near a domestic drain. The chloride the concentration at the M.P. Agro Industries where the organic manure is made from the MSW and Raslakhedi have exceeded the desirable limit of drinking water. The higher chloride content in the water leads to undesirable taste.
Presence of high concentration of chloride in these locations may be attributed to the Patra Nalla and the dumping of municipal solid waste in this area.
The presence of Nitrate and Ammoniacal-nitrogen of the ground water. In the city most of the domestic drains/sewage line are not properly lined/ maintained which may be contributing to the sewage pollution in the ground water.
Excess fluoride in the water many cause the molting of teeth, gastroenteritises and damage of lever. During the study, at 80% locations the fluoride concentration were found on higher side. There is no well defined pollution source for fluoride was observed in these areas. It may be due to the natural geological formations. At Raslakhedi and Bhanpur the concentration of Nitrates was observed in higher side. The agriculture activities around the source might have contributed to the higher concentration of nitrate in the ground water.
Total hardness (includes Calcium and Magnesium ions) combine with ions of fatty acid in soap to form soapsuds. Presence of more hardness in water, the more soap will be required to form leather. The high value of hardess has a laxative effect.
Conclusion
Perusal of the data and observation revel that the ground water in Bhopal city is having high concentration of Hardness, Sodium, Chloride, Sulphates and Nitrate. The use of groundwater source located nearby the drains should be stopped or the water should be allowed for use after proper treatment.
References
-
APHA, standard method for the examination of water and waste water, 20th edition NY.
-
Sawyer, N.C. McCarty P.L and Parkin, GF chemistry for environmental enigneer.






