Methanization Test of Water Hyacinth and Azolla in Co-digestion and Fertilizing Value of Digestates in Benin (West Africa)
Bokossa Hervé Kouessivi Janvier
*
 , Abiola Francine
, Abiola Francine
 , Hidirou Toro Moussa Tahirou
, Hidirou Toro Moussa Tahirou
 and Johnson Roch Christian
and Johnson Roch Christian

1
Environmental Chemistry Department,
Interfaculty Center of Training and Research in Environment for Sustainable Development (CIFRED),
University of Abomey-Calavi, Abomey-Calavi,
Benin,
Cotonou
Benin
Corresponding author Email: riqbokossa@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.2.14
Copy the following to cite this article:
Janvier B. H. K, Francine A, Tahirou H. T. M, Christian J. R. Methanization Test of Water Hyacinth and Azolla in Co-digestion and Fertilizing Value of Digestates in Benin (West Africa). Curr World Environ 2024;19(2). DOI:http://dx.doi.org/10.12944/CWE.19.2.14
Copy the following to cite this URL:
Janvier B. H. K, Francine A, Tahirou H. T. M, Christian J. R. Methanization Test of Water Hyacinth and Azolla in Co-digestion and Fertilizing Value of Digestates in Benin (West Africa). Curr World Environ 2024;19(2).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2024-06-04 |
|---|---|
| Accepted: | 2024-08-16 |
| Reviewed by: | 
 Devangee P. Shukla
Devangee P. Shukla
|
| Second Review by: |

 Madhu Gopal
Madhu Gopal
|
| Final Approval by: | Dr. Gangadhar Andaluri, |
Introduction
In recent years, the world has experienced an increase in energy costs which is at the origin of a collective awareness of the need for greater autonomy of societies and the use of alternative sources 1. In addition, the burning of fossil fuels contributes to increase greenhouse gas (GHG) emissions and exacerbates the air pollution and the global warming 2. Since then, several climate summits have given impetus to the use of renewable and clean energies; this is the case of the "COPs of solutions" held in New York in 2014, in Paris in 2015 (COP 21), in Marrakech (Morocco) in 2016 (COP 22), and the "COPs of Africa" held in Berlin in 2017 (COP 23) and in Poland in 2018 (COP 24). In Europe, the ever-increasing dependence of the continent's countries on the use of energy in general and fossil fuels in particular is becoming more and more worrying and raises the same concerns about the sustainability of these resources around the world. This is why, in 2008, the European Union has established the Energy-Climate Prosecutor's Office, which has set binding objectives in terms of energy consumption and GHG emissions by 2020. According to the OCDE, energy demand at the global level in 2100 will be two to five times greater than in 1990, and net carbon emissions could triple 3. The situation in Africa seems even more worrying since alongside the continent's energy challenge, in which more than half of African countries have always had an electrification rate of less than 20%, there are other challenges, including agriculture and sanitation. The restructuring of the energy policy framework thus advocates access to energy for 100% of the population by 2030 and an increase in the share of renewable energies to 10% in the energy mix 4. Several renewable energy sources exist in Africa, but plant biomass occupies a very important share of household consumption 5. This plant biomass also has a great diversity of origin, but that from wood resources is more important and has a spatial coverage that is clearly decreasing from year to year.
In this context, methanization is one of the solutions to ensure energy autonomy and reduce the use of biomass from wood resources. The use of organic waste, such as animal manure, is a clear advantage for the production of biogas, a renewable energy source. This is a promising solution for waste management strategies for health and environmental protection6. Methanization is an anaerobic decomposition of organic macro-molecules by microorganisms, resulting in the production of biogas, whose high-energy compound is methane7. This biogas is mainly composed of methane and carbon dioxide 8. It represents a source of clean, renewable energy and an alternative to conventional energy sources including fossil fuels, which have harmful implications on the environmental balance and whose reserves are decreasing at an accelerated rate 8. At the end of the digestion, a residual organic matrix called digestate is obtained. The production of biogas, and more precisely biomethane, will enable the sustainable development of rural areas and landlocked regions, consequently a diversification of energy resources 9, and the preservation of the environment 10,11.
Faced with this promising technology, the Beninese context which is characterized by excessive pressure on woody resources is an opportunity for the implementation of anaerobic digestion technologies producing clean energy in the face of the alarming increase in waste in the living environment. In fact, the growing production of organic waste in the face of a sharply rising population is a global challenge that calls for efficient responses 12-14. In addition to this, African water bodies in general and those of Benin in particular are invaded by invasive aquatic plants with their negative impacts on the reproduction and mobility of aquatic species causing anoxia and the drastic decline in the productivity of Beninese water bodies, including the Sô River, and also on the free movement of people 15. Among these invasive plants, Eichhornia crassipes and Azolla sp. are the most common and theirs impacts can be mitigated by valorizing their biomass in biogas production. Nowadays, mini bioreactors can supply methane to individual consumers and small businesses 14,16,17. Thus for communities bordering the bodies of water such as those of the Sô river in Benin, where water hyacinth and azolla create ecological and socio-economic problems, the control of the methane production potential, the conditions optimizing production yield, the number of days of fermentation and the fertilizing quality of digestates becomes an important research challenge. In fact, Mini-bioreactors can successfully transform solid organic waste directly at the point of storage18. Thus, the present scientific investigation aimed to (i) determine in co-digestion the methane production potential of water hyacinth and azolla and (ii) evaluate the fertilizing quality of the digestates obtained.
Materials and Methods
Study framework
Located in the south of Benin, the commune of Sô-Ava is home to the Sô river and is between 6°24' and 6°38' North latitude and between 2°27' and 2°30' East longitude. (figure 1). In this part of Benin, the long rainy season lasts from march to july and the short rainy season from september to november. The annual average rainfall is 1200 mm. The temperatures vary between a minimum of 22°C and a maximum of 35°C. The relative humidity is 69 % in the dry season (november to march) and 90% in the wet season. Conducive to the exploitation of fishing resources, the Sô river is today faced with pollution problems. In addition, the species of exposed lands and raised banks are, among others, Eichhornia crassipes, Azolla africana, Paspalum distichum (grass), Paspalum vaginatum, Cyperus papyrus and Typha australis. Among the invasive aquatic species found there, we can cite: Pistia stratiotes, Ceratophyllum dermersum and Nymphaea lotus. Species such as Ipomoea aquatica, Echinochloa pyramidalis and Alchornea cordifolia are among the semi-aquatic species encountered of the municipality.
 | Figure 1: Location of the plant pest collection sit
|
Collection and preparation of organic substrates
The biomass collection of the two invasive species has been carried out in June 2023 because this period usually sees the decrease in salinity allows for an increased development of the cover of the two invasive aquatic plants of the river (Figures 2 and 3). Fresh organs of the two collected species were ground into fine particles of the order of ?m. In addition, 5 volumes of water for one volume of substrates were added for the hydrolysis of organic macromolecules in order to accelerate the activity of microorganisms. The choice of organic substrates is justified by their availability on the Sô river and the need to effectively fight against the proliferation of these aquatic pests.
 | Figure 2: Azolla sp
|
 | Figure 3: Eichhornia crassipes
|
Activation of fermentation processes
From a preliminary test perspective, fresh pig excrements as substrates deriving from anaerobic decomposition processes in the digestive tract of pigs, have been identified as a source of microorganisms to accelerate the fermentative activity of the substrates similar to fresh ashes experimented by 25. To this end, for each experimental unit, a fresh quantity of 20 g of pig excrement (figure 4) has been added to the content of each biodigester as a microbial source, easy to access but necessary for the decomposition activity of organic substrates tested. These pig manure have been mixed with each plant material alone or in co-digestion depending on the treatment.
 | Figure 4: Fresh pig manure
|
Operating conditions
This is the biomethanization test or BMP in laboratory conditions for the production of biogas. The fermenters or bioreactors are jars (figure 5) loaded with substrates (plant pest + pig dejection + water) and connected to a graduated jar and subjected to a fermentation incubation time of 27 days. In fact, the substrates tested, apart from their biodegradability, included the addition of pig manure which, once in the pigs' digestive tract, are rapidly degradable by microorganisms. In view of this, a relatively short incubation time is required, given the enrichment of the bioreactors, hence the 27 days incubation period. Leak tests have been carried out to avoid air ingress which would disrupt biochemical metabolism. Monitoring of the anaerobic digestion process has been also carried out: the temperature has been set at 38°C10 and the pH has been measured at the start and end of the experiment in order to reduce any risk of disruption of the process linked to air entering the environment.
 | Figure 5: Operating conditions and experimental setup
|
Experimental apparatus
Six combinations of substrates have been constituted taking into account a single contribution of water hyacinth, a single contribution of Azolla, an equitable contribution of the two organic substrates. Each type of combination has been made in three repetitions (Table I). In order to determine the substrate combination that produces the most biogas and methane, six combinations of substrates (WHPM: 100% of water hyacinth; AZPM: 100% of azolla; AZWHPM1: 75% of water hyacinth +25 % of azolla; AZWHPM2: 25% of water hyacinth +75% of azolla; AZWHPM3: 50% of water hyacinth +50% of azolla and AZWHPM4: 66.25% of water hyacinth +33.75% of azolla) have been tested. At each interval of two days, the descent of water has been measured in the graduated jar equivalent to the volume of biogas produced. Biogas production has been monitored after 27 days. The gas analyzer has made it possible to determine the qualitative and quantitative composition of the biogas produced after 27 days.
Table 1: Proportion in (g) of mixed substrates
Biodigester/ Treatment | repetitions | Water hyacinth | Azolla | Pig manure |
WHPM AZPM AZWHPM1 | 3 3 3 | 80 0 60 | 0 80 20 | 20 20 20 |
AZWHPM2 AZWHPM3 AZWHPM4 | 3 3 3 | 20 40 53 | 60 40 27 | 20 20 20 |
Note : WHPM : 100% of water hyacinth ; AZPM : 100% of azolla ; AZWHPM1: 75% of water hyacinth +25% of azolla ; AZWHPM2: 25 % of water hyacinth +75 % of azolla ; AZWHPM3: 50% of water hyacinth +50% of azolla and AZWHPM4 : 66,25% of water hyacinth +33,75 % of azolla.
Collection, drying and analysis of the composition of digestates
At the end of the 27 days of production monitoring, collection followed by dehydration then drying in an oven at 60°C has been carried out on the digestates which were transported to the laboratory for analysis of their mineral content. The contents of P, K, Ca, Mg and Zn were determined by atomic absorption spectrophotometry at various wavelengths, the operating principle of which is as follows: At a specific wavelength, a portion of the light energy emitted by the hollow cathode lamp (lamp emitting light also specific to the element) has been absorbed by the sample solution. This quantity of energy has been used by the element to move from its “fundamental” state to a “metastable excited” state: this was the excitation energy. It was proportional to the element concentration in the solution. N has been determined by the KJELDAHL method from the micro-distillation of the mineralized sample digested with sulfuric acid in the presence of a selenium-based catalyst 19. Regarding organic carbon, its content (% Corg) has been deduced from the equation relating to the Tunisian Standard relating to the determination of organic matter given below:
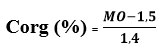
MO: organic matter
Statistical analysis of data
For the comparability of the different biogas production results according to each digester on the one hand and those of the mineral element content of each type of digestate from the biodigesters, mean values ??and standard deviations have been calculated using an analysis of variance two-factor 20. In addition, the cumulative production of biogas according to each type of biodigester has been translated and trend curves for this production were described along with equations and coefficients of determination (R2). This made it possible to test the predictability of the model in the case of continuous full scale biogas generation for populations. In addition, the mineral salt contents have been compared to various types of organic farm and conventional fertilizers. Finally, the amending power of the digestates has been evaluated by the NFU 44051 standard according to which:
if the NPK Content > 7% the product has been considered as a fertilizer;
if the NPK content <7% the product has been considered as an amending agent.
Results and discussion
Biogas content produced by the various organic substrates tested
After a period of 27 days of fermentation at a temperature of 38°C (figure 6) the mixtures composed of (40% water hyacinth, 40% azolla and 20% pig excrement) and (53% water hyacinth, 27% azolla and 20% pig excrement) has produced a maximum quantity of biogas oscillating around 1000mL per 100g of substrate mixture. On the other hand, the mixtures composed of 80% azolla and 20% pig manure and those of 80% water hyacinth and 20% pig manure have revealed the lowest production of around 800mL of biogas. Thus the microorganisms in pig manure have acted on both the labile fraction of water hyacinth and azolla, hence the high production of biogas. Thus 21 had obtained similar results but the differences revealed were linked to the optimal number of days of incubation which could oscillate around 50 days while for this study did not exceed 27 days. 22 had revealed that experimental conditions, inoculum and substrate composition are not always standardized. Indeed the inoculum, the pig manure chosen in this research were not subject to an evaluation of the microbial biomass but in the context of technologies to be transferred to farmers, the manure have the merit of coming from the digestive tract therefore rich in microorganisms. The cumulative production after 27 days have revealed for 100 g of mixture of substrates, the maximum quantity of biogas produced did not exceed 1000mL. In addition, the cumulative production trend curves have indicated a linear function with an ax+b type equation and a coefficient of determination oscillating around 98%. The different combinations of substrates could therefore induce continuous production of biogas after 27 days. So for one ton of mixture of substrates composed of water hyacinth and azolla with a microbial source, a production of 10.000 liters of biogas can be obtained. The remaining concerns was the composition of the biogas obtained. 23 had obtained 400 and 406 liters of biogas per kg of volatile solid after 30 and 50 days respectively from a combination of rice straw, water hyacinth and human excrement in semi-continuous digesters. But with water hyacinth as the only substrate, 58% of the biogas has been methane 24.
 | Figure 6: Biogas content produced by the various organic substrates tested
|
 | Figure 7: Cumulative biogas production during the experimental period
|
Quantity of methane produced after 27 days for 1 ton of substrate
The qualitative composition (Table 2) of the biogas produced from the two substrates in co-digestion after 27 days has revealed that the biogas produced was composed of H2S, CO2 and CH4 in short proportion. 24 had similar results regarding the composition of biogas which showed continuous production of methane, carbon dioxide and hydrogen sulfide. The wide range of biogas composition revealed by our work highlights the need for prior purification before use25. Likewise on solid substrates including food waste, 26 and 27 mentioned a similar composition of biogas but it is the relative mole fractions of the gases that vary. The combination of organic substrates including 75% of water hyacinth has generated the maximum quantity of methane which were 1234 liters for one ton of organic substrates. This methane production has been 1.93 times greater than that of the bioreactor containing water hyacinth alone, 1.90 times that containing azolla, 1.5 times that containing 25% of water hyacinth +75% of azolla then 3.04 times that containing the bioreactor composed of a high proportion of crushed Azolla sp. The optimal condition for methane production has been therefore a co-digestion device for the two substrates with a proportion of water hyacinth 3 times higher than that of Azolla. It therefore appears that for the establishment of mini bioreactor for the benefit of rural populations, the preferential substrate is water hyacinth. 28 and 24 demonstrated the methanogenic capacity of water hyacinth but our results, had revealed that the fermentable action observed with water hyacinth could be boosted by the addition of plant debris represented here by azolla residues in a small proportion. 13 then specifies that the production of methane had required mesophilic temperature conditions as confirmed by our results with the difference that these authors had investigated on substrates different from ours. 29, after investigating domestic waste, had noticed a remarkable production of methane. In total, this preliminary evaluation of the co-digestion of two plant pests had showed a higher production of methane in reactors with a high rate of water hyacinth but remained limited with regard to the real influence of microbial biomass and the addition of other sources of environmental waste.
Table 2: Quantitative and qualitative composition of biogas (L) for 100g of substrates and content for 1ton of substrate
Composition of biogas | CH4 | CO2 | H2S | |||
100g | 1t | 100g | 1t | 100g | 1t | |
WHPM | 0,06394 | 639,4 | 0,27179 | 2717,9 | 0,5964 | 5964 |
AZ PM | 0,06471 | 647,71 | 0.28077 | 2807,7 | 0,11816 | 1181,6 |
AZWHPM1 | 0,12346 | 1234,6 | 0,32501 | 3250,1 | 0,07178 | 717,8 |
AZWHPM2 | 0,08186 | 818,6 | 0,31600 | 3160 | 0,06808 | 680,8 |
AZWHPM3 | 0,04050 | 405,50 | 0,33263 | 3326,3 | 0,09671 | 967,1 |
Note: WHPM : 100% of water hyacinth ; AZPM : 100% of azolla ; AZWHPM1: 75% of water hyacinth +25% of azolla ; AZWHPM2: 25 % of water hyacinth +75 % of azolla ; AZWHPM3: 50% of water hyacinth +50% of azolla and AZWHPM4 : 66,25% of water hyacinth +33,75 % of azolla.
Fertilizing value of digestates
The analysis of variance carried out on the different digestates obtained from fermentation in the different bioreactors has indicated that there were a significant difference at the 5% threshold between the values ??of the mineral elements contained in these different bioreactors (table 3). The digestates from the different combinations of water hyacinth and Azolla were rich in C, N, P, K, Ca, Mg and Zn like many farm fertilizers including poultry droppings, pig droppings, crop residues and other digestate groups including Grass Silage, Corn Silage, Dairy Waste, Stomach Contents, Blood and Food Scraps 30. Our results also has confirmed those of 31 and those of 32, who had showed that soils amended with organic fertilizer based on sheep droppings significantly had improved the agronomic parameters of the soils 33. Generating biogas in Indonesia has become relevant, as digestate is often used as organic fertilizer for crops 34-37 . In addition, from our work, the digestates containing equal proportions of water hyacinth and Azolla were rich in nitrogen (24.03 ± 0.60 g/kg) against the increase in the Azolla content in the bioreactors have increased the phosphorus content (48.35 ± 2.57g/kg) of the digestates. The increased incorporation of water hyacinth in the composition of the substrates rather favors digestates rich in calcium (7.63 ± 0.14 g/kg) and magnesium (16.58 ± 3.92 g/kg). Zinc (144.89 ± 2.96 mg/kg) were highly concentrated in bioreactors enriched with Azolla. This has proved that to obtain digestates with high nutrient contents, composite substrates consisting of water hyacinth and azolla are required. 33 had obtained nutrient contents similar to ours with digestates from water hyacinth only. The Nitrogen-Phosphorus-Potassium (NPK) content of the different digestates according to the French standard NFU 44 051 has indicated that the digestates resulting from the mixture of 80% water hyacinth, and 20% pig excrement (NPK=6, 37%), mixture of 60% water hyacinth, 20% azolla and 20% pig excrement (NPK=6.61%), mixture of 20% water hyacinth, 60% azolla and 20% pig manure (NPK=6.45%) and a mixture of 53% water hyacinth, 27% azolla and 20% pig manure (NPK= 5.81%) have had average NPK contents lower than that of the French standard which were 7%, they have been therefore amenders whereas, the digestates of the mixture of 80% azolla and 20% pig excrement (NPK=7.67%) and the mixture of 40% water hyacinth, 40% azolla and 20% pig excrement (NPK= 7.39%) have had an NPK content greater than 7 % these digestates are fertilizers. 38 had showed that plants treated with composts have better growth in height and a better yield in fruit mass compared to those treated with mineral fertilizers and those without additions. Also, the richness of these composts in organic matter has improved the structure of the soil, thus ensuring better dissolution and assimilation of nutrients for good plant growth 39,40. Organic matter had retained nutrients on the surface while mineral fertilizer alone was accelerated their vertical migration 41. Organic matter had been, therefore, the best basic fertilizer 42,43. The digestates obtained have been therefore valuable fertilizers for agricultural production.
Table 3: Content of the different digestates in mineral elements
N (en g.kg-1) | C | P | K | Ca | Mg | Zn (mg.kg-1) | |
AZPM | 22.43 ± 0.60 a | 289.41 ± 6.64 ab | 48.35± 2.57 a | 5.92± 0.36c | 8.64±0.13 d | 7.93±0.27 c | 144.38±1.39 a |
WHPM | 23.87 ± 2.77 a | 308.72 ±30.37 ab | 32.27±3.06 c | 7.63±0.14 a | 14.22±0.32 ab | 16.58±3.92a | 112.64±2.60 c |
AZWHPM1 | 20.8 ± 0.12 a | 338.54 ±14.84 a | 38.56 ± 2.27bc | 6.69±0.21b | 15.47±0.39 a | 13.35±0.61 b | 118.01±2.28 c |
AZWHPM2 | 21.03 ± 096 a | 275.31 ±7.35 b | 37.65 ± 0.53 bc | 5.87±0.20 c | 11.55± 0.28 c | 10.98±0.36 bc | 144.89±2.96 a |
AZWHPM3 | 24.03 ±1.44 a | 312.15 ±16.11 ab | 43.21± 5.08 ab | 6.67±0.14b | 13.43±0.56 b | 11.9±0.43 bc | 139.87±16.83 ab |
AZWHPM4 | 21.66 ±0.79 a | 329.94 ±32.03 ab | 31.06±2.20 c | 5.43±0.27 c | 10.44± 0.76 c | 9.01±1.40 bc | 118.58±7.38c |
CV | 11,5 | 10,82 | 13,24 | 6,32 | 6,41 | 18,08 | 10,34 |
Note: WHPM: 100% of water hyacinth ; AZPM : 100% of azolla ; AZWHPM1: 75% of water hyacinth +25% of azolla ; AZWHPM2: 25 % of water hyacinth +75 % of azolla ; AZWHPM3: 50% of water hyacinth +50% of azolla and AZWHPM4 : 66,25% of water hyacinth +33,75 % of azolla.
Table 4: Nitrogen-Phosphorus-Potassium (NPK) content of the digestates analyzed
Digestat | NPK content | Characteristics according to standard NFU 44 051 | Agronomic qualification |
AZ PM | 7,67 | > 7 % | Fertilizer |
WHPM | 6,37 | < 7 % | Amendment |
AZWHPM1 | 6,61 | < 7 % | Amendment |
AZWHPM2 | 6,45 | < 7 % | Amendment |
AZWHPM3 | 7,39 | > 7 % | Fertilizer |
AZWHPM4 | 5,81 | < 7 % | Amendment |
Note: AZPM: 100% of water hyacinth ; AZPM : 100% of azolla ; AZWHPM1: 75% of water hyacinth +25% of azolla ; AZWHPM2: 25 % of water hyacinth +75 % of azolla ; AZWHPM3: 50% of water hyacinth +50% of azolla and AZWHPM4 : 66,25% of water hyacinth +33,75 % of azolla.
Conclusion
The methanization and co-digestion test of water hyacinth and Azolla has revealed a high methane production of 1234.6 liters of methane per 1000kg of substrates in the bioreactor containing 75% water hyacinth and 25% of Azolla with an addition of sources of anaerobic microorganisms consisting of fresh pig excrement, an initial mesophilic temperature of 38°C after 27 days of experimentation. These data obtained are still preliminary since the microbial kinetics, pH and temperature throughout the experiment were not followed in this research given the device adopted but have had the merit of revealing the fruitful possibilities for valorizing plant pests. for energy purposes for small communities bordering water bodies. In addition, the chemical composition of the substrates tested, in particular hemicellulose, cellulose and lignin, substances resistant to the activity of microorganisms, must be determined to better identify the organic debris to complement the two plant pests identified in the study area.
Acknowledgement
The authors would like to thank BIOGAZ-Benin for hosting the experimental phase of this research.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Approval Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Authors’ Contribution
BOKOSSA Hervé Kouessivi Janvier, ABIOLA Francine and Hidirou TORO MOUSSA TAHIROU: Formal analysis, visualization, and writing-original draft.
BONI Gratien, and JOHNSON Roch Christian: Conceptualization, supervision, and writing-review and editing.
JOHNSON Roch Christian: Investigation and resources.
All authors have read and agreed to the published version of the manuscript.
References
- Ravard B. Evaluation du potentiel méthanogène de différentes rations et des effets des digestats (fraction sèche et fraction liquide) sur des indicateurs de fonctionnement biologique du sol en lien avec le service de fertilité. Mémoire. Biologie et Ecologie pour la Forêt, l’Agronomie et l’Environnement. 2014 ; 38 p.
- Balat M., Balat H. Progress in biodiesel processing. Applied energy. 2010 ; 87(6) : 1815-1835.
CrossRef - OCDE.Energie: les cinquantes prochaines années. Rapport technique. 2010 ; 190p.
- Samah K. Diagnostique de la situation energetique au Togo. Ministère de l’environnement, Direction des eaux et forêts. République Togolaise. 2015 ; 21p.
- Magloire T. Evaluation du potentiel agronomique des digestats sur les sols de la Région de la Kara. 2019 ; 57p
- Zuhal A, Potential of biogas energy from animal waste in the Mediterranean Region of Turkey. Journal of Energy Systems. 2018 2(4): 160-167, DOI:10.30521/jes.455325
CrossRef - Mshandete A., Björnsson L., Kivaisi A. K., Rubindamayugi M. S., Mattiasson B. Effect of particle size on biogas yield from sisal fibre waste. Renewable energy. 2006 ; 31(14) : 2385-2392.
CrossRef - Igoni A. H., Ayotamuno M. J., Eze C. L., Ogaji S. O. T., Probert S. D. Designs of anaerobic digesters for producing biogas from municipal solid-waste. Applied energy. 2008 ; 85(6) : 430-438.
CrossRef - Yadvika S., Sreekrishnan T. R., Sangeeta K., Vineet R. Enhancement of biogas production from solid substrates using different technique - A Review. Bioresour. Technol. 2004 ; 95 : 1-10
CrossRef - Schievano A., D'Imporzano G., Adani F. Substituting energy crops with organic wastes and agro-industrial residues for biogas production. Journal of environmental management. 2009 ; 90(8) : 2537-2541.
CrossRef - Ward A. J., Hobbs P. J., Holliman P. J., Jones D. L. Optimisation of the anaerobic digestion of agricultural resources. Bioresource technology. 2008 ; 99(17) : 7928-7940.
CrossRef - Abbasi T., Abbasi S. A. Production of clean energy by anaerobic digestion of phytomass - new prospects for a global warming amelioration technology. Renewable and Sustainable Energy Reviews. 2010 ; 14(6) : 1653-1659.
CrossRef - Chen T., Zhang S., Yuan Z. Adoption of Solid Organic Waste Composting Products: A Critical Review. Journal of Cleaner Production. 2020 ; 272, 122712. DOI:10.1016/j.jclepro.2020.122712.
CrossRef - Alwaeli M., Alshawaf M., Klasik M. Recycling of Selected Fraction of Municipal Solid Waste as Artificial Soil Substrate in Support of the Circular Economy. Archives of Environmental Protection. 2022 ; 48 (4) : 68–77. DOI:10.24425/aep.2022.143710.
CrossRef - Hovorukha V. The effect of fermentation modes on the efficiency of organic waste treatment in batch bioreactors. Archives of Environmental Protection. 2024 ; 50(1) : 80–86. DOI:10.24425/aep.2024.149434.
CrossRef - Guézo N. C., Fiogbé E. D., Bokossa H. K. J., Ouattara A., Assogba V. A., Kouamelan P. E. Impact de l’utilisation du chlorure de sodium utilisé en pulvérisation pour lutter contre le développement de la jacinthe d’eau Eichhornia crassipes sur la qualité physico-chimique d’un écosystème semi-artificiel. Vertigo - la revue électronique en sciences de l'environnement. 2022 ; 20p. DOI: 10.4000/vertigo.34850.
CrossRef - Katinas V., Mar?iukaitis M., Perednis E., Dzenajavi?ien? E. F. Analysis of Biodegradable Waste Use for Energy Generation in Lithuania. Renewable and Sustainable Energy Reviews. 2019; 101: 559–567. DOI:10.1016/j.rser.2018.11.022.
CrossRef - Bakkaloglu S., Lowry D., Fisher R. E., France J. L., Brunner D., Chen H., Nisbet E. G. Quantification of Methane Emissions from UK Biogas Plants. Waste Management. 2021; 124: 82–93. DOI:10.1016/j.wasman.2021.01.011.
CrossRef - Khan M. A., Ngo H. H., Guo W., Liu Y., Zhang X., Guo J., Chang S.W., Nguyen D.D., Wang J. Biohydrogen Production from Anaerobic Digestion and Its Potential as Renewable Energy. Renewable Energy. 2018 ; 129 : 754–768. DOI:10.1016/j.renene.2017.04.029.
CrossRef - Rodier J., Legube B., Merlet N. Analyse de l’eau Rodier. 9ème édition. 2009 ; 1579pp.
- Dagnelie P. Théorie et Méthodes Statistiques. Applications Agronomiques. Les presses agronomiques de Gembloux. ASBL : Gembloux ; Belgique. 1986 ; 2 : 463.
- Singhal V., Rai J. P. N. Biogas production from water hyacinth and channel grass used for phytoremediation of industrial effluents. Bioresource technology. 2003 ; 86(3) : 221-225.
CrossRef - Hansen T. L., Schmidt J. E., Angelidaki I., Marca E., la Cour Jansen J, Mosbæk H, Christensen TH. Method for determination of methane potentials of solid organic waste. Waste management. 2004 ; 24(4) : 393-400.
CrossRef - Polprasert C., Edwards P., Rajput V. S., Pacharaprakiti C. Integrated biogas technology in the tropics 1. Performance of small-scale digesters. Waste management & research. 1986 ; 4(2) : 197-213.
CrossRef - Dèdonougbo D. A., N’Gobi K. G., Kouchade C. A., Kounouhewa B. B. Evaluation du pouvoir méthanogene de la jacinthe d’eau sur le Lac Nokoue à Ganvie au Benin. Journal de physique de la SOAPHYS. 2021 ; 2(2) (2020) C20A23. http://dx.doi.org/10.46411/jpsoaphys.2020.02.23.
CrossRef - Zhang J., Kan X., Shen Y., Loh K. C., Wang C. H., Dai Y, Tong Y. W. A Hybrid Biological and Thermal Wasteto-Energy System with Heat Energy Recovery and Utilization for Solid Organic Waste Treatment. Energy. 2018 ; 152 : 214-222. DOI:10.1016/j.energy.2018.03.143.
CrossRef - Fernández-Delgado Juárez M, Mostbauer P, Knapp A, Müller W, Tertsch S, Bockreis A, Insam H. Biogas purification with biomass ash Waste Management.2017. In press.
CrossRef - Parthiba K. O., Trably E., Mehariya S., Bernet N., Wong J. W. C., Carrere H. Pretreatment of Food Waste for Methane and Hydrogen Recovery: A Review. Bioresource Technology. 2018 ; 249 : 1025–1039. DOI:10.1016/j.biortech.2017.09.105.
CrossRef - Priya P., Nikhitha S. O., Anand C., Dipin N. R. S., Krishnakumar B. Biomethanation of water hyacinth biomass. Bioresource Technology. 2018. doi: https://doi.org/10.1016/j.biortech.2018.01.119.
CrossRef - Chaijak P., Sola P. ‘The New Report of Domestic Wastewater Treatment and Bioelectricity Generation Using Dieffenbachia Seguine Constructed Wetland Coupling Microbial Fuel Cell (CW-MFC)’. Archives of Environmental Protection. 2023 ; 49 (1) : 57-62. DOI:10.24425/aep.2023.144737.
CrossRef - IEA Bioénergy. Utilisation of digestate from biogas plants as biofertiliser. 2010 ; 24 p.
- Kitabala M. A., Tshala U. J., Kalenda M. A., Tshijika I. M., Mufind K. M. Effets de différentes doses de compost sur la production et la rentabilité de la tomate (Lycopersicon esculentum Mill) dans la ville de Kolwezi, Province du Lualaba (RD Congo). Journal of Applied Biosciences. 2016 ; 102 : 9669-9679.
- Batamoussi M. H., Tovihoudji P. G., Tokore O. M., Boulga J., Essegnon I. M. Effet des engrais organiques sur la croissance et le rendement de deux variétés de tomate (Lycopersicum esculentum) dans la commune de Parakou (Nord Bénin). International Journal of Innovation and Scientific Research. 2016 ; 24(1) : 86-94.
- Smith J., Abegaz A., Matthews R. B., Subedi M., Orskov E. R., Tumwesige V., Smith P. What is the potential for biogas digesters to improve soil fertility and crop production in Sub-Saharan Africa. Biomass & Bioenergy. 2014 ; 70: 58-72.
CrossRef - Almoustapha O., Millogo-Rasolodimby J., Kenfack S. Production de biogaz et de compost à partir de la jacinthe d’eau pour un développement durable en afrique sahélienne. VertigO – La revue en sciences de l'environnement. 2008 ; 8(1) : 1-8.
CrossRef - Ounnar A, Benhabyles L, Igoud S. Energetic valorization of biomethane produced from cow-dung. Procedia Eng 2012;33:330–4.
CrossRef - Nasir IM, Ghazi TIM, Omar R, Idris A. Anaerobic digestion of cattle manure: influence of Inoculum concentration. Int J Eng Technol 2013;10:22–6.
- Comparetti A, Greco C, Navickas K, Venslauskas K. Evaluation of potential biogas production in Sicily. Eng Rural Dev 2012;11:555–9.
- De Vries J, Vinken T, Hamelin L, De Boer I. Comparing environmental consequences of anaerobic mono-and co-digestion of pig manure to produce bio-energy a life cycle perspective. Bioresour Technol 2012;125:239–48.
CrossRef - Tamia J., Brou Y. B., Yéboua K., Tacra T., Konan E. D. Physico-chemical characteristics of composts based on plant residues and animal manure and their effects on growth and yield parameters of tomato on sandy soils. International Journal of Innovation and Scientific Research. 2022 ; 62(2) : 61–75.
- Duplessis J. Le compostage facilité : guide sur le compostage domestique NOVA. Envirocom. 2002 ; 107 p.
- Fagnano M., Adamo P., Zampella M., Fiorentino N. Environmental and agronomic impact of fertilization with composted organic fraction from municipal solid waste: a case study in the region of Naples, Italy. Agriculture, ecosystems & environment. 2011 ; 141(1-2) : 100-107.
CrossRef - Yoboue A. N., N’goran K. E., Ama T. J., Kouassi Y. F., Yao G. F. Effets du précédent cultural arachide (Arachis hypogaea L.) et de la charge en éléments grossiers du sol sur la production du coton (Gossypium hirsutum L.). Int. J. Biol. Chem. Sci. 2020 ; 14 (6) : 2120-2133.
CrossRef - Giller K. E., Cadisch G., Palm C. The North-South divide! Organic wastes, or resources for nutrient management? Agronomie. 2002 ; 22(7-8) : 703-709.
CrossRef - Ojetayo A. E., Olaniyi J. O., Akanbi W. B., Olabiyi T. I. Effect of fertilizer types on nutritional quality of two cabbage varieties before and after storage. Journal of Applied Biosciences. 2011 ; 48(5) : 3322-3330.







