Defluoridation Proficiency of Ocimum tenuiflorum Based Bioadsorbent against Fluoride Toxicity in Drinking Water
1
Department of Science and Humanity (Chemistry),
Dr. G.u. Pope’s College of Engineering,
Sawyerpuram, Affiliated to Anna University,
Chennai,
Tamil nadu
India
2
Department of Chemistry,
The American College,
Madurai,
Tamil nadu
India
3
R and D Division,
IB Group,
Rajnandgaon,
Chhattisgarh
India
Corresponding author Email: liviveera@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.17.2.8
Copy the following to cite this article:
Veeraputhiran V, Monica J. H. R, Manam V. K. Defluoridation Proficiency of Ocimum tenuiflorum Based Bioadsorbent against Fluoride Toxicity in Drinking Water. Curr World Environ 2022;17(2). DOI:http://dx.doi.org/10.12944/CWE.17.2.8
Copy the following to cite this URL:
Veeraputhiran V, Monica J. H. R, Manam V. K. Defluoridation Proficiency of Ocimum tenuiflorum Based Bioadsorbent against Fluoride Toxicity in Drinking Water. Curr World Environ 2022;17(2).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 27-03-2022 |
|---|---|
| Accepted: | 13-05-2022 |
| Reviewed by: | 
 Katarzyna Staszak
Katarzyna Staszak
|
| Second Review by: |

 Grigorios Kyriakopoulos
Grigorios Kyriakopoulos
|
| Final Approval by: | Dr. Amit Kumar |
Introduction
Though fluoride is one of the nutritional needs to human, the same is the major principal hazard contaminant in drinking water which exists naturally1. The availability of fluoride concentration in drinking water defines whether it is beneficial or harmful2. World Health Organization (WHO) suggests drinking water should contain fluoride less than 1.5 mg/L3. In India, more state people are consuming water with higher fluoride even after basic commercial purifications4. The excess fluoride intake by the people yielded to dental fluorosis as well as skeletal fluorosis5. The easiest identification of yellowish or brownish teeth reveals that the person is being affected by dental fluorosis. The excess fluoride not only leads to dental caries, it could also lead to the consecutive health impacts upto the inability of human’s physical movement6. Hence, for the past three decades the researcher’s attention turn towards the removal of fluoride through various chemical and physical methods. There are many traditional techniques adopted and leads to successful elimination of fluoride from drinking water7-10. Among them biosorption is a cost effective and ecofriendly method but only very few attempts were reported11-13. Therefore, to identify few more competent bioadsorbents for defluoridation, this present research team focused towards some herbal plants and presented here one of the best bioadsorbent derived from Holy Basil Ocimum tenuiflorum leaves, commonly known in India in the name of Tulsi. Ocimum tenuiflorum is one of the versatile medicinal plants from the ancient periods through holistic scripts. It is a freely available cheap herbal plant throughout India especially in Southern India. The core objectives of this project are to study the defluoridation effectiveness of prepared bioadsorbent with respect to the fluoride concentration, contact time, adsorbent dose, various sizes of adsorbent and with different natural co-anions via batch experiments. In addition to the above, the characteristic behaviour and changes in physical nature of the adsorbents were reported.
Experimental Methods
Bioadsorbent Preparation
The decided adsorbent material holy basil Ocimum tenuiflorum L. (tulsi) leaves powder is commercially available and was purchased from Siddha Pharmacy, Siddha Medical College Campus, Tirunelveli, India. It was kept aside for shadow dry for 24 hrs. After drying, the samples were further grinded well to obtain fine powdered material. Further this powder sample was air-oven dried at 378 K for 24 hrs. After that using muffle furnace, the dried herbal powder was thermally charred to get activated adsorbent at 800 °C instead of acid treatment for charring. The obtained bioadsorbent was then cooled for few hours, grinded further and sieved to the desired different particle mesh sizes and preserved in calcium desiccators upto an hour before the experiment. Analytical grade chemicals and reagents were used in this present study and used as it is. Doubly distilled water was used throughout the study. Whatmann No.41 filter papers were utilized for the filtration purposes wherever required.
Batch Adsorption Studies
In the adsorption studies, batch equilibration method is the best way to identify the efficacy of adsorbents. In this present defluoridation research, the similar pattern of batch equilibration method was performed especially at neutral pH to find the adoptability of prepared bioadsorbent at various climatic conditions. Primary NaF stock solution was prepared to 1000 mg/L as adsorbate solution and the subsequent dilutions to various fluoride concentrations were prepared using doubly distilled water for the experiments. As a pilot test, the adsorbent dose variation study was executed where 100 mL of 3.0 mg/L fluoride solution was taken up with different adsorbent doses such as 0.25-2.00 g/L with the increment of 0.25 g adsorbent at room temperature with 60 minutes contact time. It was identified that 1.5 g/L could provide optimum defluoridation and hence it was fixed as initial adsorbent dose for all the experiments except adsorbent dose effects study.
Initially the effect of contact time and fluoride concentration were studied by varying the adsorption contact time from 15 min to 3 h with the increment of 15 minutes. Parallelly the same set of experiments followed to the different initial fluoride concentrations of 2-10 mg/L solutions. From this set of studies, the optimum contact time and fluoride concentrations were finalized and traced to the subsequent batch experiments. By setting the adsorbate-adsorbent contact time and different initial fluoride concentrations, the effect of adsorbent dosage was studied as stated earlier and the effect of adsorbent particles sizes were also studied.
In addition to that, to encounter the effective defluoridation property of the prepared bioadsorbent, the set of batch experiments were carried out with natural ground water conditions in the presence co-anions viz. chloride, nitrate, sulphate and bicarbonates in addition to the fluoride. In general, due to the unavailability of good quality water, the portable drinking-ground waters of different zones contain bicarbonates in the range of 200 mg/L – 500 mg/L; Chloride concentrations upto 250 mg/L; nitrates upto 120 mg/L and sulphates upto 200 mg/L 14. Hence, the experiments were done at different concentrations of co-anions such as 100, 200, 300, 400 and 500 mg/L with 3.0 mg/L F- concentration. Remaining experiments except in effect of various fluoride concentrations study, the initial adsorbate concentration was followed with 3.0 mg/L as similar to southern India ground water condition.
In general, rotator shaker was used for the thorough shaken of the reactants and kept 200 rpm. After that the solutions were filtered off using Whatmann No. 41 filter paper. Then the supernatant liquids were analysed for fluoride concentration using Orion 9 ion-selective instrument. The adsorbent in the residue was dried at 100 °C using hot air oven and then stored for adsorptive characterization studies. The defluoridation capacity of adsorbent was calculated using the formulae15,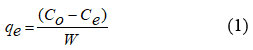
where qe is defluoridation capacity (mg/g); Co is initial concentration (mg/L); Ce is equilibrium residual concentration (mg/L); W is adsorbent weight (g). All the results were computed using MS-Excel 2013 and Origin Pro 9.0 software.
Bioadsorbent Characterization
The physical and morphological nature of the Ocimum tenuiflorum based prepared bioadsorbent were studied by Bruker AXS D8 Advance, Inst ID: OCPL/ARD/26-002 X-ray diffractometer (NIIST, Trivandrum) and Philips XL-20 electron microscope fitted with an energy dispersive X-ray analyzer (EDAX) (SAIF, Pondicherry University) that allows a qualitative detection and localization of elements in the adsorbent. In addition to that the FT-IR spectra for the experiment were recorded by using Nicolet 6700, Thermo Electronic Corporation, USA made spectrophotometer (Department of Physics, Pondicherry University).
Results and Discussion
Role of Contact Time and Fluoride Concentration
Role of contact time on Ocimum tenuiflorum based bioadsorbent–solution interaction was taken by varying the time from 15 to 180 minutes with 15 minutes time intervals to find out the optimal time for higher defluoridation while all other parameters such as adsorbent dose, particle size, temperature, pH, and fluoride concentration were kept as constant. Correspondingly, the adsorbate fluoride variation study was carried out for the fluoride concentrations 2-10 mg/L with increment of 2 mg/L. All the batch findings were depicted in Fig. 1. While analyzing Fig. 1, there are two dissimilar phases identified in this adsorption studies. Among them the initial phase is fast upto 90 minutes of contact time and the second one is very slow after 90 min. Therefore, the remaining forthcoming experiments were studied with 90 min contact period. This is due to the fact of non-availability of binding sites on bioadsorbent for further fluoride adsorption. Also, Fig. 1 elucidates that the maximum number of adsorbate fluoride was bonded on adsorbent surface at low fluoride concentration which lead to very high defluoridation rate. But in the case of higher concentration, due to availability of higher fluoride ions, the adsorbed fluoride quantity percentage will get low. The concentrations of both 2 mg/L and 3 mg/L fluoride solutions provided similar adsorption efficiency, this might due to the equilibrium between solution concentration and solid phase of Ocimum tenuiflorum based bioadsorbent.
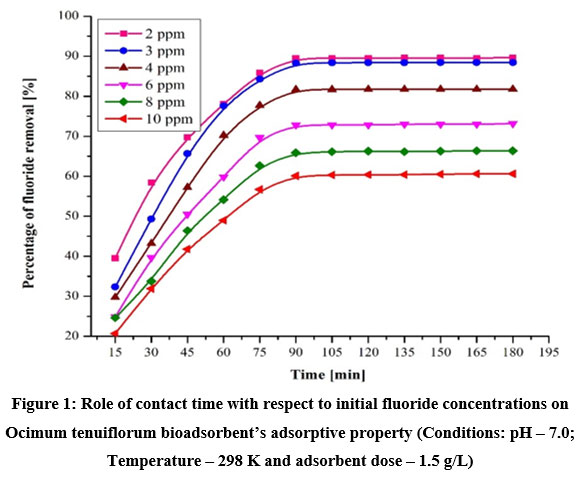 | Figure 1: Role of contact time with respect to initial fluoride concentrations on Ocimum tenuiflorum bioadsorbent’s adsorptive property (Conditions: pH – 7.0; Temperature – 298 K and adsorbent dose – 1.5 g/L) Click here to view Figure |
Effect of Biosorbent Dose and Particle Size
For the different dose of Ocimum tenuiflorum bioadsorbent, the defluoridation efficiency was evaluated with standard fluoride solutions that are having fixed values of fluoride concentration, its contact time, adsorbent particle size, pH and temperature. It could be seen from Fig. 2 that fluoride removal efficiency increased from 46% to 88.93% when the adsorbent dose was increased from 0.25 g/L to 2.0 g/L. At the value of 1.5 g/L, there was a significant fluoride removal attained to 88.3% and further improvement in adsorbent dose could not provide valuable removal efficiency. The minimum required concentration at solution phase defined its equilibrium stage and hence there was no effective observations after 1.5 g/L adsorbent dose. This dose was noted as optimum and utilized for the further experiments. The plot Fig. 3 of adsorbent dose with distribution coefficient KD gives the information about the adsorbent that it is in heterogeneous nature7.
 | Figure 2: Effect of adsorbent dosages on defluoridation capacity (Conditions: pH – 7.0; Temperature – 298 K and fluoride concentration – 3 mg/L). Click here to view Figure |
 | Figure 3: Distribution coefficient KD curve from adsorbent dose study Click here to view Figure |
With the above optimum adsorbent amount 1.5 g/L, the various particle sizes such as <53, 53–106, 106–150, 150–225 and 225–303 µm of Ocimum tenuiflorum bioadsorbent were performed for its fluoride removal efficiency by keeping remaining parameters as constant and findings are shown in Fig. 4. Obviously, the lower particle size <53 µm of adsorbent shown higher defluoridation efficiency while others show decreasing fluoride removal pattern with respect to their increasing particle sizes5,11. The results obeyed the basic adsorption principle that if particle size decreases, the adsorption capacity will get increased due to the increased surface area in the smaller sized adsorbents.
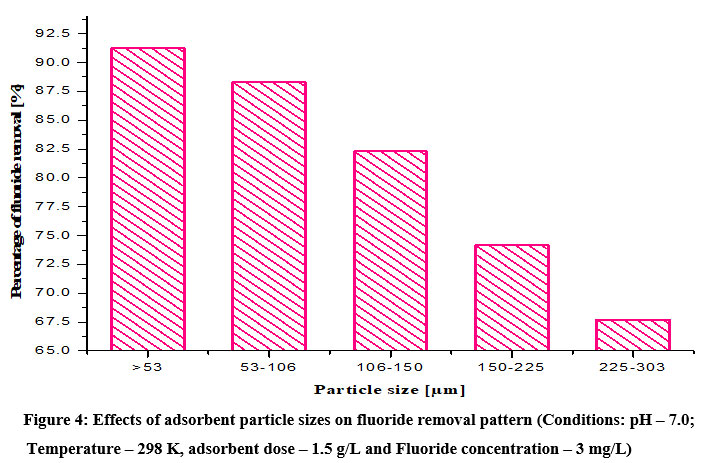 | Figure 4: Effects of adsorbent particle sizes on fluoride removal pattern (Conditions: pH – 7.0; Temperature – 298 K, adsorbent dose – 1.5 g/L and Fluoride concentration – 3 mg/L). Click here to view Figure |
Interference of Co-Existing Anions
In addition to the fluoride ions, other anions namely bicarbonate, sulphate, nitrate, chloride are present in ground water. Therefore, the defluoridation experiments were performed using the adsorbents in the presence of each of these anions separately. The effect of these interfering co-ions on defluoridation of Ocimum tenuiflorum bioadsorbent is given in Fig. 5. The observation from the figure reveals that both sulphate and bicarbonate shown remarkable interfering effect on defluoridation by this adsorbent while nitrate and chloride ions show less interference on defluoridation by Ocimum tenuiflorum bioadsorbent. This might due to that the chloride have less electronegativity than fluoride and univalent. But bicarbonates and sulphates affect the defluoridation even at its minimum concentration of 100 mg/L and 200 mg/L. This is because of their bulky structures; they protect large surface area of adsorbent’s surface from its affinity towards more number of fluoride ions and so there was unexpected drop in defluoridation capacity of prepared bioadsorbent observed6.
 | Figure 5: Interference of co- ons on defluoridation capacity of Ocimum tenuiflorum based bioadsorbent (Conditions: pH – 7.0; Temperature – 298 K, adsorbent dose – 1.5 g/L and Fluoride concentration – 3 mg/L) Click here to view Figure |
Instrumental Analysis of Morphological Characteristics
SEM morphologies of Ocimum tenuiflorum bioadsorbent and its fluoride loaded adsorbents are presented in Figs. 6a and 6b. Fig. 6a confirmed a clear morphology of adsorbent solid particles. On comparison of both images, it is quite clear that adsorption of fluoride onto the activated carbon is more prominent by the sludgy appearance. EDAX spectrum of fluoride adsorbed and untreated adsorbents gave additional evidence through Figs. 7a and 7b by revealing the presence of fluoride element identification in Fig. 7b and strongly supported the fluoride interaction with the adsorbent. The XRD pattern of both treated and untreated bioadsorbents were given in Fig. 8. Due to blocking the pores and fine surfaces of bioadsorbents by fluoride ions, the XRD of fluoride treated adsorbent missed its own prominent identifiable peaks. It confirmed the fluoride adsorption onto the Ocimum tenuiflorum bioadsorbent. FTIR spectrums in Fig. 9 indicated the –OH stretching band scale of 3261 cm?1 - 3669 cm?1; other band at 1451 cm?1 assigned by reason of inter layer water molecules16. The bands 1046 cm?1 and 566 cm?1 are attributed to metal–OH bond stretching17. After treated with fluoride solution, there were changes in the intensities of these each peak due to the fluoride interaction with metals present in the adsorbents.
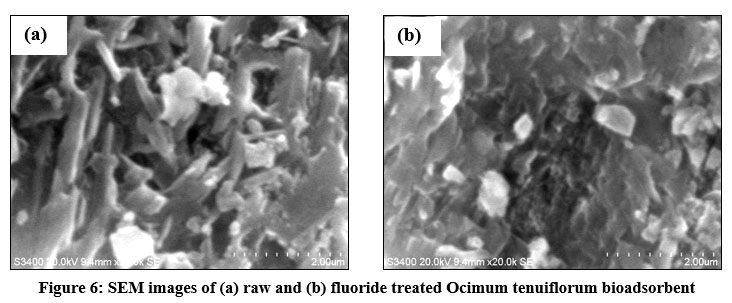 | Figure 6: SEM images of (a) raw and (b) fluoride treated Ocimum tenuiflorum bioadsorbent. Click here to view Figure |
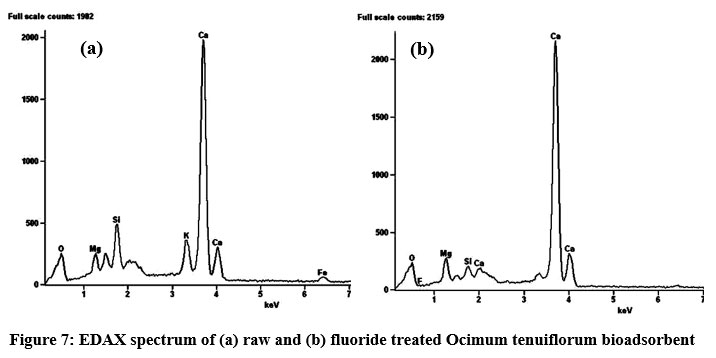 | Figure 7: EDAX spectrum of (a) raw and (b) fluoride treated Ocimum tenuiflorum bioadsorbent Click here to view Figure |
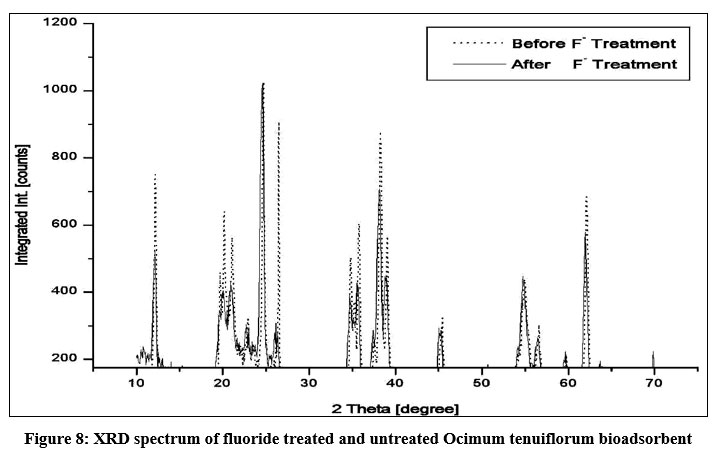 | Figure 8: XRD spectrum of fluoride treated and untreated Ocimum tenuiflorum bioadsorbent. Click here to view Figure |
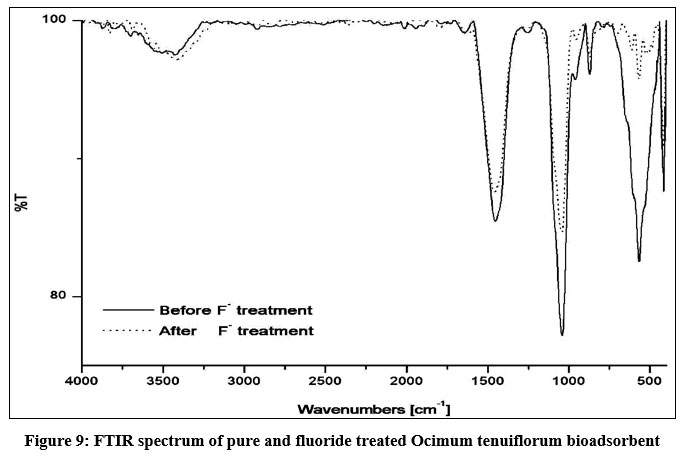 | Figure 9: FTIR spectrum of pure and fluoride treated Ocimum tenuiflorum bioadsorbent. Click here to view Figure |
Conclusion
Higher fluoride contaminant in drinking water is a serious concern since it causing numerous health impacts though it is an essential element to human health. Therefore, the removal of fluoride to its permissible limit in the drinking water have done by many researchers through different defluoridation approach, among them adsorption is a cost-effective ease operative method. This present study revealed successful preparation of bioadsorbent from Ocimum tenuiflorum commonly known as Tulsi in India. Batch equilibrium method was employed to know the adsorptive capacity of prepared bioadsorbent by varying different concentration of fluoride (adsorbate), different adsorbent dose and its different particle sizes. The findings depicted that the contact time of 90 minutes is an effective optimal time for the defluoridation using Ocimum tenuiflorum based bioadsorbent for all concentrations of fluoride in ppm range. And the lower particle size < 53 ?m could leach more fluoride from the solution medium. Importantly, the adsorbent quantity of 1.5 g/L was found that it could adsorb the maximum available fluoride at adsorbent-solution equilibrium. Apart from that to know the defluoridation efficiency with respect to real water environmental, batch studies were carried out with the presence of coexisting anions viz. chloride, nitrate, sulphate and bicarbonate. The results reveals that the presence of sulphate and bicarbonates largely interfering the adsorbent-fluoride interactions due to its occupation of large volume of adsorbent’s surface. But the presence of nitrates and chlorides along with fluoride did not affect the defluoridation capacity of the prepared bioadsorbent significantly. At all the above optimal conditions, the maximum defluoridation capacity by Ocimum tenuiflorum based bioadsorbent was achieved to 1766 mg/kg. The spectral characterizations such as SEM, EDAX, XRD and FT-IR also supported the leaching of fluoride to the adsorbent site by providing weighty findings in SEM image morphologies, presence of fluoride element in EDAX spectrum and noteworthy changes in peaks of XRD and FT-IR spectrums. Overall findings led the conclusion that the Ocimum tenuiflorum based bioadsorbent can be used not only as defluoridating agent, it can also be used for more adsorptive heavy metal removal studies.
Acknowledgement
Authors thank the management of Dr.G.U.Pope’s College of Engineering, Sawyerpuram for providing the facilities to do this work.
Conflict of interest
Authors declares that there is no conflict of interest.
Funding Sources
There is no funding or financial support for this research work.
References
- Okajima N, Tafu M, Toshima T, Takada M, Hagino Y. Enhanced reactivity of dicalcium phosphate dihydrate with fluoride ions by coating with apatite nanoparticles. J Asian Ceram Soc 2021;9(2):498-506.
CrossRef - Banerjee A. Groundwater fluoride contamination: a reappraisal. Geosci Front 2015;6(1):277-284.
CrossRef - Lennon M. A, Whelton H, O’Mullane D, Ekstrand J. Rolling Revision of the WHO Guidelines for Drinking-Water Quality, World Health Organization, Geneva, 2004.
- Ugran V, Desai N. N, Chakraborti D, Masali K.A, Mantur P. Groundwater fluoride contamination and its possible health implications in Indi taluk of Vijayapura District (Karnataka State), India. Environ Geochem Health 2017;39(1):1017-1029.
CrossRef - Alagumuthu G, Veeraputhiran V, Venkataraman R. Fluoride sorption using Cynodon dactylon based activated carbon. Hemijska industrija 2011;65(1):23-3
CrossRef - Yadav K. K, Kumar S, Pham Q. B, Gupta N, Rezania S, et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicol Environ Saf 2019;182(1):109362.
CrossRef - Lunge S, Thakre D, Kamble S, Labhsetwar N, Rayalu S. Alumina supported carbon composite material with exceptionally high defluoridation property from eggshell waste. J Hazard Mater 2012;237(1):161-169.
CrossRef - Bansiwal A, Pillewan P, Biniwale R. B, Rayalu S. S. Copper oxide incorporated mesoporous alumina for defluoridation of drinking water. Micropor Mesopor Mater 2010;129(1):54-61.
CrossRef - Borgohain X, Boruah A, Sarma G. K, Rashid M. H. Rapid and extremely high adsorption performance of porous MgO nanostructures for fluoride removal from water. J Mol Liq 2020;305(1):11279
CrossRef - Solanki Y. S, Agarwal M, Maheshwari K, Gupta S, Shukla P, Gupta A. B. Removal of fluoride from water by using a coagulant (inorganic polymeric coagulant). Environ Sci Pollut Res 2021;28(1):3897-3905.
CrossRef - Rajkumar S, Murugesh S, Sivasankar V, Darchen A, Msagati T. A. M, Chaabane T. Low-cost fluoride adsorbents prepared from a renewable biowaste: syntheses, characterization and modeling studies. Arab J Chem 2019;12(1):3004-3017.
CrossRef - Kamble S. P, Jagtap S, Labhsetwar N. K, Thakare D, Godfrey S, Devotta S, Rayalu S. S. Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem Eng J 2007;129(1):173-180.
CrossRef - Alagumuthu G, Veeraputhiran V, Venkataraman R. Adsorption isotherms on fluoride removal: batch techniques. Arch Appl Sci Res 2010;2(4):170-185.
- Veeraputhiran V, Alagumuthu G. A report on fluoride distribution in drinking water. Int J Env Sci 2010;1(4):558-566.
- Sivasankar V, Ramachandramoorthy T, Chandramohan A. Fluoride removal from water using activated andMnO2-coated Tamarind Fruit (Tamarindus indica) shell: batch and column studies. J Hazard Mater 2010;177:719-729.
CrossRef - Sujana M. G, Pradhan H. K, Anand, S. Studies on sorption of some geomaterials for fluoride removal from aqueous solutions. J Hazard Mater 2009;161(1):120-125.
CrossRef - Zhon Y, Yu C, Shan Y. Adsorption of fluoride from aqueous solution on La3+-impregnated cross-linked gelatin. Sep Purif Tech 2004;36(2):89-94.
CrossRef






