Effect of Biotic Disturbances on Herbaceous Vegetation in Cypress Mixed Oak Forests of Central Himalaya, India
Himani Karki 1 * , Pratima Rana 1 , Kiran Bargali 1 , S. S. Bargali 1 and Y.S. Rawat 1
DOI: http://dx.doi.org/10.12944/CWE.11.2.09
Herbaceous layer remains an underappreciated aspect of forest ecosystem, which serves a special role in maintaining the structure and functioning of forests. It provides important information regarding the site characteristics of forests. Present study examined the effects of site on herbaceous vegetation in an oak-mixed cypress forest in Central Himalaya, India. The study sites are located near Nainital town between 29o36’56”-29o36’79” N latitude and 79o46’03”-79o46’19” E longitude between 1600- 1850 m above mean sea level in the Central Himalaya. Both lowly and highly disturbed sites were further subdivided into three sub-sites (hill base, hill slope and hill top). A total of 35 species represented by twenty families were recorded from the studied sites. Maximum herb species belongs to the Asteraceae family. The lowly disturbed site was more diverse than the highly disturbed one. Total herb density was 344.4 ind.m-2 at highly disturbed site, whereas 481.2 ind.m-2 at lowly disturbed site.
Copy the following to cite this article:
Karki H, Rana P, Bargali K, Bargali S. S, Rawat Y. S. Department of Botany, DSB Campus. Kumaun University, Nainital-263001 (Uttarakhand), India. Curr World Environ 2016;11(2) DOI:http://dx.doi.org/10.12944/CWE.11.2.09
Copy the following to cite this URL:
Karki H, Rana P, Bargali K, Bargali S. S, Rawat Y. S. Department of Botany, DSB Campus. Kumaun University, Nainital-263001 (Uttarakhand), India. Curr World Environ 2016;11(2). Available from: http://www.cwejournal.org/?p=16066
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2016-07-16 |
|---|---|
| Accepted: | 2016-08-19 |
Introduction
Plants in the under storey layer, maintains the structure and functioning of forests (Singh and Singh, 1987; Bargali and Bargali, 2000; Augusto, 2003; Whigham, 2004; Bargali et al., 2015a). Herbaceous plant layer contains the higher number of species in the forest community and influences nutrient cycling in such a way that is disproportionate to relative biomass (Gilliam, 2007; Rana et al., 2014). This layer is also responsible for approximately 12% of the Gross Photosynthetic Production (GPP) of a forest ecosystem (Muller, 2003). In many forest ecosystems, herbaceous vegetation is a key strata and share to largest proportion of species diversity. The changes in herb layer occurred with topographic heterogeneity and biotic disturbances and harbor the majority of plant diversity in deciduous forests ecosystem (Gilliam, 2003; Jhariya et al., 2013). Herbaceous vegetation also affect regeneration of trees and therefore, has important implications for the regeneration of trees. Canopy structure have particular important influence on understory through the effects of canopy trees competing with the under story for resources both above (light) and below ground (water and nutrients) (Riegel, 1992; Okland, 1999).
It is considered that oak and oak mixed forests support the herbaceous diversity (Bargali et al., 2014, 2015b ). Natural and human disturbances are considered as major drivers of species diversity in plant communities (Kittur et al., 2014; Jhariya et al., 2014). Under extensive disturbance, species diversity normally declines, but moderate disturbance can enhance or reduce it depending on the spatial scale and types of species (Dumbrell, 2008). According to Odum (1972), those species which exert major controlling influence by their number, size, production and other activities within the community are ecological dominant. The study of the plant community is important for understanding the functioning of community (Singh and Singh, 2010). The present paper is an attempt to analyze the effect of various biotic disturbances on herbaceous vegetation in cypress mixed oak forests of Central Himalaya, India.
Study Area and Methods
Location
The study area are located near Nainital town (29o36’56”-29o36’79” N latitude and 79o46’03”-79o46’19” E longitude) between 1600- 1850m above mean sea level in the Central Himalaya. This area mainly lies in the hilly tract of the district Nainital in Uttarakhand. The study sites experienced a heavy land slide that occurred about 75-80 year ago (based on information collected from locals).
Climate
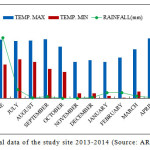
The climate of Nainital is characterized by long-cold often snowy winter and short summer. It is temperate and monsoon type (Singh and Singh, 1992) and the year have four distinct seasons viz., monsoon (July to September), post-monsoon (October to November), winter (December to January) and summer (April to mid-June). Climatic data for 2013–2014 were obtained from the State Observatory at Nainital. The annual average rainfall was 1853 mm, 60% of which occurred in the rainy season and the mean daily temperature ranged from -2.0°C to 30.5°C (Source: ARIES, Nainital) (Fig. 1).
 |
|
Sampling
Two sites having various frequencies of disturbances were selected in the cypress mixed oak forest. Canopy cover and frequency of human activities were considered to categorize the disturbance. With the help of disturbance index (based on the minimum and maximum values of observed disturbance parameters) following Misra et al., (2004), sites were classified as: (i) lowly disturbed (LD), where the human activities were not a regular feature but people used these forests in a particular time of the year with closed canopy (ii) highly disturbed (HD), where frequency of human activities is high (e.g. lopping, grazing, litter removal and fire) with open canopy. Each site was further subdivided into three sub sites viz. Hill Base (HB), Hill Slope (HS) and Hill Top (HT) (1 ha area of each sub-site).
The vegetational analysis of herb species was conducted by placing randomly 10 quadrats of 1m x 1m size at different locations on each of the three sub-sites (viz., hill base, hill slope and hill top) of each site. The size and number of samples were determined following Saxena and Singh, (1982). Grasses were sampled through tiller analysis and each tiller of grass was considered as an individual plant (Singh, 1967) and creeping plants were counted on the basis of presence of functional roots (Singh, 1969). The vegetational data were quantitatively analyzed for abundance, density and frequency (Curtis and Mcintosh, 1950). The Provenance value (PV) of herbs was determined as the sum of the relative frequency and relative density (Curtis, 1959). The ratio of abundance to frequency indicates regular distribution if < 0.025, random distribution between 0.025 and 0.05 and contagious distribution if A/F ratio is >0.05 (Curtis and Cottam, 1956). Similarity between sites was calculated following Muller – Dombois and Ellenberg (1974) using species richness in different forests:

where, C is the common species in comparison forests/sites; A is the total number of species in forest A and B is the total number of species in forest B.
Species diversity for each stand was determined with the Shannon and Wiener (1963), information function, which reads
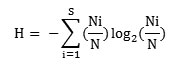
where, Ni is the total number of individuals of species i and N is the total number of individuals of all species in that stand.
Concentration of dominance was measured by Simpson's index (Simpson, 1949):
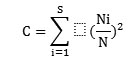
where, Ni and N were the same as i=1 for the Shannon-Wiener information function.
Species richness was determined following Whittaker (1960) as the total number of species in a given community and the equitability was calculated following Pielou (1966). Dominance diversity curves (Whittaker, 1975) were employed to interpret the community organization in terms of resource share or ecological niche.
Results
Floristic Composition
A total of 69 plant species were recorded across all the forest sites (16 trees, 18 shrubs and 35 herbs) distributed in 66 genera and 47 families. In herb layer, 35 species belonging to 20 families were recorded. Lowly disturbed site was more diverse with 27 species distributed in 18 families as compared to highly disturbed site with 23 species distributed in 13 families (Table 1). The most dominant family was Asteraceae with maximum number of species (40% of the total species).
Table 1: Species richness in lowly and highly disturbed cypress mixed oak forest
|
Forest sites |
Trees |
Shrubs |
Herbs |
Total |
|
Lowly disturbed |
15 |
13 |
27 |
55 |
|
Highly disturbed |
07 |
14 |
23 |
44 |
Soil Conditions
The rocks present in lowly disturbed and highly disturbed forest site are commonly called infra krol. These rocks are carbonaceous, locally oxidized into an ash-grey colour, with characteristic oxidization rings on primary planes. The soil is residual to fairly deep and sandy loam in texture. The bulk density was higher in the lowly disturbed site as compared to highly disturbed site. The soil pH was slightly higher (6.27-6.67) at lowly disturbed site as compared to the highly disturbed site (6.13-6.26). The soil in the highly disturbed site had markedly lower moisture level than in the lowly disturbed site. The surface soil temperature measured in the daytime was markedly higher on highly disturbed site than lowly disturbed site. The disturbed oak sites on debris are part of the lower and upper krol formation. The pyritic carbonaceous rocks exposed belong to the infra krol member.
Herb Density and Provenance Value
In lowly disturbed site, total herb density ranged from 142.4 ind.m-2 (hill base) to 174.0 ind.m-2 (hill slope). Contrary to this, in highly disturbed site it varied from 100.8 ind.m-2 (hill base) to 135.6 ind.m-2 (hill top). At lowely disturbed site, S. bryopteris (62 ind.m-2) showed maximum individual herb density at hill top, followed by C. rotundus (45 ind.m-2) at hill slope. However, at highly disturbed site, C. rotundus (40 ind.m-2) showed maximum individual herb density at hill top followed by A. lanceolatus (32 ind.m-2) at hill slope (Table 2). According to PV value S. bryopteris was the most dominant and C. rotundus was the pre-dominant herb species in lowly disturbed site whereas, C. rotundus showed dominance and A. lanceolatus showed pre-dominance in highly disturbed site (Table 2). The mean value of total herb density was maximum in lowly disturbed site (160.4 ind.m-2) and minimum in highly disturbed site (114.8 ind.m-2).
 |
|
Distribution Pattern
Regular distribution was totally absent, while contagious distribution was most common in both the sites and random distribution was rarely observed. In lowly disturbed site, 11% herb species showed random distribution and 89% showed contagious distribution. In highly disturbed site, 2% herbs showed random distribution and 98% herbs showed contagious distribution (Table 3) indicating that. disturbance caused a slight shift in the distribution pattern of herbs.
Table 3: Distribution pattern of herb species in lowly and highly disturbed sites in cypress mixed oak forest
|
Site |
Hill base |
Hill slope |
Hill top |
||||||
|
|
R |
r |
C |
R |
r |
C |
R |
r |
C |
|
Lowly disturbed |
- |
5.88 |
94.12 |
- |
14.29 |
85.71 |
- |
13.33 |
86.67 |
|
Highly disturbed |
- |
6.25 |
93.75 |
- |
- |
100.00 |
- |
- |
100.00 |
Note: R = Regular, r = random, C = Contagious
Similarity Index
The herb layer composition of the study sites was compared by similarity coefficients calculated on the basis of species richness (Table 4). According to it, the herb species showed maximum similarity (34.5%) between hill slope and hill top of lowly disturbed site, while only 12.9% herb species were similar between hill top of lowly disturbed site and hill base of highly disturbed site.
Table 4: Similarity coefficients calculated on the basis of species richness of herb species for different forests (% of total species)
|
LD-HB |
LD-HS |
LD-HT |
HD-HB |
HD-HS |
HD-HT |
|
|
LD-HB |
100 |
25.81 |
21.88 |
21.21 |
17.24 |
17.86 |
|
LD-HS |
100 |
34.48 |
23.33 |
19.23 |
24.00 |
|
|
LD-HT |
100 |
12.90 |
14.81 |
23.08 |
||
|
HD-HB |
100 |
28.57 |
25.93 |
|||
|
HD-HS |
100 |
30.43 |
||||
|
HD-HT |
100 |
LD: lowly disturbed site, HD: highly disturbed site
Table 5: Diversity index of herbs layer in lowly and highly disturbed site in cypress mixed oak forest
|
Forest type |
Altitude |
Species richness(d) |
Simpson index(Cd) |
Shannon Index(H') |
Equitability(e) |
|
Lowly disturbed |
Hill base |
17 |
0.15 |
3.19 |
1.13 |
|
Hill slope |
14 |
0.14 |
3.23 |
1.22 |
|
|
Hill top |
15 |
0.02 |
2.89 |
1.07 |
|
|
Highly disturbed |
Hill base |
16 |
0.12 |
3.47 |
1.25 |
|
Hill slope |
12 |
0.21 |
2.65 |
1.07 |
|
|
Hill top |
11 |
0.19 |
2.74 |
1.15 |
Table 5 indicates that the maximum species richness was observed at hill base of the lowly disturbed site. However minimum species richness was observed at hill top of the highly disturbed site. Concentration of dominance was highest at hill slope of highly disturbed site and lowest at hill top of lowly disturbed site. Maximum diversity was observed at hill base, while minimum diversity was observed at hill slope of highly disturbed site. Equitability was greater at hill slope of lowly disturbed site and lowest at the hill top of the lowly disturbed as well as hill slope of highly disturbed site.
Herb Biomass
The maximum total biomass of herbs was observed at hill top, whereas minimum biomass was reported at hill slope of both the sites. It ranged from 435.2 gm-2 to 498.0 gm-2 in lowly disturbed site and 216.6 gm-2 to 571.8 gm-2 in highly disturbed site. C. buchaniana at hill base and V. serpens at hill top showed minimum (0.02 gm-2) biomass, contrary to this C. rotundus at hill slope showed maximum (172.4 gm-2) biomass at lowly disturbed site. Highest biomass (253.3 gm-2) was observed for E. adenophorum at hill top and lowest (0.08 gm-2) for R. cordifolia as well as for F. vesca at hill slope at highly disturbed site (Table 6).
Table 6: Total biomass of different herb species in lowly and highly disturbed sites in cypress mixed oak forest
|
Species |
LD |
HD |
||||
|
Hill base |
Hill slope |
Hill top |
Hill base |
Hill slope |
Hill top |
|
|
Achyranthes bidentata |
75.00 |
38.09 |
6.80 |
- |
17.65 |
15.15 |
|
Ainsliaea aptera |
77.90 |
15.20 |
61.74 |
- |
- |
- |
|
Androsace lanuginose |
- |
- |
- |
74.25 |
- |
- |
|
Artemisia annua |
10.30 |
- |
- |
- |
0.92 |
- |
|
Arthraxon lanceolatus |
27.54 |
38.64 |
103.97 |
5.38 |
11.21 |
14.55 |
|
Bidens pilosa |
- |
- |
- |
- |
0.14 |
- |
|
Clematis buchaniana |
0.02 |
- |
0.03 |
- |
- |
- |
|
Craniotome versicolor |
54.17 |
30.78 |
- |
49.2 |
46.39 |
122.22 |
|
Cynodon dactylon |
9.29 |
1.08 |
- |
12.09 |
- |
- |
|
Cyperus rotundus |
- |
172.36 |
35.24 |
45.14 |
68.1 |
50.68 |
|
Dicliptera bupleuroides |
- |
41.08 |
13.02 |
- |
- |
- |
|
Dioscorea deltoidea |
2.59 |
- |
- |
- |
- |
- |
|
Erigeron billidioides |
104.78 |
- |
- |
7.3 |
- |
- |
|
Eupatorium adenophorum |
89.66 |
- |
- |
10.61 |
- |
253.30 |
|
Fragaria vesca |
0.11 |
- |
- |
- |
0.09 |
- |
|
Galium aparina |
0.51 |
- |
- |
0.54 |
- |
- |
|
Galium rotundifolium |
- |
13.40 |
2.72 |
- |
- |
0.20 |
|
Geranium nepalense |
- |
- |
- |
58.01 |
3.73 |
0.37 |
|
Gerbera gossypina |
- |
3.36 |
1.05 |
1.39 |
57.74 |
110.12 |
|
Goldfussia dalhousiana |
5.03 |
10.53 |
52.63 |
6.77 |
- |
- |
|
Hedychium spicatum |
- |
- |
137.88 |
- |
- |
- |
|
Justicia simplex |
- |
- |
- |
0.46 |
2.18 |
1.62 |
|
Lepidagathis cristata |
- |
- |
- |
4.32 |
8.35 |
- |
|
Melaxia acuminata |
0.04 |
- |
- |
- |
- |
- |
|
Micromeria biflora |
- |
- |
- |
2.71 |
- |
- |
|
Onychium cryptogrammoides |
- |
40.24 |
- |
7.01 |
- |
- |
|
Rubia cordifolia |
- |
- |
- |
0.19 |
0.09 |
- |
|
Selaginella bryopteris |
19.04 |
30.09 |
76.26 |
- |
- |
3.16 |
|
Stellaria media |
- |
0.07 |
- |
- |
- |
- |
|
Thalictrum foliolosum |
- |
- |
0.18 |
- |
- |
- |
|
Tragopogon gracile |
- |
- |
5.32 |
- |
- |
- |
|
Tridax procumbens |
- |
- |
- |
- |
- |
0.45 |
|
Viola canescens |
0.70 |
- |
- |
- |
- |
- |
|
Viola serpens |
- |
- |
0.02 |
- |
- |
- |
|
Vitis himalyana |
3.90 |
0.27 |
1.18 |
- |
- |
- |
|
Total |
480.58 |
435.19 |
498.04 |
285.82 |
216.58 |
571.82 |
LD: Lowly disturbed, HD: Highly disturbed
Discussion
Floristic inventory and diversity studies help us to understand the species composition and diversity status of forests (Phillips et al., 2003) which also offer vital information for forest conservation. In the present study, species richness was higher in lowly disturbed site as compared to highly disturbed site. The low species richness at highly disturbed site may be attributed to environmental stress in disturbed sites (Usman et al., 1998). Forest disturbance can alter environmental condition by changing light availability and soil conditions (Fredericksen and Mostacedo, 2000). The disturbance produce spatio-temporal mosaic of patches at different successional states and plays an important role in structuring of ecosystem. Recurrent human intervention for the collection of fuel wood, fodder, litter and minor forest products as well as grazing, browsing and trampling can substantially alter species habitat (Pandey and Shukla, 1999). Both natural and human disturbances influence forest dynamics and plant diversity at local and regional scales (Sheil, 1999). The species richness of a site subjected to disturbance depends on the differential responses of species to such disturbances; some species may tolerate the disturbances, while others may become locally extinct (Sagar et al., 2003).
The total herb density was maximum in lowly disturbed site as compared to highly disturbed site. Disturbance caused by lopping and felling of trees create gaps in the stands, which generally increase light intensity and soil temperature, while reduce competition for water and nutrients, compared with undisturbed site (Danslow et al., 1998). Therefore, the combined effects of increased light intensity, increased soil temperature and reduced competition for resources, might favour the regeneration process of many species. Among distribution pattern of herbaceous layer the contagious distribution was the most common in both sites. Odum (1971) opined that contagious distribution is the most common pattern, while random distribution is found only in very uniform environment. The contagious pattern of distribution may be due to the fact that the majority of herb species reproduce vegetatively in addition to sexually. The vegetative reproduction may not be the only reason for contagious distribution, as the recent researches indicate that the contagious vegetation is due to multitude of factors. Several workers (Kershaw, 1973; Singh and Yadava, 1974; Saxena, 1999; Bargali, 1986; Tewari, 1982 and Kharkwal, 2002) have also observed that among regular, random and contagious distribution, the contagious distribution was the most common in the herb layer.
The lowly disturbed site showed high similarity with its sub-sites as compared to highly disturbed site. Chandra et al., (1989) have observed that the herb strata under Q. leucotrichophora forest are similar to the present study. The diversity of herb was higher in the lowly disturbed site.
Forest diversity is the main source of livelihood of the people living in Uttarakhand, Central Himalaya. The increasing population over the last few decades has led to the vast exploitation of natural flora of this region. Thus biodiversity of these forests is under great anthropogenic pressure. From conservation prospective, it is necessary to protect the forests for sustainable management of non-timber forest resource which in turn maintains understory composition and diversity as well as habitat heterogeneity.
References
- Singh, J.S. and Singh, S.P. Forest vegetation of Himalaya. Botanical Review. 53(1): 80-192 (1987).
CrossRef - Bargali, S.S. and Bargali, K. Diversity and biomass of the under story vegetation in an age series of Eucalyptus tereticornis International Journal of Ecology and Environmental Science. 26: 173-181 (2000).
- Augusto, L., Dupouey, J.L. and Ranger, J. Effects of tree species on understorey vegetation and environmental conditions in temperate forests. Annals of Forest Science. 60: 823-831 (2003).
CrossRef - Whigham, D.F. Ecology of woodland herbs in temperate deciduous forests. Annual Review of Ecology, Evolution, and Systematics. 35: 583-621 (2004).
CrossRef - Bargali, K., Maurya, N.R. and Bargali, S. Effect of a Nitrogen-fixing Actinorhizal Shrub on Herbaceous Vegetation in a Mixed Conifer Forest of Central Himalaya. Current World Environment 10(3): 957-966. (2015a).
CrossRef - Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. 57: 845-858. (2007).
- Rana S., Bargali, K. and Bargali, S. Assessment of plant diversity, regeneration status, biomass and carbon stock in a Central Himalayan cypress forest. International Journal of Biodiversity and Conservation. 7(6):321-329 (2015).
CrossRef - Muller, R.N. Nutrient relations of the herbaceous layer in in deciduous forest ecosystems. In Gilliam, F.S., Roberts, M.R.(Eds.), The Herbacious Layer in Forests of Eastern North America. Oxford University Press, New York. 15-37 (2003).
- Gilliam, F.S. and Roberts, M.R. Conceptual framework for studies of the herbaceous layer. In: Gilliam FS, Roberts MR (eds) The herbaceous layer in forests of Eastern North America. Oxford University Press, Oxford, 3-11 (2003).
- Jhariya, M.K., Bargali, S., Swamy, S.L. and Oraon, P.R. Herbaceous diversity in Rowghat, Narayanpur District of Chhattisgarh, India. Journal of Plant Development Sciences. 5 (4): 385-393(2013).
- Riegel, G.M., Miller, R.F. and Krueger, W.C. Competition for resources between understorey vegetation and overstorey Pinus ponderosain northeastern Oregon. Ecological Applications. 2: 71-85 (1992).
CrossRef - Okland, R.H., Rydgren, K. and Okland, T. Single-tree influence on understorey vegetation in a Norwegian boreal spruce forest. Oikos. 87: 488-499 (1999).
- Bargali, K., Joshi, B., Bargali S.S. and Singh, S.P. Diversity within Oaks. International Oaks. 25: 57-70 (2014).
- Bargali, K., Joshi, B., Bargali, S.S. and Singh, S.P. Oaks and Biodiversity They Sustain. International Oaks. 26:65-76 (2015).
- Kittur, B., Swamy, S.L., Bargali, S.S. and Jhariya, M.K. Wildland Fires and Moist Deciduous Forests of Chhattisgarh, India: Divergent Component Assessment. Journal of Forestry Research. 25(4): 857-866 (2014).
- Jhariya, M.K., Bargali, S., Swamy, S.L., Kittur, B., Bargali, K. and Pawar, G.V. Impact of forest fire on biomass and Carbon storage pattern of Tropical Deciduous Forests in Bhoramdeo Wildlife Sanctuary, Chhattisgarh. International Journal of Ecology and Environmental Science. 40(1): 57-74 (2014).
- Dumbrell, A.J., Clarke, E.S., Frost, G.A., Randel, T.E., Pitchford, J.W. and Hill, J.K. Changes in species diversity following habitat disturbance are dependent on Spatial scale: theoretical and empirical evidence. Journal of Applied Ecology. 45(5): 1531-1539 (2008).
- Odum, E.P., Fundamentals of ecology. W.B. Saumders Co. Philadelphia, U.S.A. (1972).
- Singh, E. and Singh, M.P. Biodiversity and phytosociological analysis of plants around the municipal drains in Janupur. International Journal of Biological and Life Sciences. 6(2): 77-82 (2010).
- Singh, V.P. and Singh, J.S. Energetics and environmental coasts of agriculture in a dry tropical region of India. Environmental Management. 16: 495-503 (1992).
CrossRef - Misra, B.P., Tripathi, O.P. and Pandey, H.N. Effects of anthropogenic disturbance on plant diversity and community structure of a sacred groove in Meghalaya, Northeast India. Biodiversity and Conservation. 13: 421-436 (2004).
- Saxena, A.K. and Singh, J.S. A phytosociological analysis of woody species in forest communities of a part of Kumaun Himalaya, India. Indian Journal of Management. 1: 13-32 (1982).
- Singh, J.S. Seasonal variation in composition, plant biomass and net primary productivity in the grass land of Varansi. Ph. D. Thesis, Banaras Hindu University, Varanasi, India. 318 (1967).
- Singh, J.S. Influence of biotic disturbance on the preponderance and interspecific association of two common herbs in the grasslands at Varanasi, India. Tropical Ecology. 10: 59-71 (1969).
- Curtis, J.T. and Mcintosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology. 31: 438-455 (1950).
- Curtis, J.T. The vegetation of Wisconsin. An ordination of plant community University Wisconsin Press, Madison, Wisconsin. 657 (1959).
- Curtis, J.T. and Cottam, G. Plant ecology work book. Laboratory field reference manual. Burgess Publication Co., Minnesota. 193(1956).
- Muller-Dombois, D. and Ellenberg. H. Aims and methods of vegetation ecology. Wiley, New York 547 (1974).
- Shannon, C.E. and Wiener, W. The Mathematical Theory of Communication. University of Illinois Press, Urbana (1963).
- Simpson, E.H. Measurement of diversity. Nature, 168:688 (1949).
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecological Monographs. 30: 279-338 (1960).
- Pielou, E.C. The measurement of diversity in different types of biological collections. Journal of Theoretical biology, 13: 131-144 (1966).
CrossRef - Whittaker, R.H. Communities and Ecosystem. 2nd edition. Macmillon Publishing co., Newyork. 385 (1975).
- Phillips, O.L., Martinez, R.V., Vargas, P.N., Monteagudo, A.L., Zans, M.C., Sanchez, W.G., Cruz, A.P., Timana, M., Yli-Halla and Rose, S. Efficientplot-based floristic assessment of tropical forests. Journal of Tropical Ecology, 19: 629-645 (2003).
- Usman, S., Chandra G., Singh, S. and Tewari A. Vegetational Analysis of herb layer in Oak and Pine forests of Central Himalaya. Journal of Environmental biology. 10(4):251-256 (1998).
- Freclericksen, T.S. and Mostacedo, B. Regeneration of timber species following selection logging in a Bolivian tropical dry forest. Forest Ecology and Management.131: 47-55 (2000).
- Pandey, S.K. and Shukla, R.P. Plant diversity and community pattern along the disturbance gradient in plantation forests of Sal (Shorea robusta). Current Science. 77: 814-818 (1999).
- Sheil, D. Tropical forest diversity environmental change and species augmentation after the intermediate disturbance hypothesis. Journal of Vegetation Science. 10: 851-860 (1999).
- Sagar, R., Raghubanshi, A.S. and Singh, J.S. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. Forest Ecology and Management. 186: 61-71 (2003).
CrossRef - Denslow, J.S., Aaron, M.E. and Sanford, R.E. Tree fall gap size effects on above and below ground processes in a tropical wet forest. Journal of Ecology. 86: 597–609 (1998).
CrossRef - Odum, E.P. Fundamentals of Ecology. W.B. Saunders Co.Philadelphia, USA. (1971).
- Kershaw, K.R. Quantitative and dynamic plant ecology. Edward Arnold Ltd., London. 308(1973).
- Singh, J.S. and Yadava, P.S. Seasonal variation in composition, plant biomass, and net primary productivity of a tropical grassland at Kurukshetra, India. Ecological Monographs. 44(3): 351-376 (1974).
CrossRef - Saxena, A.K. Ecology of Vegetation complex of North Western catchment of River Gola, Ph.D. Thesis, Kumaun University, Nainital (1999).
- Bargali, S.S. Composition of woody vegetation in high elevation blue pine mixed oak forest, Kumaun Himalaya, M.Sc. Thesis, Kumaun University, Nainital. (1986).
- Tewari, J.C. Vegetational Analysis along altitudinal gradients around Nainital. Ph.D. Thesis, Kumaun University, Nainital. (1982).
- Kharkwal, G. Spatial Pattern of Plant Species Diversity With Particular Reference to Forest Herbs along an Altitudinal Transect In Central Himalaya. Ph.D. Thesis, Kumaun University, Nainital. (2002).
- Chandra, R., Upadhyay, V.P. and Bargali, S.S. Analysis of herbaceous vegetation under oak and pine forests of Central Himalaya. Environment and Ecology. 7: 521-525 (1989).






